A Rapid One-Pot Synthesis of Novel High-Purity Methacrylic Phosphonic Acid (PA)-Based Polyhedral Oligomeric Silsesquioxane (POSS) Frameworks via Thiol-Ene Click Reaction
Abstract
:1. Introduction
2. Material Synthesis
2.1. Materials
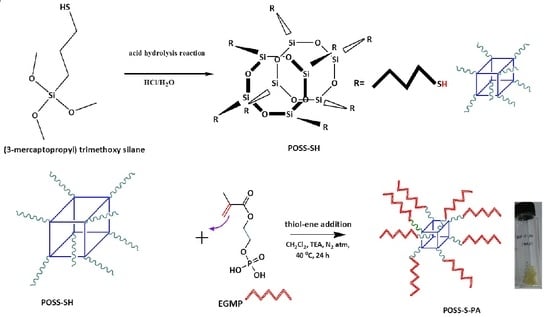
2.2. Synthesis of POSS-S-PA
3. Results and Discussions
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chinnam, P.R.; Zhang, H.; Wunder, S.L. Blends of pegylated polyoctahedralsilsesquioxanes (POSS-PEG) and methyl cellulose as solid polymer electrolytes for lithium batteries. Electrochim. Acta 2015, 170, 191–201. [Google Scholar] [CrossRef]
- Gupta, D.; Madhukar, A.; Choudhary, V. Effect of functionality of polyhedral oligomeric silsesquioxane [POSS] on the properties of sulfonated poly(ether ether ketone) [SPEEK] based hybrid nanocomposite proton exchange membranes for fuel cell applications. Int. J. Hydrogen Energy 2013, 38, 12817–12829. [Google Scholar] [CrossRef]
- Zhang, W.; Li, J.; Jiang, S.; Wang, Z.-S. POSS with eight imidazolium iodide arms for efficient solid-state dye-sensitized solar cells. Chem. Commun. 2014, 50, 1685–1687. [Google Scholar] [CrossRef] [PubMed]
- Blanco, I.; Abate, L.; Bottino, F.A. Variously substituted phenyl hepta cyclopentyl-polyhedral oligomeric silsesquioxane (ph,hcp-POSS)/polystyrene (PS) nanocomposites: The influence of substituents on the thermal stability. J. Therm. Anal. Calorim. 2013, 112, 421–428. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, J.; Wang, C.; Jia, T.; Li, Y.; Fang, S. Substituents Effects on the Properties of Polyhedral Oligomeric Silsesquioxanes(POSS)/Poly(l-lactic acid) Hybrid Films. J. Macromol. Sci. Part A 2012, 49, 73–80. [Google Scholar] [CrossRef]
- Blanco, I.; Bottino, F.A.; Cicala, G.; Latteri, A.; Recca, A. Synthesis and Characterization of Differently Substituted Phenyl Hepta Isobutyl-Polyhedral Oligomeric Silsesquioxane/Polystyrene Nanocomposites. Polym. Compos. 2014, 35, 151–157. [Google Scholar]
- Li, Y.; Zhang, W.B.; Hsieh, I.F.; Zhang, G.; Cao, Y.; Li, X.; Wesdemiotis, C.; Lotz, B.; Xiong, H.; Cheng, S.Z.D. Breaking symmetry toward nonspherical janus particles based on polyhedral oligomeric silsesquioxanes: Molecular design, “click” synthesis, and hierarchical structure. J. Am. Chem. Soc. 2011, 133, 10712–10715. [Google Scholar] [PubMed]
- Li, Y.; Dong, X.H.; Guo, K.; Wang, Z.; Chen, Z.; Wesdemiotis, C.; Quirk, R.P.; Zhang, W.B.; Cheng, S.Z.D. Synthesis of shape amphiphiles based on POSS tethered with two symmetric/asymmetric polymer tails via sequential “grafting-from” and thiol-ene “click” chemistry. ACS Macro Lett. 2012, 1, 834–839. [Google Scholar] [CrossRef]
- Markovic, E.; Clarke, S.; Matisons, J.; Simon, G.P. Synthesis of POSS-Methyl Methacrylate-Based Cross-Linked Hybrid Materials. Macromolecules 2008, 41, 1685–1692. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, Y.; You, H.; Wang, D. Hedgehog buckyball: A high-symmetry complete polyhedral oligomeric silsesquioxane (POSS). Polymers 2016, 8. [Google Scholar] [CrossRef]
- Blanco, I.; Abate, L.; Bottino, F.A.; Bottino, P. Synthesis, characterization and thermal stability of new dumbbell-shaped isobutyl-substituted POSSs linked by aromatic bridges. J. Therm. Anal. Calorim. 2014, 117, 243–250. [Google Scholar] [CrossRef]
- Araki, H.; Naka, K. Syntheses and properties of dumbbell-shaped POSS derivatives linked by luminescent π-conjugated units. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 4170–4181. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Zheng, J.; Su, H.; Lin, F.; Guo, K.; Feng, X.; Wesdemiotis, C.; Becker, M.L.; Cheng, S.Z.D.; Zhang, W. Bin Cascading one-pot synthesis of single-tailed and asymmetric multitailed giant surfactants. ACS Macro Lett. 2013, 2, 1026–1032. [Google Scholar] [CrossRef]
- Yu, X.; Li, Y.; Dong, X.H.; Yue, K.; Lin, Z.; Feng, X.; Huang, M.; Zhang, W.B.; Cheng, S.Z.D. Giant surfactants based on molecular nanoparticles: Precise synthesis and solution self-assembly. J. Polym. Sci. Part B Polym. Phys. 2014, 52, 1309–1325. [Google Scholar] [CrossRef]
- Su, H.; Li, Y.; Yue, K.; Wang, Z.; Lu, P.; Feng, X.; Dong, X.-H.; Zhang, S.; Cheng, S.Z.D.; Zhang, W.B. Macromolecular structure evolution toward giant molecules of complex structure: tandem synthesis of asymmetric giant gemini surfactants. Polym. Chem. 2014, 5, 3697. [Google Scholar] [CrossRef]
- Kaneko, Y.; Shoiriki, M.; Mizumo, T. Preparation of cage-like octa(3-aminopropyl)silsesquioxane trifluoromethanesulfonate in higher yield with a shorter reaction time. J. Mater. Chem. 2012, 22, 14475–14478. [Google Scholar] [CrossRef]
- Lowe, A.B. Thiol-yne “click”/coupling chemistry and recent applications in polymer and materials synthesis and modification. Polymer 2014, 55, 5517–5549. [Google Scholar] [CrossRef]
- Han, J.; Zheng, Y.; Zheng, S.; Li, S.; Hu, T.; Tang, A.; Gao, C. Water soluble octa-functionalized POSS: all-click chemistry synthesis and efficient host-guest encapsulation. Chem. Commun. 2014, 50, 8712–8714. [Google Scholar] [CrossRef] [PubMed]
- Kotal, A.; Satyabrata, S.; Paira, T.K.; Mandal, T.K. Polymer synthesis of semitelechelic POSS-polymethacrylate hybrids by thiol-mediated controlled radical polymerization with unusual thermal behaviors. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 1111–1123. [Google Scholar] [CrossRef]
- Matsumura, S.; Hlil, A.R.; Lepiller, C.; Gaudet, J.; Guay, D.; Shi, Z.; Holdcroft, S.; Hay, A.S. Ionomers for proton exchange membrane fuel cells with sulfonic acid groups on the end-groups: Novel branched poly(ether-ketone)s. Am. Chem. Soc. Polym. Prepr. Div. Polym. Chem. 2008, 49, 511–512. [Google Scholar] [CrossRef]
- Lee, S.-I.I.; Yoon, K.-H.H.; Song, M.; Peng, H.G.; Page, K.a.; Soles, C.L.; Yoon, D.Y. Structure and properties of polymer electrolyte membranes containing phosphonic acids for anhydrous fuel cells. Chem. Mater. 2012, 24, 115–122. [Google Scholar] [CrossRef]
- Shibata, M.; Nagashima, S. Trehalose-incorporated organic-inorganic hybrid nanocomposites produced by thiol-ene photopolymerization. Polym. J. 2016, 48, 111–116. [Google Scholar] [CrossRef]
- Li, Z.; He, G.; Zhang, B.; Cao, Y.; Wu, H.; Jiang, Z.; Tiantian, Z. Enhanced proton conductivity of nafion hybrid membrane under different humidities by incorporating metal-organic frameworks with high phytic acid loading. ACS Appl. Mater. Interfaces 2014, 6, 9799–9807. [Google Scholar] [CrossRef] [PubMed]
- Goldmann, A.S.A.; Walther, A.; Nebhani, L.; Joso, R.; Ernst, D.; Loos, K.; Barner-Kowollik, C.; Earner, L.; Müller, A.H.E. Surface Modification of Poly(divinylbenzene) Microspheres via Thiol-Ene Chemistry and Alkyne-Azide Click Reactions. Macromolecules 2009, 42, 3707–3714. [Google Scholar] [CrossRef]
- Li, L.; Liu, H. Rapid Preparation of Silsesquioxane-Based Ionic Liquids. Chem.-A Eur. J. 2016, 22, 4713–4716. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.W.; Gao, S.X.; Xu, K.; Zhang, Y.X.; Peng, J.; Chen, M.C. Synthesis and characterization of silsesquioxane-cored star-shaped hybrid polymer via “grafting from” RAFT polymerization. Chin. Chem. Lett. 2016, 27, 1696–1700. [Google Scholar] [CrossRef]
- Karuppasamy, K.; Prasanna, K.; Kim, D.; Kang, Y.H.; Rhee, H.W. Headway in rhodanide anion based ternary gel polymer electrolytes (TILGPEs) for applications in rechargeable lithium ion batteries: an efficient route to achieve high electrochemical and cycling performances. RSC Adv. 2017, 7, 19211–19222. [Google Scholar] [CrossRef]
- Karuppasamy, K.; Thanikaikarasan, S.; Antony, R.; Balakumar, S.; Shajan, X.S. Effect of nanochitosan on electrochemical, interfacial and thermal properties of composite solid polymer electrolytes. Ionics. 2012, 18, 737–745. [Google Scholar] [CrossRef]
- Blanco, I.; Abate, L.; Antonelli, M.L.; Bottino, F.A.; Bottino, P. Phenyl hepta cyclopentyl-Polyhedral oligomeric silsesquioxane (ph,hcp-POSS)/polystyrene (PS) nanocomposites: The influence of substituents in the phenyl group on the thermal stability. Express Polym. Lett. 2012, 6, 997–1006. [Google Scholar] [CrossRef]
- Fina, A.; Tabuani, D.; Carniato, F.; Frache, A.; Boccaleri, E.; Camino, G. Polyhedral oligomeric silsesquioxanes (POSS) thermal degradation. Thermochim. Acta 2006, 440, 36–42. [Google Scholar] [CrossRef]
- Cramer, N.B.; Bowman, C.N. Kinetics of thiol-ene and thiol-acrylate photopolymerizations with real-time Fourier transform infrared. J. Polym. Sci. Part A Polym. Chem. 2001, 39, 3311–3319. [Google Scholar] [CrossRef]
- Rozga-Wijas, K.; Stanczyk, W.A.; Kurjata, J.; Kazmierski, S. Star-Shaped and Linear POSS-Polylactide Hybrid Copolymers. Materials 2015, 8, 4400–4420. [Google Scholar] [CrossRef]




| FTIR Frequency Wavenumber (cm−1) | Assignments | |
|---|---|---|
| POSS-SH | POSS-S-PA | |
| 1095–1056 | 1096–1058 | Si–O–Si |
| - | 990, 950 | P–O stretching & P–C stretching |
| - | 1736 | C=O |
| 2690 | - | S–H |
| 3100–2880 | 3050–2850 | C–H stretching |
| - | 3200–3600 | P–OH stretching |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karuppasamy, K.; Prasanna, K.; Vikraman, D.; Kim, H.-S.; Kathalingam, A.; Mitu, L.; Rhee, H.W. A Rapid One-Pot Synthesis of Novel High-Purity Methacrylic Phosphonic Acid (PA)-Based Polyhedral Oligomeric Silsesquioxane (POSS) Frameworks via Thiol-Ene Click Reaction. Polymers 2017, 9, 192. https://doi.org/10.3390/polym9060192
Karuppasamy K, Prasanna K, Vikraman D, Kim H-S, Kathalingam A, Mitu L, Rhee HW. A Rapid One-Pot Synthesis of Novel High-Purity Methacrylic Phosphonic Acid (PA)-Based Polyhedral Oligomeric Silsesquioxane (POSS) Frameworks via Thiol-Ene Click Reaction. Polymers. 2017; 9(6):192. https://doi.org/10.3390/polym9060192
Chicago/Turabian StyleKaruppasamy, K., K. Prasanna, Dhanasekaran Vikraman, Hyun-Seok Kim, A. Kathalingam, Liviu Mitu, and Hee Woo Rhee. 2017. "A Rapid One-Pot Synthesis of Novel High-Purity Methacrylic Phosphonic Acid (PA)-Based Polyhedral Oligomeric Silsesquioxane (POSS) Frameworks via Thiol-Ene Click Reaction" Polymers 9, no. 6: 192. https://doi.org/10.3390/polym9060192
APA StyleKaruppasamy, K., Prasanna, K., Vikraman, D., Kim, H.-S., Kathalingam, A., Mitu, L., & Rhee, H. W. (2017). A Rapid One-Pot Synthesis of Novel High-Purity Methacrylic Phosphonic Acid (PA)-Based Polyhedral Oligomeric Silsesquioxane (POSS) Frameworks via Thiol-Ene Click Reaction. Polymers, 9(6), 192. https://doi.org/10.3390/polym9060192