Microwave Assisted Reduction of Pt-Catalyst by N-Phenyl-p-Phenylenediamine for Proton Exchange Membrane Fuel Cells
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials
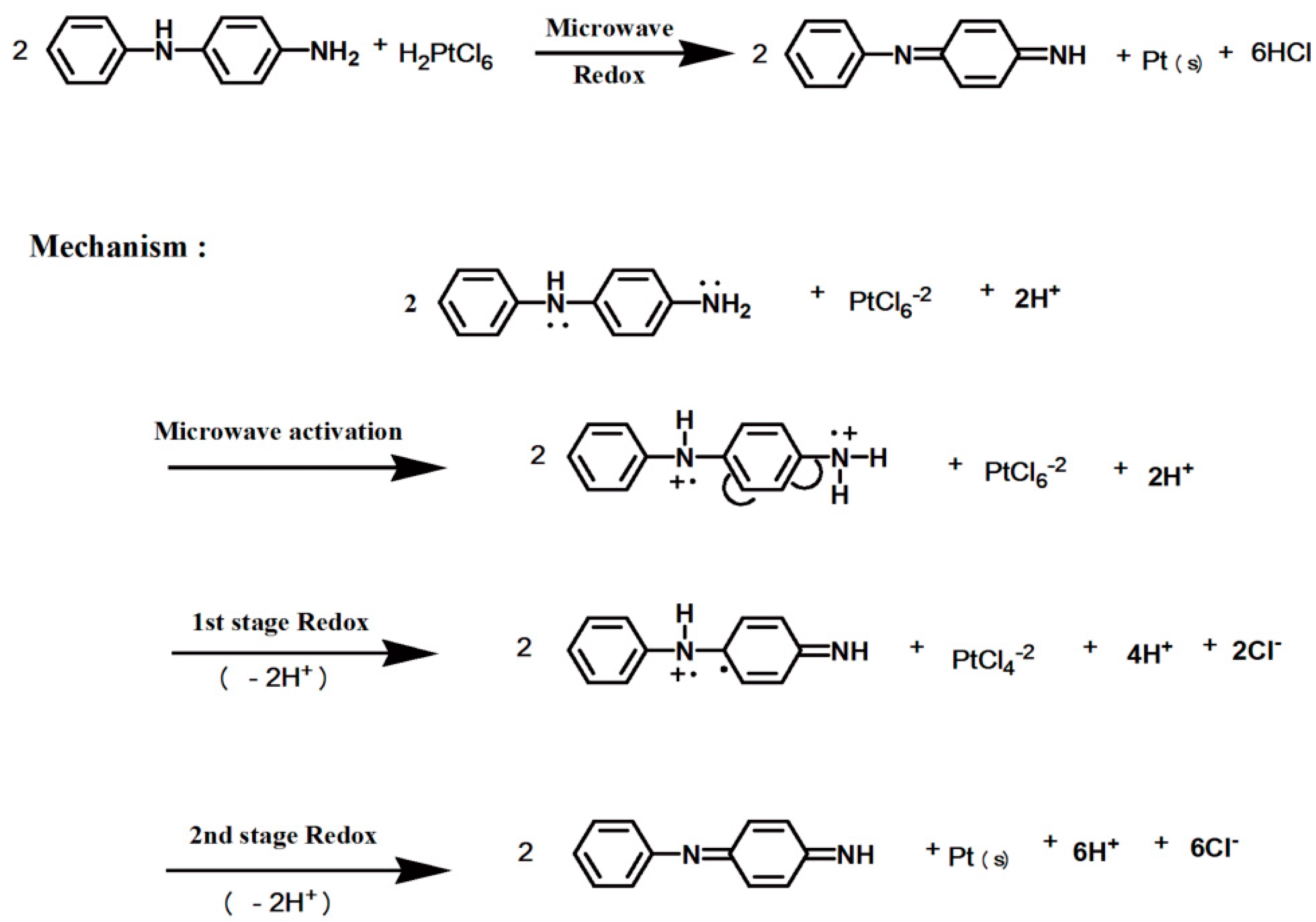
2.2. Preparation of Pt/XC72-PPDA-MW
2.3. Basic Characterization
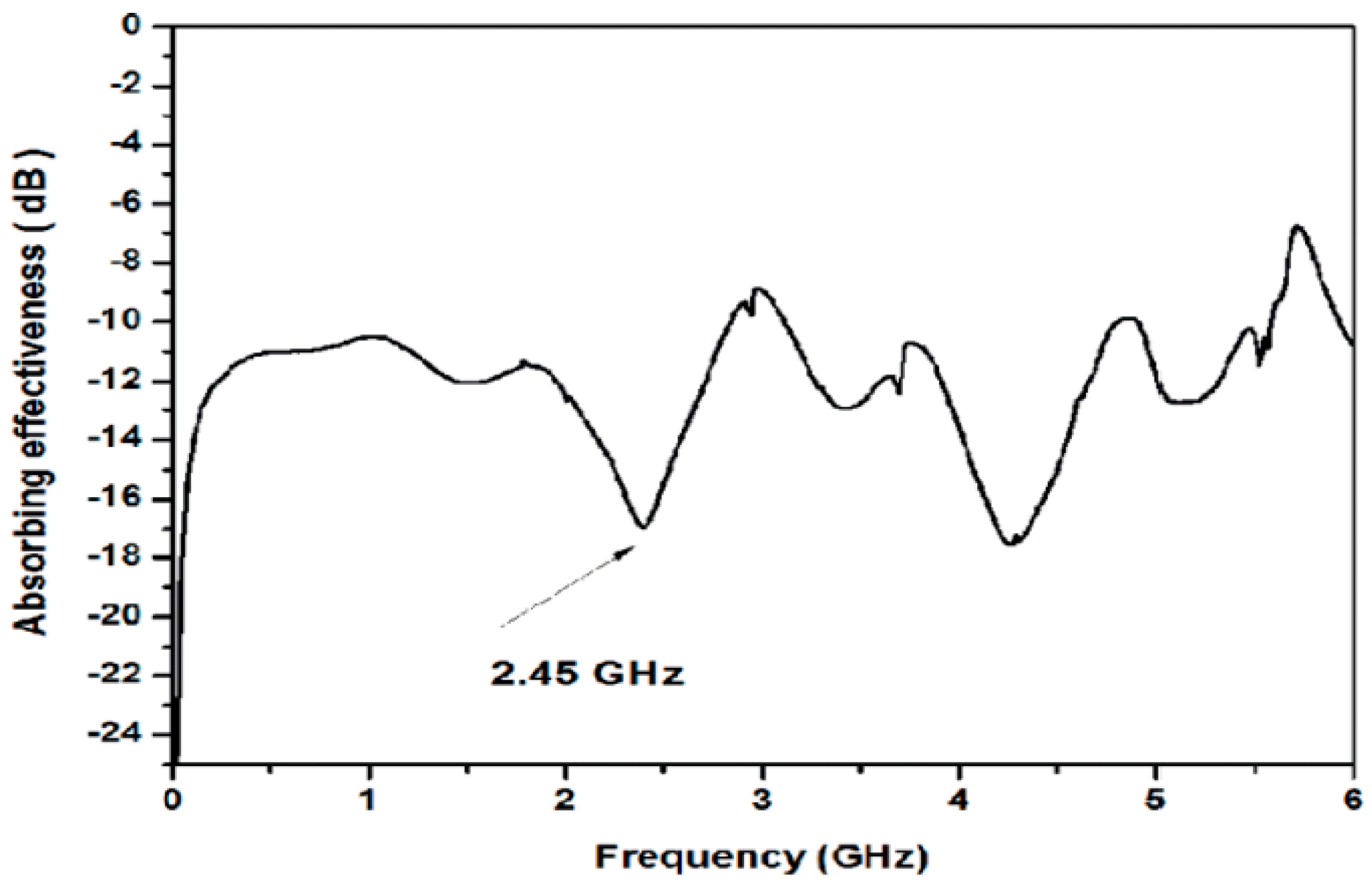
2.3.1. Microwave Vector Network Analyzer, VNA
2.3.2. Microwave Oven
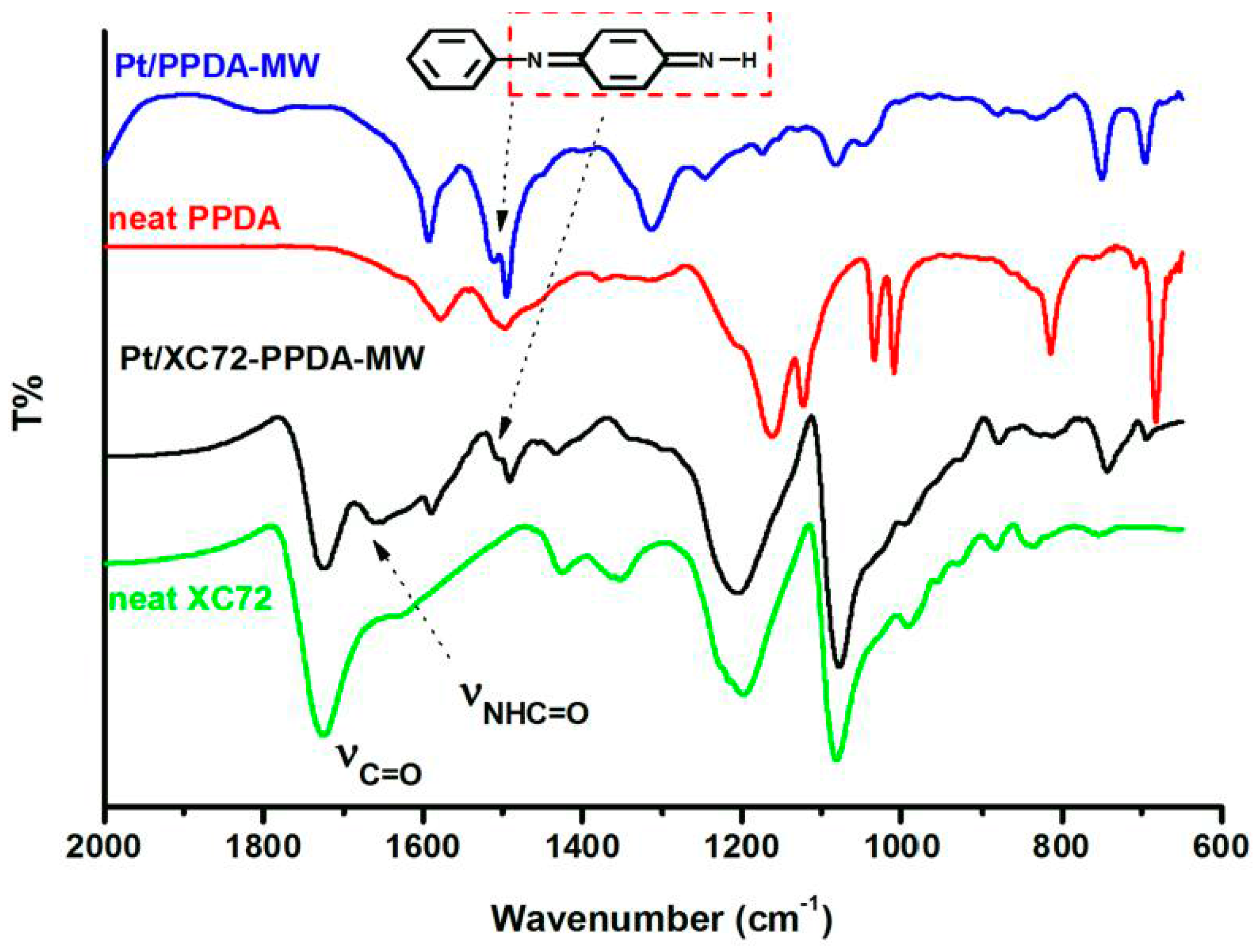
2.3.3. FTIR Spectroscopy
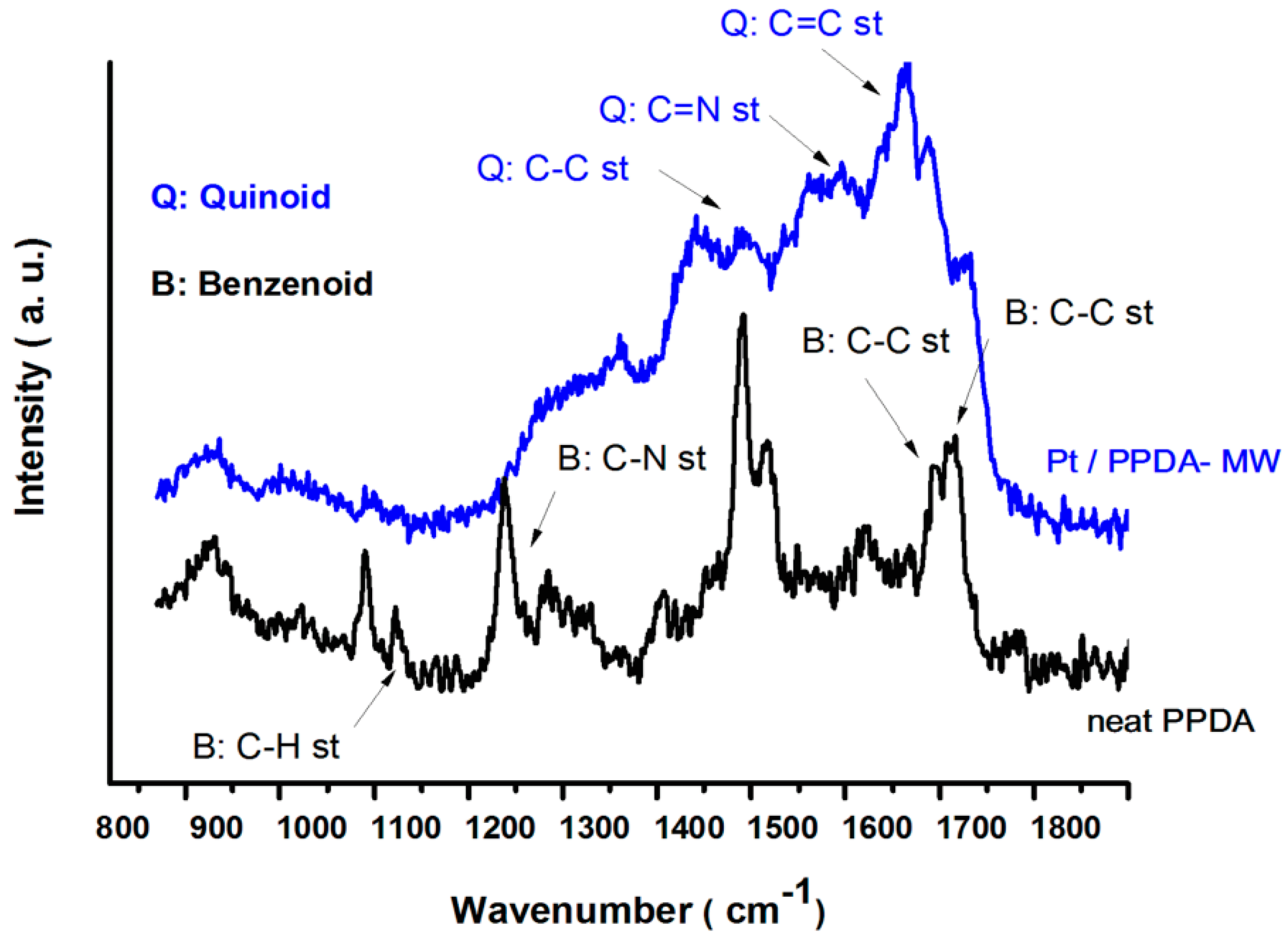
2.3.4. Raman Spectroscopy
2.3.5. UV-VIS-NIR Spectroscopy
2.3.6. TEM (Transmission Electron Microscopy)
2.3.7. TGA (Thermogravimetric Analysis)
2.3.8. WXRD (Wide-Angle X-ray Diffraction: Powder X-ray Diffraction)
2.4. Electrochemical Characterization
2.5. MEA Preparation
2.6. Single-Cell Performance Testing
3. Results and Discussion
3.1. Microwave Absorption of PPDA
3.2. FTIR Spectroscopy
3.3. Raman Spectroscopy
3.4. UV-VIS Spectroscopy
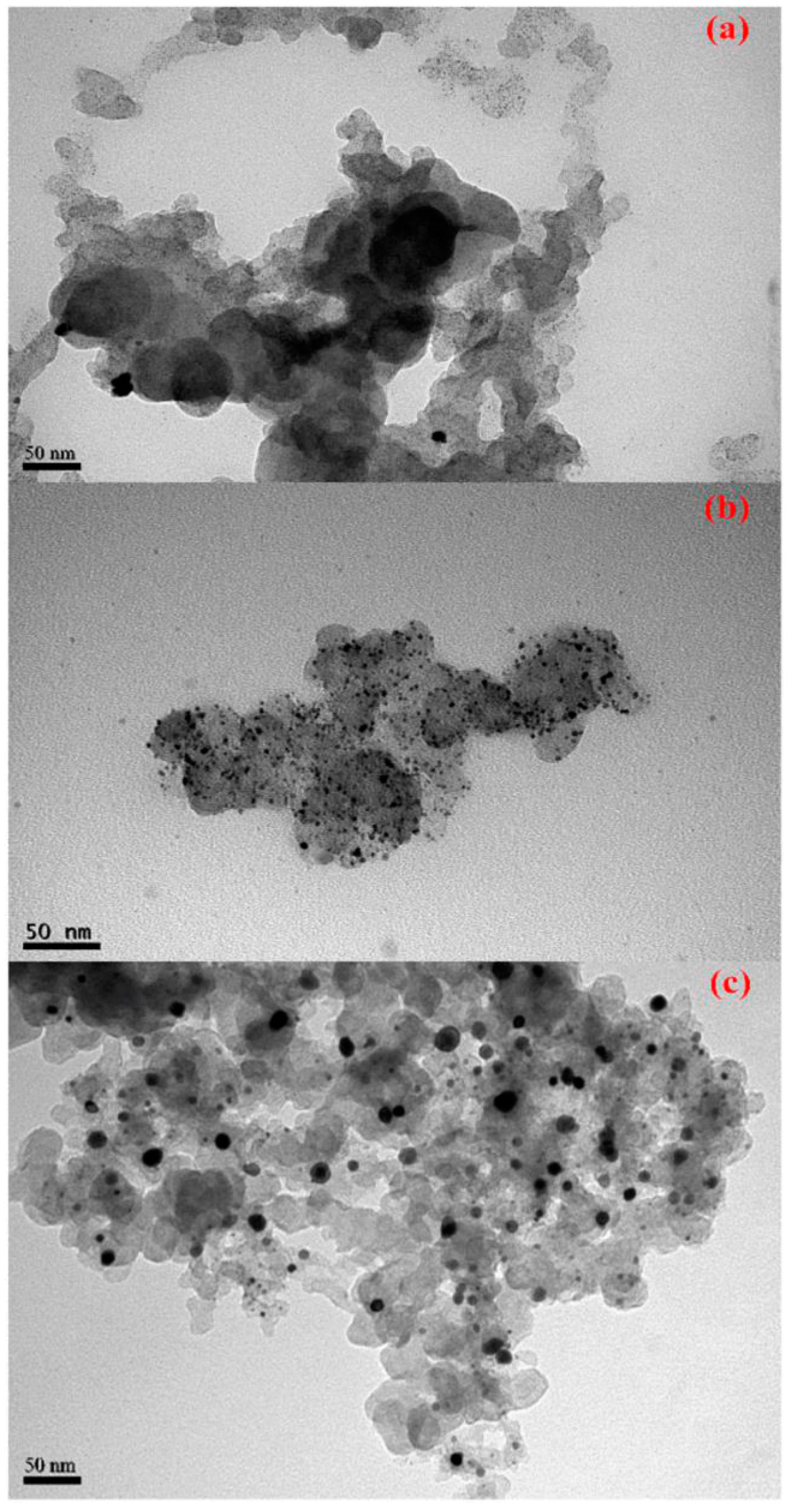
3.5. Transmission Electronic Microscopy (TEM)
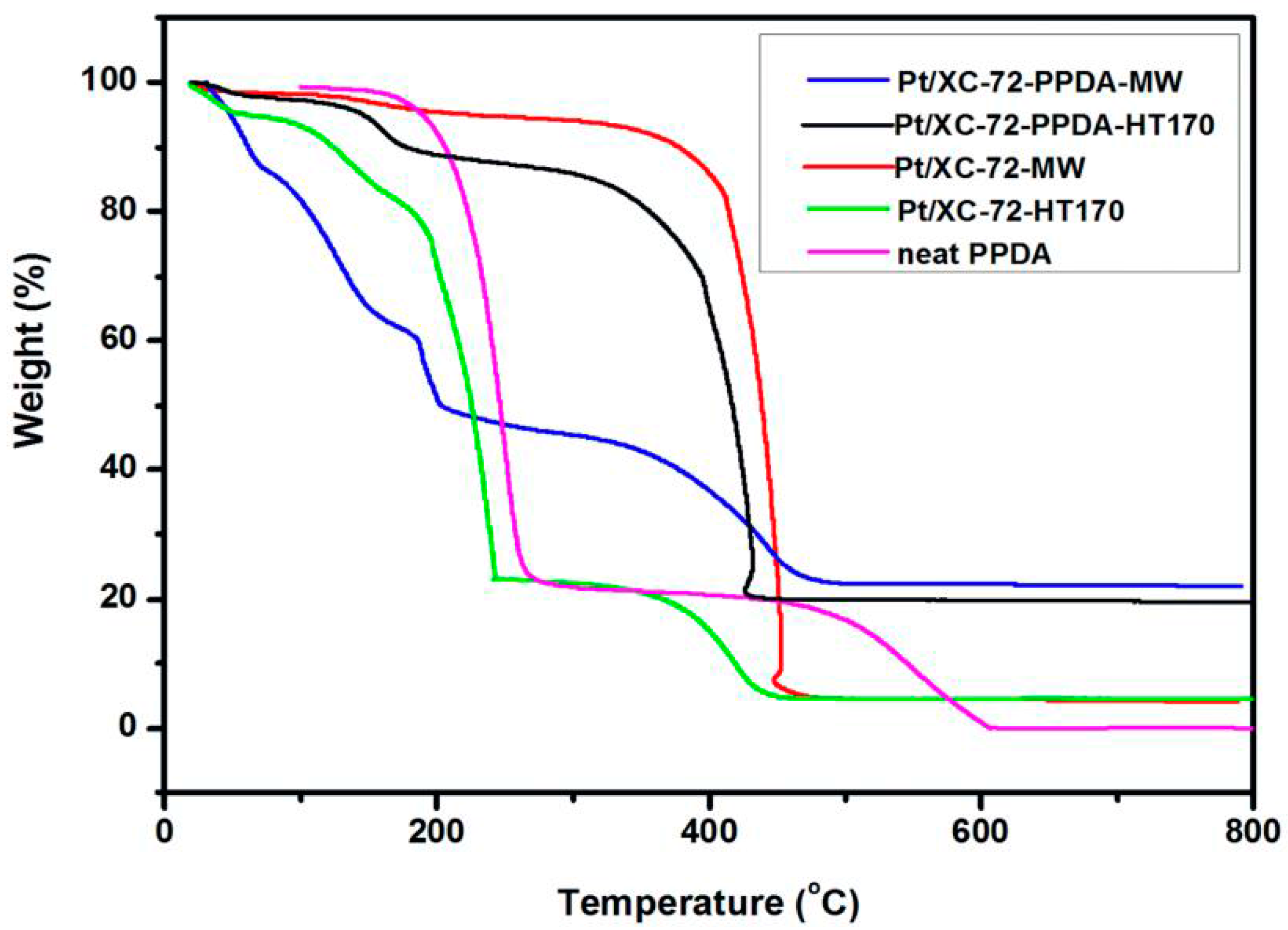
3.6. Thermogravimetric Analysis (TGA)
3.7. X-ray Diffraction Pattern of the Electrocatalyst Electrode Materials
3.8. Electrochemical Analysis
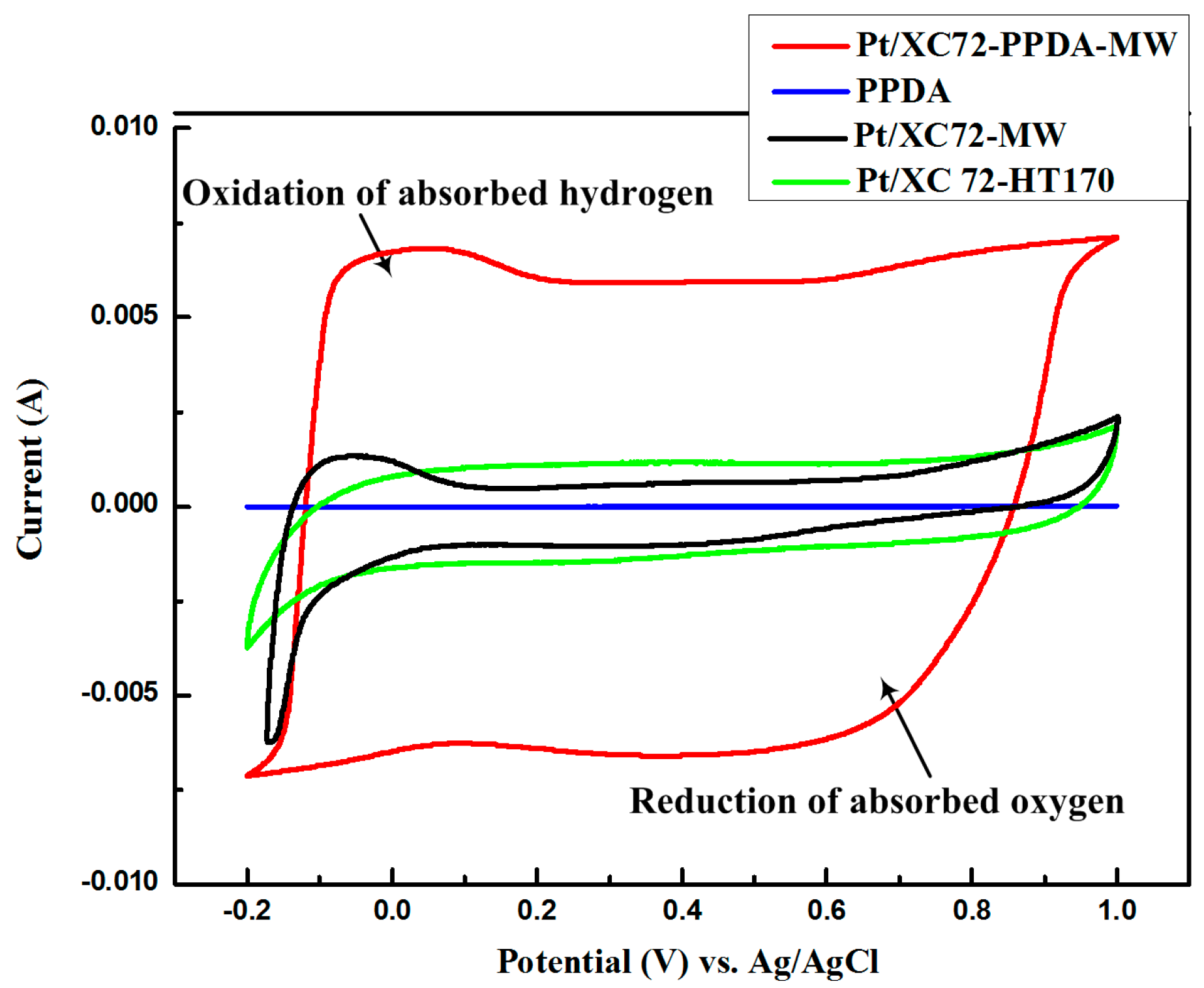
3.8.1. Cyclic Voltammetry (CV)
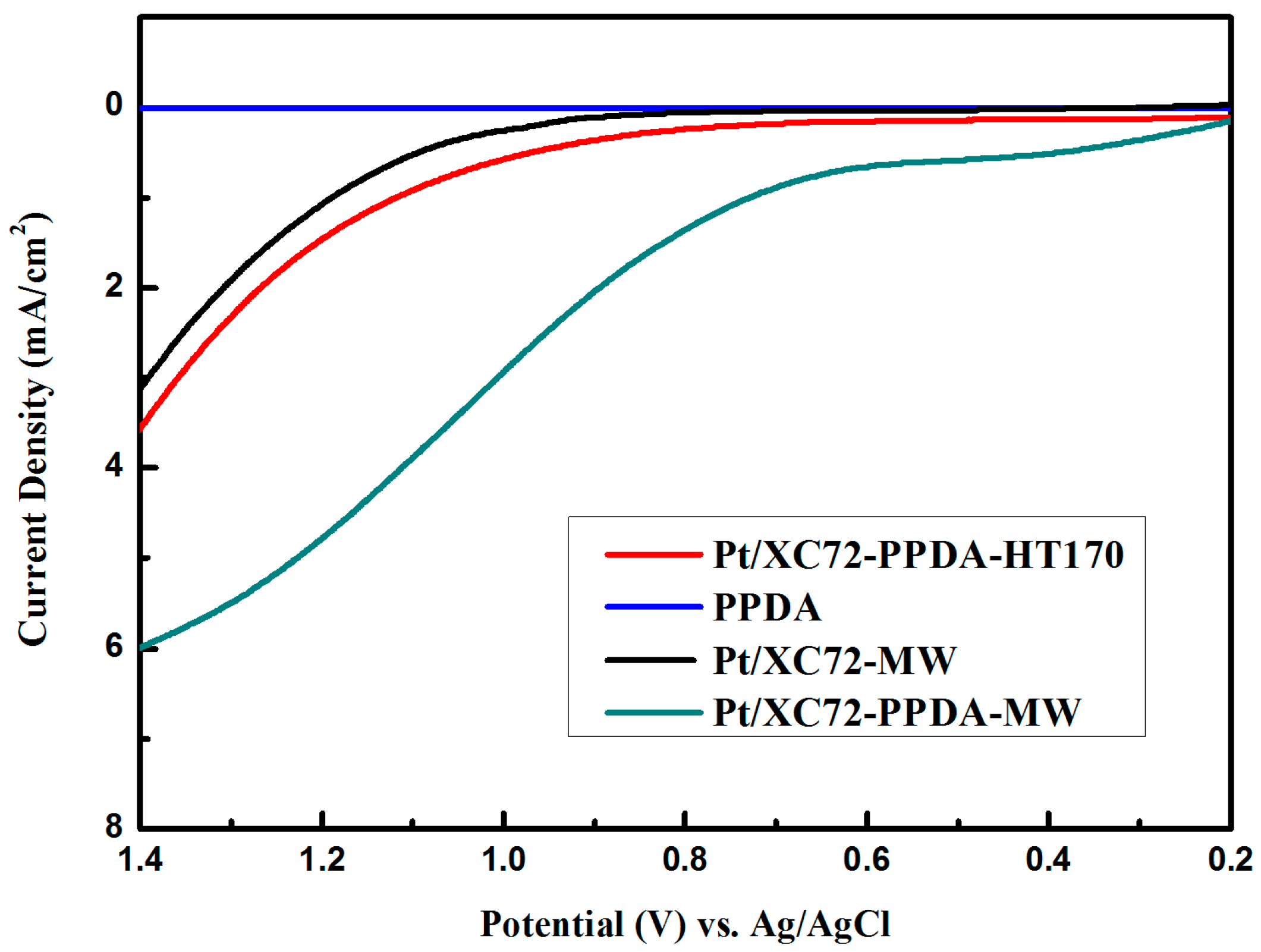
3.8.2. Oxygen Reduction Reaction (ORR) Performance
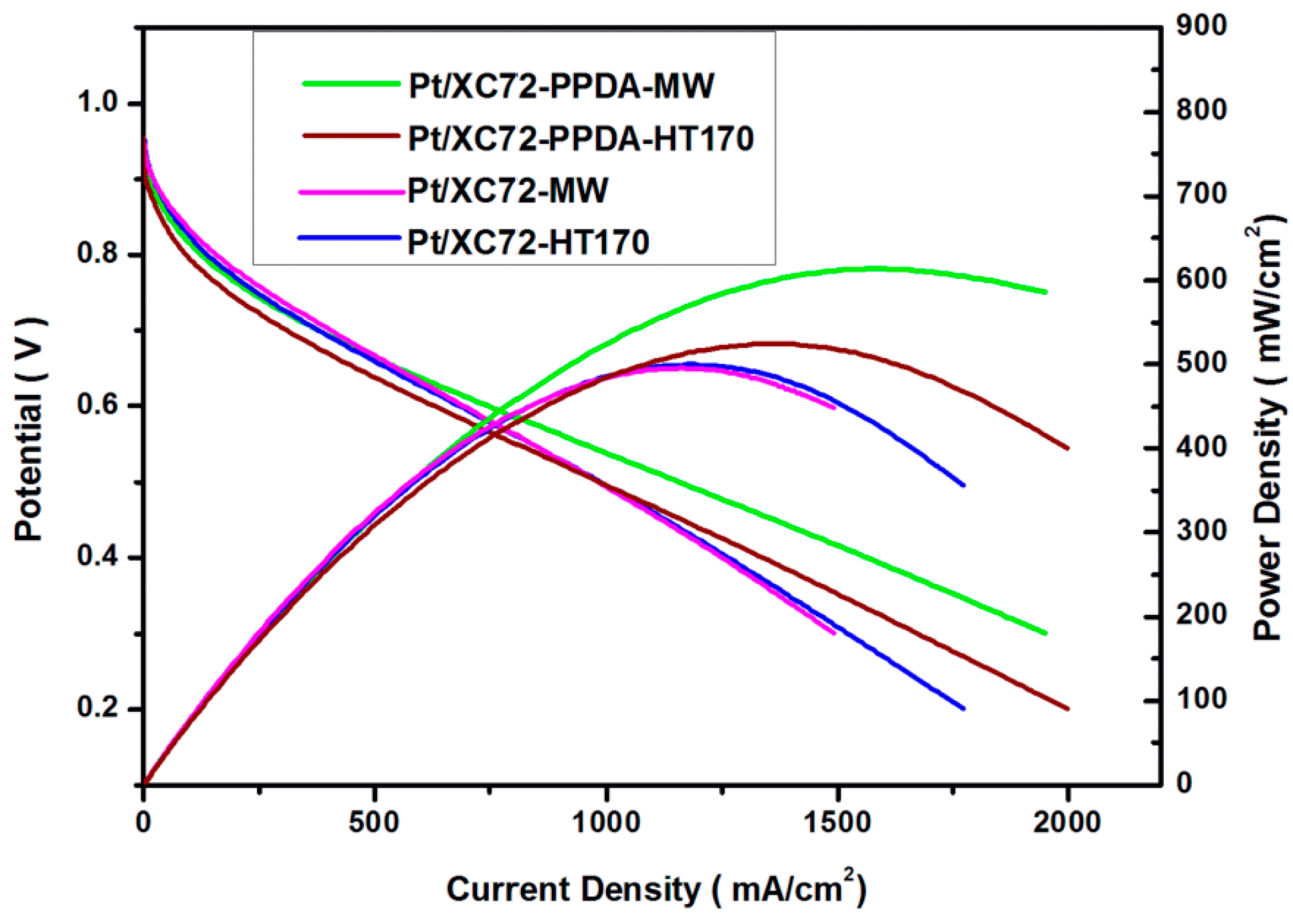
3.8.3. Single Cell Performance Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Brentner, L.B.; Peccia, J.; Zimmerman, J.B. Challenges in Developing Biohydrogen as a Sustainable Energy Source. Environ. Sci. Technol. 2010, 44, 2243–2254. [Google Scholar] [CrossRef] [PubMed]
- Das, D. Advances in bio-hydrogen production processes: An approach towards commercialization. Int. J. Hydrog. Energy 2009, 34, 7349–7357. [Google Scholar] [CrossRef]
- Logan, B.E. Biologically extracting energy from wastewater: Biohydrogen production and microbial fuel cells. Environ. Sci. Technol. 2004, 38, 160A–167A. [Google Scholar] [CrossRef] [PubMed]
- Ho, L.Q.; Sugano, Y.; Yoshikawa, H.; Saito, M.; Tamiya, E. Structural assembly effects of Pt nanoparticle-carbon nanotube-polyaniline nanocomposites on the enhancement of biohydrogen fuel cell performance. Electrochim. Acta 2011, 56, 9875–9882. [Google Scholar] [CrossRef]
- He, D.; Zeng, C.; Xu, C.; Cheng, N.; Li, H.; Mu, S.; Pan, M. Polyaniline-functionalized carbon nanotube supported platinum catalysts. Langmuir 2011, 27, 5582–5588. [Google Scholar] [CrossRef] [PubMed]
- Cindrella, L.; Kannan, A.M. Membrane electrode assembly with doped polyaniline interlayer for proton exchange membrane fuel cells under low relative humidity conditions. J. Power Sources 2009, 193, 447–453. [Google Scholar] [CrossRef]
- Michel, M.; Ettingshausen, F.; Scheiba, F.; Wolz, A.; Roth, C. Using layer-by-layer assembly of polyaniline fibers in the fast preparation of high performance fuel cell nanostructured membrane electrodes. Phys. Chem. Chem. Phys. 2008, 10, 3796–3801. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, K. Carbon: Electrochemical and Physicochemical Properties, 1st ed.; Wiley: New York, NY, USA, 1988; pp. 5–10. [Google Scholar]
- Kinoshita, K.; Bett, J.A.S. Potentiodynamic analysis of surface oxides on carbon blacks. Carbon 1973, 11, 403–411. [Google Scholar] [CrossRef]
- Pyun, S.I.; Lee, E.J.; Kim, T.Y.; Lee, S.J.; Ryu, Y.G.; Kim, C.S. Role of surface oxides in corrosion of carbon black in phosphoric acid solution at elevated temperature. Carbon 1994, 32, 155–159. [Google Scholar]
- Chandrasekhar, P.; Naishadham, K. Broadband microwave adsorption and shielding properties of a poly(aniline). Synth. Met. 1999, 105, 115–120. [Google Scholar] [CrossRef]
- Abbas, S.M.; Chandra, M.; Verma, A.; Chatterjee, R.; Goel, T.C. Complex permittivity and microwave adsorption properties of a composite dielectric absorber. Compos. Part A Appl. Sci. Manuf. 2006, 37, 2148–2154. [Google Scholar] [CrossRef]
- Faez, R.; Martin, I.M.; De Paoli, M.-A.; Rezende, M.C. Microwave properties of EPDM/PAni-DBSA blends. Synth. Met. 2001, 119, 435–436. [Google Scholar] [CrossRef]
- John, H.R.; Thomas, M.; Jacob, J.; Mathew, K.T.; Joseph, R. Conducting polyaniline composites as microwave absorbers. Polym. Comp. 2007, 28, 588–592. [Google Scholar] [CrossRef]
- Oyharcabal, M.; Olinga, T.; Foulc, M.-P.; Lacomme, S.; Gontier, E.; Vigneras, V. Influence of the morphology of polyaniline on the microwave absorption properties of epoxy polyaniline composites. Comp. Sci. Technol. 2013, 74, 107–112. [Google Scholar] [CrossRef]
- Qu, B.; Xu, Y.; Deng, Y.; Peng, X.; Chen, J.; Dai, L. Polyaniline/carbon black composite as Pt electrocatalyst supports for methanol oxidation: Synthesis and characterization. J. Appl. Polym. Sci. 2010, 118, 2034–2042. [Google Scholar] [CrossRef]
- Mentus, S.; Ćirić-Marjanović, G.; Trchová, M.; Stejskal, J. Conducting carbonized polyaniline nanotubes. Nanotechnology 2009, 20, 245601. [Google Scholar] [CrossRef] [PubMed]
- Gavrilov, N.; Dašić-Tomić, M.; Pašti, I.; Ćirić-Marjanović, G.S. Carbonized polyaniline nanotubes/nanosheets-supported Pt nanoparticles: Synthesis, characterization and electrocatalysis. Mater. Lett. 2011, 65, 962–965. [Google Scholar] [CrossRef]
- Yan, X.; Liu, H.; Liew, K.Y. Size control of polymer-stabilized ruthenium nanoparticles by polyol reduction. J. Mater. Chem. 2001, 11, 3387–3391. [Google Scholar] [CrossRef]
- Liu, Z.; Gan, L.M.; Hong, L.; Chen, W.; Lee, J.Y. Carbon-supported Pt nanoparticles as catalysts for proton exchange membrane fuel cells. J. Power Sources 2005, 139, 73–78. [Google Scholar] [CrossRef]
- Esmaeilifar, A.; Rowshanzamir, S.; Eikani, M.H.; Ghazanfari, E. Synthesis methods of low-Pt-loading electrocatalysts for proton exchange membrane fuel cell systems. Energy 2010, 35, 3941–3957. [Google Scholar] [CrossRef]
- Higgins, D.C.; Meza, D.; Chen, Z. Nitrogen-doped carbon nanotubes as platinum catalyst supports for oxygen reduction reaction in proton exchange membrane fuel cells. J. Phys. Chem. C 2010, 114, 21982–21988. [Google Scholar] [CrossRef]
- Gong, K.; Du, F.; Xia, Z.; Durstock, M.; Dai, L. Nitrogen-doped carbon nanotube arrays with high electrocatalytic activity for oxygen reduction. Science 2009, 323, 760–764. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Higgins, D.; Chen, Z. Electrocatalystic activity of nitrogen doped carbon nanotubes with different morphologies for oxygen reduction reaction. Electrochim. Acta 2010, 55, 4799–4804. [Google Scholar] [CrossRef]
- Chen, Z.; Higgins, D.; Tao, H.; Hsu, R.S.; Chen, Z.J. Highly active nitrogen-doped carbon nanotubes for oxygen reduction reaction reaction in fuel cell applications. J. Phys. Chem. C 2009, 113, 21008–21013. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Tsai, M.J.; Hsieh, T.H.; Tseng, P.H.; Ho, K.S. Studies on the 1D polyanilines prepared with N-dodecylbenzenesulfonic and camphorsulfonic acids. Polym. Int. 2015, 64, 1568–1577. [Google Scholar] [CrossRef]
- Wu, R.H.; Tsai, M.J.; Ho, K.S.; Wei, T.E.; Hsieh, T.H.; Han, Y.K.; Kuo, C.W.; Tseng, P.H.; Wang, Y.Z. Sulfonated Polyaniline Nanofiber as Pt-Catalyst Conducting Support for Proton Exchange Membrane Fuel Cell. Polymer 2014, 55, 2035–2043. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Chang, K.J.; Hung, L.F.; Ho, K.S.; Chen, J.P.; Hsieh, T.H.; Chao, L. Carboxylated Carbonized Polyaniline nanofibers as Pt-Catalyst Conducting Support for Proton Exchange Membrane Fuel Cell. Synth. Met. 2014, 188, 21–29. [Google Scholar] [CrossRef]
- Yang, S.W.; Hu, C.S.; Liu, D.; Zhang, T.T.; Guo, T.T.; Liao, F. Synthesis of Platinum Nanoparticles-Decorated Poly(p-Phenylenediamine) Colloids with a High Performance for Methanol Electrocatalysis for Direct Methanol Fuel Cells. J. Clust. Sci. 2014, 25, 337–348. [Google Scholar] [CrossRef]
- Genies, E.M.; Boyle, A.; Lapkowski, M.; Tsintavis, C. Polyaniline: A historical survey. Synth. Met. 1990, 36, 139–281. [Google Scholar] [CrossRef]
- Zhao, M.; Wu, X.; Cai, C. Crystal chemistry and dehydrogenation/rehydrogenation properties of perovskite hydrides RbMgH3 and RbCaH3. J. Phys. Chem. C 2009, 113, 15091–15098. [Google Scholar]
- Hsu, C.H.; Liao, H.Y.; Kuo, P.L. Aniline as a Dispersant and Stabilizer for the Preparation of Pt Nanoparticles Deposited on Carbon Nanotubes. J. Phys. Chem. C 2010, 114, 7933–7939. [Google Scholar] [CrossRef]
- Maiyalagan, T.; Viswanathan, B.; Varadaraju, U.V. Nitrogen containing carbon nanotubes as supports for Pt—Alternate anodes for fuel cell applications. Electrochem. Commun. 2005, 7, 905–912. [Google Scholar] [CrossRef]


| Method | Pt particle size a (nm) | Pt residual weight b % | Pt surface area per unit support c (cm2·mg−1) |
|---|---|---|---|
| HT | 3.5 | 19.47 | 193.23 |
| MW | 5.2 | 21.98 | 342.62 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, M.-J.; Hsieh, T.-H.; Wang, Y.-Z.; Ho, K.-S.; Chang, C.-Y. Microwave Assisted Reduction of Pt-Catalyst by N-Phenyl-p-Phenylenediamine for Proton Exchange Membrane Fuel Cells. Polymers 2017, 9, 104. https://doi.org/10.3390/polym9030104
Tsai M-J, Hsieh T-H, Wang Y-Z, Ho K-S, Chang C-Y. Microwave Assisted Reduction of Pt-Catalyst by N-Phenyl-p-Phenylenediamine for Proton Exchange Membrane Fuel Cells. Polymers. 2017; 9(3):104. https://doi.org/10.3390/polym9030104
Chicago/Turabian StyleTsai, Ming-Jer, Tar-Hwa Hsieh, Yen-Zen Wang, Ko-Shan Ho, and Chia-Yun Chang. 2017. "Microwave Assisted Reduction of Pt-Catalyst by N-Phenyl-p-Phenylenediamine for Proton Exchange Membrane Fuel Cells" Polymers 9, no. 3: 104. https://doi.org/10.3390/polym9030104
APA StyleTsai, M.-J., Hsieh, T.-H., Wang, Y.-Z., Ho, K.-S., & Chang, C.-Y. (2017). Microwave Assisted Reduction of Pt-Catalyst by N-Phenyl-p-Phenylenediamine for Proton Exchange Membrane Fuel Cells. Polymers, 9(3), 104. https://doi.org/10.3390/polym9030104






