Applications of Three Dithienylpyrroles-Based Electrochromic Polymers in High-Contrast Electrochromic Devices
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Electrochemical Synthesis
2.1.1. Synthesis of 1-(4-(Methylthio)phenyl)-2,5-di(thiophen-2-yl)-pyrrole (MPS)
2.1.2. Synthesis of 1-(4-Methoxyphenyl)-2,5-di(thiophen-2-yl)-pyrrole (MPO)
2.1.3. Synthesis of 4-(2,5-Di(thiophen-2-yl)-pyrrol-1-yl)benzonitrile (ANIL)
2.2. Construction of ECDs and Spectroelectrochemical Characterizations
3. Results and Discussion
3.1. Electrochemical Polymerizations of Anodic Polymer Films
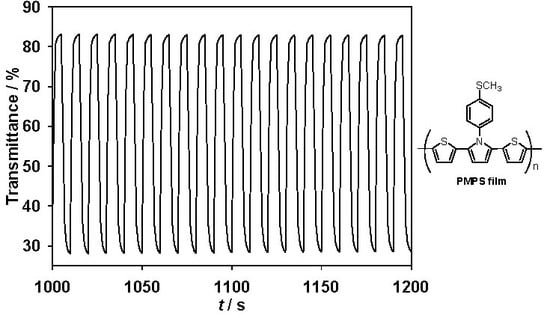
3.2. Electrochromic Properties of PMPS, PMPO, and PANIL Films
3.3. Spectroelectrochemistry of ECDs
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Hacioglu, S.O.; Yiğit, D.; Ermis, E.; Soylemez, S.; Güllü, M.; Toppare, L. Syntheses and electrochemical characterization of low oxidation potential nitrogen analogs of pedot as electrochromic materials. J. Electrochem. Soc. 2016, 163, E293–E299. [Google Scholar] [CrossRef]
- Du, Q.; Wei, Y.; Zheng, J.; Xu, C. Donor-π-bridge-acceptor type polymeric materials with pendant electron-withdrawing groups for electrochromic applications. Electrochim. Acta 2014, 132, 258–264. [Google Scholar] [CrossRef]
- Carbas, B.B.; Kivrak, A.; Teke, E.; Zora, M.; Önal, A.M. Electrochemical polymerization of a new low-voltage oxidized thienylenepyrrole derivative and its electrochromic device application. J. Electroanal. Chem. 2014, 729, 15–20. [Google Scholar] [CrossRef]
- Yu, G.; Gao, J.; Hummelen, J.C.; Wudl, F.; Heeger, A.J. Polymer photovoltaic cells: Enhanced efficiencies via a network of internal donor-acceptor heterojunctions. Science 1995, 270, 1789–1791. [Google Scholar] [CrossRef]
- Wu, T.Y.; Sheu, R.B.; Chen, Y. Synthesis, optically acid-sensory and electrochemical properties of novel polyoxadiazole derivatives. Macromolecules 2004, 37, 725–733. [Google Scholar] [CrossRef]
- Wu, T.Y.; Chen, Y. Poly(phenylene vinylene)-based copolymers containing 3,7-phenothiazylene and 2,6-pyridylene chromophores: Fluorescence sensors for acids, metal ions, and oxidation. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 1272–1284. [Google Scholar]
- Yen, H.J.; Chang, J.H.; Wu, J.H.; Liou, G.S. The steric effect of α- and β-substituted anthraquinone units on high performance polymeric memory devices. Polym. Chem. 2015, 6, 7758–7763. [Google Scholar] [CrossRef]
- Huang, T.T.; Tsai, C.L.; Hsiao, S.H.; Liou, G.S. Linkage and donor-acceptor effects on resistive switching memory devices of 4-(N-carbazolyl)triphenylamine-based polymers. RSC Adv. 2016, 6, 28815–28819. [Google Scholar] [CrossRef]
- Wu, T.Y.; Kuo, C.W.; Chen, Y.L.; Chang, J.K. Copolymers based on indole-6-carboxylic acid and 3,4-ethylenedioxythiophene as platinum catalyst support for methanol oxidation. Catalysts 2015, 5, 1657–1672. [Google Scholar] [CrossRef]
- Kuo, C.W.; Kuo, Z.Y.; Jow, J.J.; Wu, T.Y.; Chen, J.Y.; Zhu, X.X. Enhanced electrocatalytic performance for methanol oxidation via insertion of ruthenium oxide particles into Pt and polyaniline-poly(acrylic acid-co-maleic acid) composite electrode. Int. J. Electrochem. Sci. 2012, 7, 4974–4987. [Google Scholar] [CrossRef]
- Kuo, C.W.; Chen, S.J.; Chen, P.R.; Tsai, W.T.; Wu, T.Y. Doping process effect of polyaniline doped with poly(styrenesulfonic acid) supported platinum for methanol oxidation. J. Taiwan Inst. Chem. Eng. 2013, 44, 497–504. [Google Scholar] [CrossRef]
- Wu, T.Y.; Chen, Y. Synthesis, optical and electrochemical properties of novel copolymers containing alternate 2,3-quinoxaline and hole-transporting units. J. Polym. Sci. Part. A Polym. Chem. 2002, 40, 4570–4580. [Google Scholar] [CrossRef]
- Wu, T.Y.; Chen, Y. Synthesis and characterization of novel luminescent polymers with alternate phenothiazine and divinylbenzene units. J. Polym. Sci. Part A Polym. Chem. 2002, 40, 4452–4462. [Google Scholar] [CrossRef]
- Seshadri, V.; Padilla, J.; Bircan, H.; Radmard, B.; Draper, R.; Wood, M.; Otero, T.F.; Sotzing, G.A. Optimization, preparation, and electrical short evaluation for 30 cm2 active area dual conjugated polymer electrochromic windows. Org. Electron. 2007, 8, 367–381. [Google Scholar] [CrossRef]
- Mortimer, R.J. Organic electrochromic materials. Electrochim. Acta 1999, 44, 2971–2981. [Google Scholar] [CrossRef]
- Krukiewicz, K.; Jarosz, T.; Herman, A.P.; Turczyn, R.; Boncel, S.; Zak, J.K. The effect of solvent on the synthesis and physicochemical properties of poly(3,4-ethylenedioxypyrrole). Synth. Met. 2016, 217, 231–236. [Google Scholar] [CrossRef]
- Lin, K.; Zhang, S.; Liu, H.; Zhao, Y.; Wang, Z.; Xu, J. Effects on the electrochemical and electrochromic properties of 3 linked polythiophene derivative by the introduction of polyacrylate. Int. J. Electrochem. Sci. 2015, 10, 7720–7731. [Google Scholar]
- Ming, S.; Zhang, S.; Liu, H.; Zhao, Y.; Mo, D.; Xu, J. Methacrylate modified polythiophene: Electrochemistry and electrochromics. Int. J. Electrochem. Sci. 2015, 10, 6598–6609. [Google Scholar]
- Kuo, C.W.; Chen, B.K.; Li, W.B.; Tseng, L.Y.; Wu, T.Y.; Tseng, C.G.; Chen, H.R.; Huang, Y.C. Effects of supporting electrolytes on spectroelectrochemical and electrochromic properties of polyaniline-poly(styrene sulfonic acid) and poly(ethylenedioxythiophene)-poly(styrene sulfonic acid)-based electrochromic device. J. Chin. Chem. Soc. 2014, 61, 563–570. [Google Scholar] [CrossRef]
- Hsiao, S.H.; Hsueh, J.C. Electrochemical synthesis and electrochromic properties of new conjugated polycarbazoles from di(carbazol-9-yl)-substituted triphenylamine and N-phenylcarbazole derivatives. J. Electroanal. Chem. 2015, 758, 100–110. [Google Scholar] [CrossRef]
- Hsiao, S.H.; Lin, S.W. The electrochemical fabrication of electroactive polymer films from diamide- or diimide-cored N-phenylcarbazole dendrons for electrochromic applications. J. Mater. Chem. C 2016, 4, 1271–1280. [Google Scholar] [CrossRef]
- Rende, E.; Kilic, C.E.; Udum, Y.A.; Toffoli, D.; Toppare, L. Electrochromic properties of multicolored novel polymer synthesized via combination of benzotriazole and N-functionalized 2,5-di(2-thienyl)-1H-pyrrole units. Electrochim. Acta 2014, 138, 454–463. [Google Scholar] [CrossRef]
- Cansu-Ergun, E.G.; Onal, A.M.; Cihaner, A. Propylenedioxy and benzimidazole based electrochromic polymers. J. Electrochem. Soc. 2016, 163, G53–G60. [Google Scholar] [CrossRef]
- Wu, T.Y.; Su, S.G.; Gung, S.T.; Lin, M.W.; Lin, Y.C.; Lai, C.A.; Sun, I.W. Ionic liquids containing an alkyl sulfate group as potential electrolytes. Electrochim. Acta 2010, 55, 4475–4482. [Google Scholar] [CrossRef]
- Kuo, C.W.; Hao, L.; Kuo, P.L.; Chen, P.R.; Wu, T.Y. Influence of lithium salt addition on ionic conductivity and transporting properties of lithium bis(trifluoromethanesulfonyl)imide-doped glycine-based ionic liquid electrolyte. J. Taiwan Inst. Chem. Eng. 2014, 45, 1270–1279. [Google Scholar] [CrossRef]
- Wu, T.Y.; Wang, H.C.; Su, S.G.; Gung, S.T.; Lin, M.W.; Lin, C.B. Characterization of ionic conductivity, viscosity, density, and self-diffusion coefficient for binary mixtures of polyethyleneglycol (or polyethyleneimine) organic solvent with room temperature ionic liquid BMIBF4 (or BMIPF6). J. Taiwan Inst. Chem. Eng. 2010, 41, 315–325. [Google Scholar] [CrossRef]
- Sun, I.W.; Wang, H.P.; Teng, H.; Su, S.G.; Lin, Y.C.; Kuo, C.W.; Chen, P.R.; Wu, T.Y. Cyclic ammonium-based ionic liquids as potential electrolytes for dye-sensitized solar cells. Int. J. Electrochem. Sci. 2012, 7, 9748–9764. [Google Scholar]
- Chen, B.-K.; Wu, T.-Y.; Wong, J.-M.; Chang, Y.-M.; Lee, H.-F.; Huang, W.-Y.; Chen, A.F. Highly Sulfonated Diamine Synthesized Polyimides and Protic Ionic Liquid Composite Membranes Improve PEM Conductivity. Polymers 2015, 7, 1046–1065. [Google Scholar] [CrossRef]
- Welsh, D.M.; Kumar, A.; Meijer, E.W.; Reynolds, J.R. Enhanced contrast ratio and rapid switching in electrochromics based on poly(3,4-propylenedioxythiophene) derivatives. Adv. Mater. 1999, 11, 1379–1382. [Google Scholar] [CrossRef]
- Wu, T.Y.; Chen, B.K.; Hao, L.; Lin, K.F.; Sun, I.W. Thermophysical properties of a room temperature ionic liquid (1-methyl-3-pentyl-imidazolium hexafluorophosphate) with poly(ethylene glycol). J. Taiwan Inst. Chem. Eng. 2011, 42, 914–921. [Google Scholar] [CrossRef]
- Tarkuc, S.; Sahmetlioglu, E.; Tanyeli, C.; Akhmedov, I.M.; Toppare, L. Electrochromic properties of a soluble conducting polymer of 1-benzyl-2,5-di(thiophene-2-yl)-1H-pyrrole. Sens. Actuators B 2007, 121, 622–628. [Google Scholar] [CrossRef]
- Wu, T.Y.; Liao, J.W.; Chen, C.Y. Electrochemical synthesis, characterization and electrochromic properties of indan and 1,3-benzodioxole-based poly(2,5-dithienylpyrrole) derivatives. Electrochim. Acta 2014, 150, 245–262. [Google Scholar] [CrossRef]
- Wu, T.Y.; Chung, H.H. Applications of tris(4-(thiophen-2-yl)phenyl)amine- and dithienylpyrrole-based conjugated copolymers in high-contrast electrochromic devices. Polymers 2016, 8, 206. [Google Scholar] [CrossRef]
- Tsao, M.H.; Wu, T.Y.; Wang, H.P.; Sun, I.W.; Su, S.G.; Lin, Y.C.; Chang, C.W. An efficient metal free sensitizer for dye-sensitized solar cells. Mater. Lett. 2011, 65, 583–586. [Google Scholar] [CrossRef]
- Wu, T.Y.; Chen, Y. Synthesis and characterization of luminescent copolymers containing iminodibenzyl and divinylbenzene chromophores. J. Polym. Sci. Part A Polym. Chem. 2002, 40, 3847–3857. [Google Scholar] [CrossRef]
- Soyleyici, S.; Karakus, M.; Ak, M. Transparent-blue colored dual type electrochromic device: Switchable glass application of conducting organic-inorganic hybrid carbazole polymer. J. Electrochem. Soc. 2016, 163, H679–H683. [Google Scholar] [CrossRef]
- Chang, K.H.; Wang, H.P.; Wu, T.Y.; Sun, I.W. Optical and electrochromic characterizations of four 2,5-dithienylpyrrole-based conducting polymer films. Electrochim. Acta 2014, 119, 225–235. [Google Scholar] [CrossRef]
- Wu, T.Y.; Su, Y.S. Electrochemical synthesis and characterization of a 1,4-benzodioxan-based electrochromic polymer and its application in electrochromic devices. J. Electrochem. Soc. 2015, 162, G103–G112. [Google Scholar] [CrossRef]
- Camurlu, P.; Gültekin, C. A comprehensive study on utilization of N-substituted poly(2,5-dithienylpyrrole) derivatives in electrochromic devices. Sol. Energy Mater. Sol. Cells 2012, 107, 142–147. [Google Scholar] [CrossRef]
- Kuo, C.W.; Wu, T.L.; Lin, Y.C.; Chang, J.K.; Chen, H.R.; Wu, T.Y. Copolymers based on 1,3-bis(carbazol-9-yl)benzene and three 3,4-ethylenedioxythiophene derivatives as potential anodically coloring copolymers in high-contrast electrochromic devices. Polymers 2016, 8, 368. [Google Scholar] [CrossRef]












| Polymer Films and ECDs | Reduction (0 V) | Oxidation (+1.6 V) |
|---|---|---|
| PMPS |  |  |
| PMPO |  |  |
| PANIL |  |  |
| PMPS/PProDOT-Et2 |  |  |
| PMPO/PProDOT-Et2 |  |  |
| PANIL/PProDOT-Et2 |  |  |
| Polymers | E/V | L | a | b | L* | a* | b* | x | y |
|---|---|---|---|---|---|---|---|---|---|
| PMPS | −0.4 | 80.25 | −1.00 | 25.76 | 84.17 | 0.09 | 31.17 | 0.3726 | 0.3828 |
| −0.2 | 80.24 | −0.92 | 25.75 | 84.17 | 0.17 | 31.16 | 0.3727 | 0.3827 | |
| 0 | 80.37 | −1.16 | 25.83 | 84.27 | −0.08 | 31.25 | 0.3724 | 0.383 | |
| 0.2 | 80.26 | −0.93 | 25.73 | 84.18 | 0.16 | 31.12 | 0.3726 | 0.3826 | |
| 0.4 | 80.32 | −1.06 | 25.72 | 84.23 | 0.03 | 31.1 | 0.3723 | 0.3826 | |
| 0.6 | 80.19 | −1.35 | 25.35 | 84.13 | −0.28 | 30.55 | 0.3708 | 0.3819 | |
| 0.8 | 79.38 | −1.78 | 23.81 | 83.45 | −0.73 | 28.4 | 0.3663 | 0.3784 | |
| 1.0 | 76.51 | −1.99 | 18.99 | 81.03 | −1.01 | 21.93 | 0.3541 | 0.3666 | |
| 1.2 | 72.95 | −1.61 | 13.51 | 78 | −0.66 | 14.98 | 0.3413 | 0.3526 | |
| 1.4 | 67.69 | −0.39 | 6.78 | 73.42 | 0.57 | 6.93 | 0.3269 | 0.3346 | |
| 1.6 | 64.25 | 1.41 | 3.69 | 70.37 | 2.52 | 3.40 | 0.3227 | 0.3251 | |
| PMPO | −0.4 | 84.19 | 0.76 | 24.26 | 87.42 | 1.91 | 28.09 | 0.3677 | 0.3731 |
| −0.2 | 84.19 | 0.74 | 24.29 | 87.42 | 1.9 | 28.13 | 0.3678 | 0.3732 | |
| 0 | 84.16 | 0.7 | 24.31 | 87.4 | 1.85 | 28.15 | 0.3677 | 0.3733 | |
| 0.2 | 84.16 | 0.56 | 24.23 | 87.4 | 1.71 | 28.04 | 0.3673 | 0.3733 | |
| 0.4 | 83.98 | −0.05 | 23.49 | 87.25 | 1.1 | 27.01 | 0.3645 | 0.3719 | |
| 0.6 | 83.12 | −0.97 | 21.06 | 86.55 | 0.15 | 23.77 | 0.3571 | 0.3667 | |
| 0.8 | 81 | −1.31 | 16.05 | 84.8 | −0.22 | 17.39 | 0.3447 | 0.3549 | |
| 1.0 | 75.3 | −0.14 | 5.62 | 80.01 | 0.92 | 5.23 | 0.3228 | 0.3296 | |
| 1.2 | 67.81 | 2.85 | −2.19 | 73.53 | 4.07 | −3.2 | 0.31 | 0.3087 | |
| 1.4 | 61.26 | 3.77 | −4.04 | 67.67 | 5.12 | −5.21 | 0.3069 | 0.3024 | |
| 1.6 | 61.06 | 4.08 | −3.24 | 67.49 | 5.46 | −4.36 | 0.3095 | 0.3041 | |
| 1.8 | 61.94 | 4.08 | −2.58 | 68.29 | 5.45 | −3.64 | 0.3113 | 0.306 | |
| PANIL | −0.4 | 87.66 | −0.42 | 20 | 90.25 | 0.76 | 21.69 | 0.3525 | 0.3606 |
| −0.2 | 87.71 | −0.44 | 20.01 | 90.29 | 0.74 | 21.7 | 0.3525 | 0.3606 | |
| 0 | 87.74 | −0.47 | 20.03 | 90.32 | 0.71 | 21.71 | 0.3525 | 0.3606 | |
| 0.2 | 87.79 | −0.52 | 20.03 | 90.35 | 0.66 | 21.71 | 0.3524 | 0.3606 | |
| 0.4 | 87.78 | −0.64 | 19.96 | 90.35 | 0.55 | 21.62 | 0.352 | 0.3606 | |
| 0.6 | 87.69 | −0.93 | 19.58 | 90.28 | 0.25 | 21.13 | 0.3507 | 0.3599 | |
| 0.8 | 87.11 | −1.29 | 18.28 | 89.81 | −0.11 | 19.55 | 0.3473 | 0.3573 | |
| 1.0 | 85.34 | −1.45 | 15.21 | 88.36 | −0.3 | 15.9 | 0.3406 | 0.3508 | |
| 1.2 | 80.64 | −0.96 | 8.74 | 84.5 | 0.13 | 8.55 | 0.3277 | 0.3365 | |
| 1.4 | 74.34 | 0.8 | 3.1 | 79.19 | 1.9 | 2.44 | 0.3185 | 0.3229 | |
| 1.6 | 70.62 | 3.46 | 1.04 | 75.99 | 4.7 | 0.25 | 0.3186 | 0.3161 | |
| 1.8 | 69.85 | 5.35 | 1.53 | 75.32 | 6.69 | 0.81 | 0.3231 | 0.3158 | |
| 2.0 | 70.35 | 5.88 | 2.85 | 75.76 | 7.24 | 2.26 | 0.3272 | 0.3185 |
| Polymer Films and ECDs | λmax/nm | Cycle No. | Optical Contrast/% | τc/s | Optical Contrast/% | τb/s |
|---|---|---|---|---|---|---|
| ΔT/% | T95% | ΔT/% | T95% | |||
| PMPS film in [EPI+][TFSI−] | 600 | 1 | 17.59 | 2.22 | 17.59 | 2.01 |
| 50 | 17.27 | 2.16 | 17.28 | 2.08 | ||
| 100 | 18.62 | 2.21 | 18.61 | 1.93 | ||
| 940 | 1 | 53.94 | 1.98 | 53.94 | 2.09 | |
| 50 | 53.1 | 1.96 | 53.1 | 2.07 | ||
| 100 | 54.47 | 1.97 | 54.47 | 2.01 | ||
| PMPO film in [EPI+][TFSI−] | 584 | 1 | 18.02 | 2.05 | 18.02 | 1.76 |
| 50 | 18.01 | 2.04 | 18.03 | 1.69 | ||
| 100 | 16.98 | 1.92 | 16.98 | 1.76 | ||
| 890 | 1 | 43.99 | 1.85 | 43.99 | 2.02 | |
| 50 | 43.45 | 1.74 | 43.45 | 2.13 | ||
| 100 | 43.72 | 1.87 | 43.87 | 2.01 | ||
| PANIL film in [EPI+][TFSI−] | 566 | 1 | 15.83 | 2.05 | 15.82 | 2.05 |
| 50 | 15.26 | 2.06 | 15.25 | 2.09 | ||
| 100 | 15.09 | 2.01 | 15.09 | 2.08 | ||
| 950 | 1 | 46.17 | 1.97 | 46.17 | 2.10 | |
| 50 | 44.63 | 1.94 | 44.63 | 2.28 | ||
| 100 | 39.44 | 2.08 | 39.44 | 2.14 | ||
| PMPS/PProDOT-Et2 ECD | 590 | 1 | 32.51 | 1.00 | 32.51 | 1.10 |
| 50 | 30.43 | 0.94 | 30.43 | 1.00 | ||
| 100 | 31.92 | 0.99 | 31.91 | 1.01 | ||
| PMPO/PProDOT-Et2 ECD | 626 | 1 | 41.13 | 1.54 | 41.13 | 1.10 |
| 50 | 39.43 | 1.45 | 39.43 | 0.98 | ||
| 100 | 38.50 | 1.42 | 38.50 | 1.12 | ||
| PANIL/PProDOT-Et2 ECD | 628 | 1 | 25.00 | 1.21 | 25.00 | 1.06 |
| 50 | 22.23 | 1.14 | 22.23 | 1.03 | ||
| 100 | 21.71 | 1.17 | 21.71 | 1.06 |
| ECDs | E/V | L | a | b | L* | a* | b* | x | y |
|---|---|---|---|---|---|---|---|---|---|
| PMPS/PProDOT-Et2 | −0.6 | 78.12 | −1.07 | 11.74 | 82.39 | −0.02 | 12.28 | 0.3354 | 0.3449 |
| −0.4 | 78.13 | −1.13 | 11.79 | 82.4 | −0.08 | 12.33 | 0.3355 | 0.345 | |
| −0.2 | 78.13 | −1.27 | 11.77 | 82.4 | −0.23 | 12.31 | 0.3352 | 0.3451 | |
| 0 | 78 | −1.67 | 11.35 | 82.3 | −0.65 | 11.8 | 0.3335 | 0.3444 | |
| 0.2 | 77.44 | −2.18 | 10.21 | 81.82 | −1.19 | 10.48 | 0.33 | 0.3421 | |
| 0.4 | 75.55 | −1.87 | 7.83 | 80.22 | −0.9 | 7.77 | 0.3252 | 0.3364 | |
| 0.6 | 69.94 | 0.1 | 1.09 | 75.4 | 1.13 | 0.32 | 0.3129 | 0.3189 | |
| 0.8 | 64.41 | −0.34 | −6.29 | 70.52 | 0.58 | −7.49 | 0.2942 | 0.3009 | |
| 1.0 | 61.4 | 0.1 | −10.71 | 67.8 | 1.05 | −11.97 | 0.2839 | 0.2893 | |
| 1.2 | 60.73 | 0.72 | −12.22 | 67.18 | 1.73 | −13.46 | 0.2814 | 0.2851 | |
| 1.4 | 60.42 | 1.34 | −13.27 | 66.91 | 2.43 | −14.47 | 0.2801 | 0.2822 | |
| 1.6 | 60.06 | 1.91 | −13.56 | 66.57 | 3.07 | −14.76 | 0.2804 | 0.2809 | |
| PMPO/PProDOT-Et2 | −0.4 | 69.99 | −1.97 | 16.09 | 75.44 | −1.11 | 18.83 | 0.3498 | 0.3626 |
| −0.2 | 69.92 | −2.32 | 15.75 | 75.38 | −1.49 | 18.37 | 0.3482 | 0.362 | |
| 0 | 69.45 | −2.75 | 14.84 | 74.97 | −1.97 | 17.17 | 0.3449 | 0.3599 | |
| 0.2 | 67.75 | −2.44 | 12.62 | 73.48 | −1.68 | 14.35 | 0.3397 | 0.3538 | |
| 0.4 | 61.31 | −1.16 | 5.04 | 67.72 | −0.38 | 5.12 | 0.322 | 0.332 | |
| 0.6 | 51.91 | −1.54 | −7.26 | 58.93 | −1 | −8.84 | 0.2851 | 0.2958 | |
| 0.8 | 46.45 | −0.32 | −14.88 | 53.58 | 0.38 | −16.8 | 0.2641 | 0.2704 | |
| 1.0 | 42.8 | 1.68 | −19.68 | 49.88 | 2.84 | −21.55 | 0.2532 | 0.2526 | |
| 1.2 | 40.43 | 3.4 | −22.6 | 47.43 | 5.03 | −24.36 | 0.2476 | 0.2409 | |
| 1.4 | 38.94 | 4.52 | −24.8 | 45.86 | 6.47 | −26.39 | 0.2431 | 0.2326 | |
| 1.6 | 38.48 | 4.79 | −25.17 | 45.37 | 6.82 | −26.75 | 0.2423 | 0.2307 | |
| 1.8 | 38.23 | 5.13 | −25.7 | 45.1 | 7.26 | −27.22 | 0.2415 | 0.2288 | |
| PANIL/PProDOT-Et2 | −0.4 | 74.98 | −4.17 | 19.46 | 79.74 | −3.36 | 22.84 | 0.3526 | 0.3715 |
| −0.2 | 75 | −4.33 | 19.37 | 79.75 | −3.53 | 22.71 | 0.352 | 0.3713 | |
| 0 | 74.85 | −4.6 | 19.02 | 79.63 | −3.83 | 22.24 | 0.3506 | 0.3706 | |
| 0.2 | 74.5 | −4.9 | 18.3 | 79.33 | −4.16 | 21.27 | 0.3482 | 0.369 | |
| 0.4 | 73.33 | −4.61 | 17.25 | 78.33 | −3.89 | 19.97 | 0.3463 | 0.3664 | |
| 0.6 | 66.93 | −2.76 | 11.09 | 72.76 | −2.05 | 12.41 | 0.3349 | 0.3498 | |
| 0.8 | 55.45 | −4.98 | −2.59 | 62.29 | −5.02 | −3.69 | 0.2916 | 0.3135 | |
| 1.0 | 48.59 | −4.69 | −11.81 | 55.7 | −5.01 | −13.66 | 0.2634 | 0.2843 | |
| 1.2 | 44.1 | −2.96 | −18.32 | 51.2 | −3.01 | −20.21 | 0.2465 | 0.2615 | |
| 1.4 | 40.04 | −0.32 | −24.33 | 47.01 | 0.28 | −25.89 | 0.2334 | 0.2392 | |
| 1.6 | 35.72 | 3.14 | −30.97 | 42.4 | 4.81 | −31.75 | 0.2204 | 0.214 | |
| 1.8 | 32.91 | 5.48 | −35.32 | 39.29 | 7.97 | −35.34 | 0.212 | 0.1975 |
| Polymer Films and ECDs | λ/nm | Eg/eV | ΔTmax/% | ΔODmax/% | ηmax/cm2·C−1 |
|---|---|---|---|---|---|
| PMPS | 940 | 2.17 | 54.74 | 46.40 | 298.28 |
| PMPO | 890 | 2.25 | 43.87 | 30.04 | 142.48 |
| PANIL | 950 | 2.21 | 44.63 | 34.94 | 279.19 |
| PMPS/PProDOT-Et2 ECD | 590 | - | 32.51 | 54.45 | 637.25 |
| PMPO/PProDOT-Et2 ECD | 626 | - | 41.13 | 52.40 | 674.67 |
| PANIL/PProDOT-Et2 ECD | 628 | - | 25.00 | 29.80 | 401.63 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, Y.-S.; Chang, J.-C.; Wu, T.-Y. Applications of Three Dithienylpyrroles-Based Electrochromic Polymers in High-Contrast Electrochromic Devices. Polymers 2017, 9, 114. https://doi.org/10.3390/polym9030114
Su Y-S, Chang J-C, Wu T-Y. Applications of Three Dithienylpyrroles-Based Electrochromic Polymers in High-Contrast Electrochromic Devices. Polymers. 2017; 9(3):114. https://doi.org/10.3390/polym9030114
Chicago/Turabian StyleSu, Yuh-Shan, Jui-Cheng Chang, and Tzi-Yi Wu. 2017. "Applications of Three Dithienylpyrroles-Based Electrochromic Polymers in High-Contrast Electrochromic Devices" Polymers 9, no. 3: 114. https://doi.org/10.3390/polym9030114
APA StyleSu, Y.-S., Chang, J.-C., & Wu, T.-Y. (2017). Applications of Three Dithienylpyrroles-Based Electrochromic Polymers in High-Contrast Electrochromic Devices. Polymers, 9(3), 114. https://doi.org/10.3390/polym9030114