Improvement of Dielectric, Magnetic and Thermal Properties of OPEFB Fibre–Polycaprolactone Composite by Adding Ni–Zn Ferrite
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Ni0.5Zn0.5Fe2O4 Composite Preparation
2.3. Preparation of NZF + OPEFB + PCL Composites
2.4. Characterisations
2.4.1. Fourier Transform Infrared (FTIR)
2.4.2. Thermogravimetric Analysis (TGA) and Differential Thermogravimetric (DTG) Curves
2.4.3. Morphology
2.4.4. Variation in S-Parameter Coefficients with Frequency
2.4.5. Effective (Relative) Permittivity and Effective Magnetic Permeability of NZF Composites
3. Results and Discussion
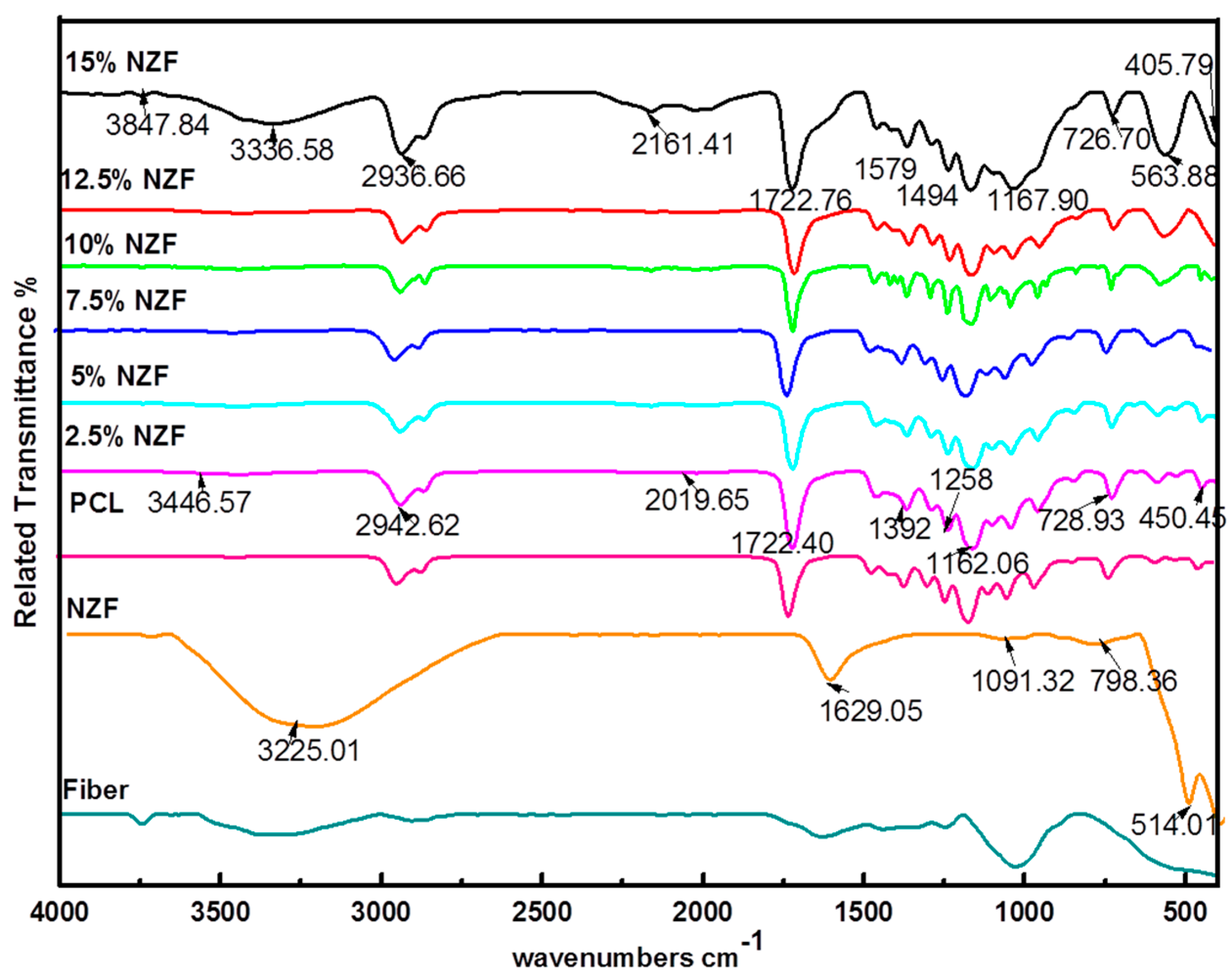
3.1. Fourier Transform Infrared (FTIR)
3.2. Thermogravimetric Analysis of Composites
3.3. Morphological Study of NZF + OPEFB + PCL Composites for Various NZF Filler Contents
3.4. Effective Permittivity of NZF + OPEFB + PCL Composites
3.5. Effective Permeability of NZF Composites
3.6. Absorption Measurement
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Madhuri, G.; Anand, M.; Ajay, S. Blending Strategies of Natural Polymers–A Review. Trends Biomater. Artif. Org. 2016, 30, 61–76. [Google Scholar]
- Kong, L.B.; Li, Z.W.; Liu, L.; Huang, R.; Abshinova, M.; Yang, Z.H.; Tang, C.B.; Tan, P.K.; Deng, C.R.; Matitsine, S. Recent progress in some composite materials and structures for specific electromagnetic applications. Int. Mater. Rev. 2013, 58, 203–259. [Google Scholar] [CrossRef]
- Jamalian, M.; Ghasemi, A. A Study of the Effect of Annealing Temperature on Structural, Magnetic, and Microwave Properties of Barium Hexaferrite Nanoparticles With and Without Substitutions. J. Supercond. Novel Magn. 2015, 28, 3293–3299. [Google Scholar] [CrossRef]
- Phan, C.H.; Mariatti, M.; Koh, Y.H. Electromagnetic interference shielding performance of epoxy composites filled with multi walled carbon nanotubes/manganese zinc ferrite hybrid fillers. Magn. Magn. Mater. 2016, 401, 472–478. [Google Scholar] [CrossRef]
- Mdarhri, A.; Brosseau, C.; Carmona, F. Microwave dielectric properties of carbon black filled polymers under uniaxial tension. J. Appl. Phys. 2007, 101, 084111. [Google Scholar] [CrossRef]
- Mdarhri, A.; Carmona, F.; Brosseau, C.; Delhaes, P. Direct current electrical and microwave properties of polymer-multiwalled carbon nanotubes composites. J. Appl. Phys. 2008, 103, 054303. [Google Scholar] [CrossRef]
- Adohi, B.J.-P.; Mdarhri, A.; Prunier, C.; Haidar, B.; Brosseau, C. A comparison between physical properties of carbon black-polymer and carbon nanotubes-polymer composites. J. Appl. Phys. 2010, 108, 074108. [Google Scholar] [CrossRef]
- Brosseau, C.; Molinié, P.; Boulic, F.; Carmona, F. Mesostructure, electron paramagnetic resonance, and magnetic properties of polymer carbon black composites. J. Appl. Phys. 2001, 89, 8297–8310. [Google Scholar] [CrossRef]
- Ahmad, A.F.; Abbas, Z.; Obaiys, S.J.; Ibrahim, N.A.; Zainuddin, M.F.; Salem, A. Permittivity Properties of Nickel Zinc Ferrite-Oil Palm Empty Fruit Bunch-Polycaprolactone Composite. Proc. Chem. 2016, 19, 603–610. [Google Scholar] [CrossRef]
- Flaifel, M.H.; Ahmad, S.H.; Abdullah, M.H.; Rasid, R.; Shaari, A.H.; El-Saleh, A.A.; Appadu, S. Preparation, thermal, magnetic and microwave absorption properties of thermoplastic natural rubber matrix impregnated with NiZn ferrite nanoparticles. Compos. Sci. Technol. 2010, 96, 103–108. [Google Scholar] [CrossRef]
- Raju, P.; Murthy, S.R. Preparation and characterization of Ni–Zn ferrite + polymer nanocomposites using mechanical milling method. Appl. Nanosci. 2013, 3, 469–475. [Google Scholar] [CrossRef]
- Puryanti, D.; Ahmad, S.H.; Abdullah, M.H. Effect of nickel-cobalt-zinc ferrite filler on electrical and mechanical properties of thermoplastic natural rubber composites. Polym. Plast. Technol. Eng. 2006, 45, 561–567. [Google Scholar] [CrossRef]
- Teber, A.; Unver, I.; Kavas, H.; Aktas, B.; Bansal, R. Knitted radar absorbing materials (RAM) based on nickel–cobalt magnetic materials. J. Magn. Magn. Mater. 2016, 406, 228–232. [Google Scholar] [CrossRef]
- Wurm, A.; Zhuravlev, E.; Eckstein, K.; Jehnichen, D.; Pospiech, D.; Androsch, R.; Wunderlich, B.; Schick, C. Crystallization and homogeneous nucleation kinetics of poly (ε-caprolactone)(PCL) with different molar masses. Macromolecules 2012, 45, 3816–3828. [Google Scholar] [CrossRef]
- Vilay, V.; Mustapha, M.; Ahmad, Z.; Mitsugu, T. Characterization of the microstructure and mode I fracture property of biodegradable poly (L-lactic acid) and poly (ϵ-caprolactone) polymer blends with the additive lysine triisocyanate. Polym. Plast. Technol. Eng. 2013, 52, 768–773. [Google Scholar] [CrossRef]
- Mohammed, L.; Ansari, M.N.M.; Pua, G.; Jawaid, M.; Islam, M.S. A review on natural fiber reinforced polymer composite and its applications. Int. J. Polym. Sci. 2015, 2015, 243947. [Google Scholar] [CrossRef]
- Jovalekić, Č.; Nikolić, A.S.; Gruden-Pavlović, M.; Pavlović, M.B. Mechano–chemical synthesis of stoichiometric nickel and nickel–zinc ferrite powders with Nicolson–Ross analysis of the absorption coefficients. J. Serbian Chem. Soc. 2012, 77, 497–505. [Google Scholar] [CrossRef]
- Ibrahim, N.A.; Hashim, N.; Rahman, M.Z.A.; Yunus, W.M.Z.W. Mechanical Properties and Morphology of Oil Palm Empty Fruit Bunch-Polypropylene Composites: Effect of Adding ENGAGE™ 7467. J. Thermoplast. Compos. Mater. 2011, 24, 713–732. [Google Scholar] [CrossRef]
- Karmarkar, A.; Chauhan, S.S.; Modak, J.M.; Chanda, M. Mechanical properties of wood–fiber reinforced polypropylene composites: Effect of a novel compatibilizer with isocyanate functional group. Compos. Part A Appl. Sci. Manuf. 2007, 38, 227–233. [Google Scholar] [CrossRef]
- Giudicianni, P.; Cardone, G.; Ragucci, R. Cellulose, hemicellulose and lignin slow steam pyrolysis: Thermal decomposition of biomass components mixtures. J. Anal. Appl. Pyrolysis 2013, 100, 213–222. [Google Scholar] [CrossRef]
- Wang, H.; Xiang, F.; Yang, H.; Li, K. Multifunctional Low Loss Ceramic–Polymer Composites for Microwave Applications. In Wiley Encyclopedia of Composites; John Wiley & Sons, Inc.: Chichester, UK, 2012. [Google Scholar]
- Choy, T.C. Effective Medium Theory: Principles and Applications; Oxford University Press: New York, NY, USA, 2015; Volume 165. [Google Scholar]
- Wan, Y.-J.; Yang, W.-H.; Yu, S.-H.; Sun, R.; Wong, C.-P.; Liao, W.-H. Covalent polymer functionalization of graphene for improved dielectric properties and thermal stability of epoxy composites. Compos. Sci. Technol. 2016, 122, 27–35. [Google Scholar] [CrossRef]
- Ahmed, M.A.; Imam, N.G.; Hefny, M.M.; Gomaa, H.R. Synthesis and Characterization of Nanoparticulate Crystallite Cobalt Ferrite for Permanent Magnet Applications. J. Ceram. Sci. Technol. 2016, 7, 235–241. [Google Scholar]
- Fahad, A.; Abbas, Z.; Zainuddin, M.F.; Jabbar, S.; Yakubu, A.B. Dielectric Characterization of Oil Palm Fiber Reinforced Polycaprolactone-nickel Oxide Composite at Microwave Frequency. Proc. Environ. Sci. 2015, 30, 273–278. [Google Scholar] [CrossRef]
- Saini, P. Electrical properties and electromagnetic interference shielding response of electrically conducting thermosetting nanocomposites. In Thermoset Nanocomposites; John Wiley & Sons, Inc.: New York, NY, USA, 2013. [Google Scholar]
- Das, C.K.; Mandal, A. Microwave Absorbing Properties of DBSA-doped Polyaniline/BaTiO3-Ni0.5Zn0.5Fe2O4 Nanocomposites. J. Mater. Sci. Res. 2015, 1, 45. [Google Scholar] [CrossRef]
- Micheli, D.; Apollo, C.; Pastore, R.; Marchetti, M. X-Band microwave characterization of carbon-based nanocomposite material, absorption capability comparison and RAS design simulation. Compos. Sci. Technol. 2010, 70, 400–409. [Google Scholar] [CrossRef]








| Percentage of NZF wt % | Percentage of OPEFB wt % | Percentage of PCL wt % |
|---|---|---|
| 2.5 | 12.2 | 85.3 |
| 5 | 23.8 | 71.2 |
| 7.5 | 34.7 | 57.8 |
| 10 | 45 | 45 |
| 12.5 | 54.7 | 32.8 |
| 15 | 63.8 | 21.2 |
| Filler (%) | First peak (°C) | Second peak (°C) | Third peak (°C) | Fourth peak (°C) | Fifth peak (°C) |
|---|---|---|---|---|---|
| Pure NZF | 51.51 | 285.80 | - | - | - |
| 2.5 | - | - | - | 410.50 | 667.25 |
| 5.0 | - | 283.61 | - | 406.50 | - |
| 7.5 | - | - | - | 405.94 | 692.79 |
| 10.0 | 121.74 | 286.27 | 354.37 | 403.69 | 698.03 |
| 12.5 | 61.17 | 287.74 | 354.74 | 402.10 | 649.22 |
| 15.0 | 51.51 | 285.80 | - | - | - |
| Percentage of NZF wt % | ε′ | ε″ |
|---|---|---|
| 100% PCL | 2.844 | 0.367 |
| 2.5% | 3.058 | 0.390 |
| 5% | 3.257 | 0.434 |
| 7.5% | 3.592 | 0.506 |
| 10% | 3.856 | 0.560 |
| 12.5% | 4.446 | 0.683 |
| 15% | 4.868 | 0.758 |
| 100% NZF | 5.632 | 0.962 |
| Percentage of NZF wt % | μ′ | μ″ |
|---|---|---|
| 2.5 | 0.92 | 0.042 |
| 5 | 0.95 | 0.045 |
| 7.5 | 0.98 | 0.046 |
| 10 | 1.00 | 0.048 |
| 12.5 | 1.02 | 0.051 |
| 15 | 1.05 | 0.054 |
| Pure NZF | 1.17 | 0.073 |
| NZF% | 8 GHz | 9 GHz | 10 GHz | 11 GHz | 12 GHz |
|---|---|---|---|---|---|
| 2.5% | −9.43 | −10.80 | −10.61 | −10.96 | −10.75 |
| 5% | −9.83 | −11.41 | −10.97 | −11.51 | −10.96 |
| 7.5% | −10.09 | −11.71 | −11.63 | −12.50 | −11.60 |
| 10% | −10.46 | −12.16 | −12.08 | −13.00 | −12.01 |
| 12.5% | −10.75 | −12.53 | −12.44 | −13.40 | −12.41 |
| 15% | −11.36 | −13.10 | −12.66 | −14.37 | −13.97 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, A.F.; Abbas, Z.; Obaiys, S.J.; Abdalhadi, D.M. Improvement of Dielectric, Magnetic and Thermal Properties of OPEFB Fibre–Polycaprolactone Composite by Adding Ni–Zn Ferrite. Polymers 2017, 9, 12. https://doi.org/10.3390/polym9020012
Ahmad AF, Abbas Z, Obaiys SJ, Abdalhadi DM. Improvement of Dielectric, Magnetic and Thermal Properties of OPEFB Fibre–Polycaprolactone Composite by Adding Ni–Zn Ferrite. Polymers. 2017; 9(2):12. https://doi.org/10.3390/polym9020012
Chicago/Turabian StyleAhmad, Ahmad F., Zulkifly Abbas, Suzan J. Obaiys, and Daw M. Abdalhadi. 2017. "Improvement of Dielectric, Magnetic and Thermal Properties of OPEFB Fibre–Polycaprolactone Composite by Adding Ni–Zn Ferrite" Polymers 9, no. 2: 12. https://doi.org/10.3390/polym9020012
APA StyleAhmad, A. F., Abbas, Z., Obaiys, S. J., & Abdalhadi, D. M. (2017). Improvement of Dielectric, Magnetic and Thermal Properties of OPEFB Fibre–Polycaprolactone Composite by Adding Ni–Zn Ferrite. Polymers, 9(2), 12. https://doi.org/10.3390/polym9020012







