Human Mesenchymal Stem Cells Differentiation Regulated by Hydroxyapatite Content within Chitosan-Based Scaffolds under Perfusion Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Preparation
2.2. Scaffolds Characterization
Microstructure Imaging
2.3. Dynamic hMSCs Cell Culture in Perfusion Bioreactor
2.3.1. Human Mesenchymal Stem Cells Isolation and Expansion
2.3.2. Osteogenic Induction in Perfusion Bioreactor
2.3.3. Hoechst 33342 Staining
2.3.4. Histological Analysis
2.3.5. Isolation of Total Cellular RNA and Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR) Analysis
2.3.6. Immunohistochemical Detection of Type I Collagen and Osteocalcin
2.4. Statistical Analysis
3. Results
3.1. Scaffolds’ Microstructure
3.2. Biological Evaluation of Construct (Grafts) Cultured in Perfusion Bioreactor
3.2.1. Histological Analysis of the Grafts
3.2.2. Quantitative Evaluation of Osteoinduction
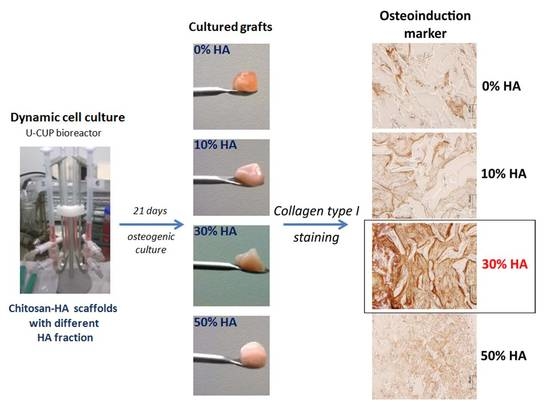
3.2.3. Immunohistochemical Staining of the Grafts
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Burdick, J.; Vunjak-Novakovic, G. Engineered microenvironments for controlled stem cell differentiation. Tissue Eng. A 2009, 15, 205–219. [Google Scholar] [CrossRef] [PubMed]
- Ivkovic, A.; Marijanovic, I.; Hudetz, D.; Porter, R.M.; Pecina, M.; Evans, C.H. Regenerative medicine and tissue engineering in orthopaedic surgery. Front. Biosci. 2011, E3, 923–944. [Google Scholar] [CrossRef]
- Majumdar, M.K.; Keane-Moore, M.; Buyaner, D.; Hardy, W.B.; Moorman, M.A.; Mclntosh, K.R.; Mosca, J.D. Characterization and functionality of cell surface molecules on human mesenchymal stem cells. J. Biomed. Sci. 2003, 10, 228–241. [Google Scholar] [CrossRef] [PubMed]
- Koegler, P.; Clayton, A.; Thissen, H.; Santos, G.N.C.; Kingshott, P. The influence of nanostructured materials on biointerfacial interactions. Adv. Drug Deliv. Rev. 2012, 64, 1820–1839. [Google Scholar] [CrossRef] [PubMed]
- Sellgren, K.L.; Ma, T. Perfusion conditioning of hydroxyapatite–chitosan–gelatin scaffolds for bone tissue regeneration from human mesenchymal stem cells. J. Tissue Eng. Regen. Med. 2012, 6, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Grayson, W.L.; Ma, T.; Brunnell, B.; Lu, W.W. Effects of hydroxyapatite in 3-D chitosan–gelatin polymer network on human mesenchymal stem cell construct development. Biomaterials 2006, 27, 1859–1867. [Google Scholar] [CrossRef] [PubMed]
- Smith, I.O.; McCabe, L.R.; Baumann, M.J. MC3T3-E1 osteoblast attachment and proliferation on porous hydroxyapatite scaffolds fabricated with nanophase powder. Int. J. Nanomed. 2006, 1, 189–194. [Google Scholar] [CrossRef]
- Wang, J.; De Boer, J.; De Groot, K. Proliferation and differentiation of osteoblast-like MC3T3-E1 cells on biomimetically and electrolytically deposited calcium phosphate coatings. J. Biomed. Mater. Res. A 2009, 90, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Imaizumi, H.; Sakurai, M.; Kashimoto, O.; Kikawa, T.; Suzuki, O. Comparative study on osteoconductivity by synthetic octacalcium phosphate and sintered hydroxyapatite in rabbit bone marrow. Calcif. Tissue Int. 2006, 78, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Pistone, A.; Iannazzo, D.; Panseri, S.; Montesi, M.; Tampieri, A.; Galvagno, S. Hydroxyapatite-magnetite-MWCNT nanocomposite as a biocompatible multifunctional drug delivery system for bone tissue engineering. Nanotechnology 2014, 25. [Google Scholar] [CrossRef] [PubMed]
- Pistone, A.; Iannazzo, D.; Espro, C.; Galvagno, S.; Tampieri, A.; Montesi, M.; Panseri, S.; Sandri, M. Tethering of Gly-Arg-Gly-Asp-Ser-Pro-Lys Peptides on Mg-Doped Hydroxyapatite. Engineering 2017, 3, 55–59. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A. Chitins and chitosans for the repair of wounded skin, nerve, cartilage and bone. Carbohydr. Polym. 2009, 76, 167–182. [Google Scholar] [CrossRef]
- Kim, T.; Jiang, H.; Jere, D.; Park, P.; Cho, M.; Nah, J.; Choi, Y.; Akaike, T.; Cho, C. Chemical modification of chitosan as a gene carrier in vitro and in vivo. Prog. Polym. Sci. 2007, 32, 726–753. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A. Chitosan composites with inorganics, morphogenetic proteins and stem cells, for bone regeneration. Carbohydr. Polym. 2011, 83, 1433–1445. [Google Scholar] [CrossRef]
- Hunter, K.T.; Ma, T. In vitro evaluation of hydroxyapatite–chitosan–gelatin composite membrane in guided tissue regeneration. J. Biomed. Mater. Res. A 2013, 101, 1016–1025. [Google Scholar] [CrossRef] [PubMed]
- Palazzo, B.; Gall, A.; Casillo, A.; Nitti, P.; Ambrosio, L.; Piconi, C. Fabrication, characterization and cell cultures on novel composite chitosan-nano-hydroxyapatite scaffold. Int. J. Immunopathol. Pharmacol. 2011, 24, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Lai, G.; Shalumon, K.T.; Chen, J. Response of human mesenchymal stem cells to intrafibrillar nanohydroxyapatite content and extrafibrillar nanohydroxyapatite in biomimetic chitosan/silk fibroin/nanohydroxyapatite nanofibrous membrane scaffolds. Int. J. Nanomed. 2015, 10, 567–584. [Google Scholar] [CrossRef]
- Zhao, F.; Ma, T. Perfusion bioreactor system for human mesenchymal stem cell tissue engineering: Dynamic cell seeding and construct development. Biotechnol. Bioeng. 2005, 91, 482–493. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.C.; Arns, C.H.; Hutmacher, D.W.; Milthorpe, B.K.; Sheppard, A.P.; Knackstedt, M.A. The correlation of pore morphology, interconnectivity and physical properties of 3D ceramic scaffolds with bone ingrowth. Biomaterials 2009, 30, 1440–1451. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Ma, T. Perfusion regulation of hMSC microenvironment and osteogenic differentiation in 3D scaffold. Biotechnol. Bioeng. 2012, 109, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Chella, R.; Ma, T. Effects of shear stress on 3-D human mesenchymal stem cell construct development in a perfusion bioreactor system: Experiments and hydrodynamic modeling. Biotechnol. Bioeng. 2007, 96, 584–595. [Google Scholar] [CrossRef] [PubMed]
- Rogina, A.; Rico, P.; Ferrer, G.G.; Ivanković, M.; Ivanković, H. In situ hydroxyapatite content affects the cell differentiation on porous chitosan/hydroxyapatite scaffolds. Ann. Biomed. Eng. 2016, 44, 1107–1119. [Google Scholar] [CrossRef] [PubMed]
- Rogina, A.; Rico, P.; Ferrer, G.G.; Ivanković, M.; Ivanković, H. Effect of in situ formed hydroxyapatite on microstructure of freeze-gelled chitosan-based biocomposite scaffolds. Eur. Polym. J. 2016, 68, 278–287. [Google Scholar] [CrossRef]
- Matic, I.; Antunović, M.; Brkic, S.; Josipovic, P.; Mihalic, K.C.; Karlak, I.; Ivkovic, A.; Marijanovic, I. Expression of OCT-4 and SOX-2 in bone marrow-derived human mesenchymal stem cells during osteogenic differentiation. Open Access Maced. J. Med. Sci. 2016, 4, 9–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valenti, M.T.; Dalle Carbonare, L.; Donatelli, L.; Bertoldo, F.; Zanatta, M.; Lo Cascio, V. Gene expression analysis in osteoblastic differentiation from peripheral blood mesenchymal stem cells. Bone 2008, 43, 1084–1092. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, M.; Fujisawa, R.; Kuboki, Y. Type I collagen-induced osteoblastic differentiation of bone-marrow cells mediated by collagen-α2β1 integrin interaction. J. Cell. Physiol. 2000, 184, 207–213. [Google Scholar] [CrossRef]
- Qin, C.; D’Souza, R.; Feng, J.Q. Dentin matrix protein 1 (DMP1): New and important roles for biomineralization and phosphate homeostasis. J. Dent. Res. 2007, 86, 1134–1141. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.Q.; Huang, H.; Lu, Y.; Ye, L.; Xie, Y.; Tsutsui, T.W.; Kunieda, T.; Castranio, T.; Scott, G.; Bonewald, L.B.; et al. The dentin matrix protein 1 (DMP1) is specifically expressed in mineralized but not soft tissues during development. J. Dent. Res. 2003, 82, 776–780. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, Y.; Suzawa, M.; Kikuchi, T.; Nishida, E.; Fujita, T.; Matsumoto, T. Differentiation and transforming growth factor-beta receptor down regulation by collagen-alpha 2 beta 1 integrin interaction is mediated by focal adhesion kinase and its downstreamsignals in murine osteoblastic cells. J. Biol. Chem. 1997, 272, 29309–29316. [Google Scholar] [CrossRef] [PubMed]
- Kundu, A.K.; Putnam, A.J. Vitronectin and collagen I differentially regulate osteogenesis in mesenchymal stem cells. Biochem. Biophy. Res. Commun. 2006, 347, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Stein, G.S.; Lian, J.B.; Stein, J.L.; Van Wijnen, A.J.; Montecino, M. Transcriptional control of osteoblast growth and differentiation. Physiol. Rev. 1996, 76, 593–629. [Google Scholar] [PubMed]
- Gundberg, C.M. Biochemical markers of bone formation. Clin. Lab. Med. 2000, 20, 489–501. [Google Scholar] [PubMed]
- Dworetzky, S.I.; Fey, E.G.; Penman, S.; Lian, J.B.; Stein, J.L.; Stein, G.S. Progressive changes in the protein composition of the nuclear matrix during rat osteoblast differentiation. Proc. Natl. Acad. Sci. USA 1990, 87, 4605–4609. [Google Scholar] [CrossRef] [PubMed]
- Stein, G.S.; Lian, J.B.; Van Wijnen, A.J.; Stein, J.L. The osteocalcin gene: A model for multiple parameters of skeletal-specific transcriptional control. Mol. Biol. Rep. 1997, 24, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Shin, Y.C.; Lee, S.M.; Jin, O.S.; Kang, S.H.; Hong, S.W.; Jeong, C.M.; Huh, J.B.; Han, D.W. Enhanced osteogenesis by reduced graphene oxide/hydroxyapatite nanocomposites. Sci. Rep. 2015, 5, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Sadat-Shojai, M.; Khorasani, M.; Dinpanah-Khoshdargi, E.; Jamshidi, A. Synthesis methods for nanosized hydroxyapatite with diverse structures. Acta Biomater. 2013, 9, 7591–7621. [Google Scholar] [CrossRef] [PubMed]
- Sundelacruz, S.; Kaplan, D. Stem cell and scaffold-based tissue engineering approaches to osteochondral regenerative medicine. Semin. Cell Dev. Biol. 2009, 20, 646–655. [Google Scholar] [CrossRef] [PubMed]
- Hulbert, S.F.; Young, F.A.; Mathews, R.S.; Klawitter, J.J.; Talbert, C.D.; Stelling, F.G. Potential of ceramic materials as permanently implantable skeletal prostheses. J. Biomed. Mater. Res. 1970, 4, 433–456. [Google Scholar] [CrossRef] [PubMed]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Yamamoto, M.; Tabata, Y. Osteogenic differentiation of mesenchymal stem cells in biodegradable sponges composed of gelatin and b-tricalcium phosphate. Biomaterials 2005, 26, 3587–3596. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.Y.; Feng, Y.F.; Ma, Z.S.; Li, X.; Wang, J.; Wang, L.; Lei, W. The promotion of osteointegration under diabetic conditions using chitosan/hydroxyapatite composite coating on porous titanium surfaces. Biomaterials 2014, 35, 7259–7270. [Google Scholar] [CrossRef] [PubMed]
- Barrère, F.; Van Blitterswijk, C.A.; De Groot, K. Bone regeneration: Molecular and cellular interactions with calcium phosphate ceramics. Int. J. Nanomed. 2006, 1, 317–332. [Google Scholar]
- Tang, S.; Tian, B.; Guo, Y.J.; Zhu, Z.A.; Guo, Y.P. Chitosan/carbonated hydroxyapatite composite coatings: Fabrication, structure and biocompatibility. Surf. Coat. Technol. 2014, 251, 210–216. [Google Scholar] [CrossRef]
- Marinucci, L.; Balloni, S.; Becchetti, E.; Bistoni, G.; Calvi, E.M.; Lumare, E.; Ederli, F.; Locci, P. Effects of hydroxyapatite and Biostite® on osteogenic induction of hMSC. Ann. Biomed. Eng. 2010, 38, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Spadaccio, C.; Rainer, A.; Trombetta, M.; Vadalá, G.; Chello, M.; Covino, E.; Denaro, V.; Toyoda, Y.; Genovese, J.A. Poly-l-lactic acid/hydroxyapatite electrospun nanocomposites induce chondrogenic differentiation of human MSC. Ann. Biomed. Eng. 2009, 37, 1376–1389. [Google Scholar] [CrossRef] [PubMed]
- Dimitrievska, S.; Bureau, M.N.; Antoniou, J.; Mwale, F.; Petit, A.; Lima, R.S.; Marple, B.R. Titania-hydroxyapatite nanocomposite coatings support human mesenchymal stem cells osteogenic differentiation. J. Biomed. Mater. Res. A 2011, 98, 576–588. [Google Scholar] [CrossRef] [PubMed]
- Polini, A.; Pisignano, D.; Parodi, M.; Quarto, R.; Scaglione, S. Osteoinduction of human mesenchymal stem cells by bioactive composite scaffolds without supplemental osteogenic growth factors. PLoS ONE 2011, 6, e26211. [Google Scholar] [CrossRef] [PubMed]
- Depan, D.; Pesacreta, T.C.; Misra, R.D.K. The synergistic effect of a hybrid grapheme oxide–chitosan system and biomimetic mineralization on osteoblast functions. Biomater. Sci. 2014, 2, 264–274. [Google Scholar] [CrossRef]
- Lee, S.H.; Chung, H.Y.; Shin, H.I.; Park, D.J.; Choi, J.H. Osteogenic activity of chitosan-based hybrid scaffold prepared by polyelectrolyte complex formation with alginate. Tissue Eng. Regen. Med. 2014, 11, 1–7. [Google Scholar] [CrossRef]







| Primers | 5′- | Sequences | Annealing Temperature (°C) |
|---|---|---|---|
| COL1A1 | forward | GCTATGATGAGAAATCAACCG | 61.1 |
| reverse | TCATCTCCATTTCCAGG | 61.6 | |
| IBSP | forward | GGAGACTTCAAATGAAGGAG | 57.9 |
| reverse | CAGAAAGTGTGGTATTCTCAG | 56.4 | |
| DMP1 | forward | CAACTATGAAGATCAGCATCC | 58.8 |
| reverse | CTTCCATTCTTCAGAATCCTC | 59.3 | |
| BGLAP | forward | TTCTTTCCTCTTCCCCTTG | 60.8 |
| reverse | CCTCTTCTGGAGTTTATTTGG | 59.3 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rogina, A.; Antunović, M.; Pribolšan, L.; Caput Mihalić, K.; Vukasović, A.; Ivković, A.; Marijanović, I.; Gallego Ferrer, G.; Ivanković, M.; Ivanković, H. Human Mesenchymal Stem Cells Differentiation Regulated by Hydroxyapatite Content within Chitosan-Based Scaffolds under Perfusion Conditions. Polymers 2017, 9, 387. https://doi.org/10.3390/polym9090387
Rogina A, Antunović M, Pribolšan L, Caput Mihalić K, Vukasović A, Ivković A, Marijanović I, Gallego Ferrer G, Ivanković M, Ivanković H. Human Mesenchymal Stem Cells Differentiation Regulated by Hydroxyapatite Content within Chitosan-Based Scaffolds under Perfusion Conditions. Polymers. 2017; 9(9):387. https://doi.org/10.3390/polym9090387
Chicago/Turabian StyleRogina, Anamarija, Maja Antunović, Lidija Pribolšan, Katarina Caput Mihalić, Andreja Vukasović, Alan Ivković, Inga Marijanović, Gloria Gallego Ferrer, Marica Ivanković, and Hrvoje Ivanković. 2017. "Human Mesenchymal Stem Cells Differentiation Regulated by Hydroxyapatite Content within Chitosan-Based Scaffolds under Perfusion Conditions" Polymers 9, no. 9: 387. https://doi.org/10.3390/polym9090387
APA StyleRogina, A., Antunović, M., Pribolšan, L., Caput Mihalić, K., Vukasović, A., Ivković, A., Marijanović, I., Gallego Ferrer, G., Ivanković, M., & Ivanković, H. (2017). Human Mesenchymal Stem Cells Differentiation Regulated by Hydroxyapatite Content within Chitosan-Based Scaffolds under Perfusion Conditions. Polymers, 9(9), 387. https://doi.org/10.3390/polym9090387