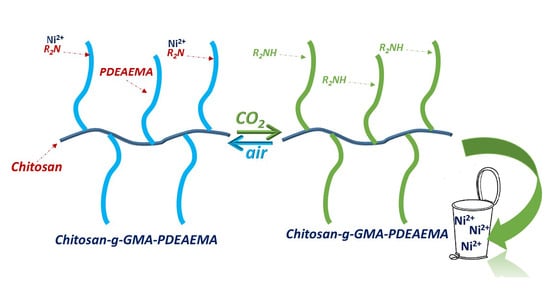
CO2-Responsive Graft Modified Chitosan for Heavy Metal (Nickel) Recovery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instrumentation
2.3. Synthesis of Chitosan–g–glycidyl Methacrylate (CTS–g–GMA)
2.4. Synthesis of Poly(diethylamino)ethyl Methacrylate (PDEAEMA) via Nitroxide Mediated Polymerization (NMP)
2.5. Synthesis of CTS-g-GMA-PDEAEMA
2.6. Ni(II) Adsorption Equilibrium Studies
2.7. CO2 Regeneration Studies
3. Results & Discussion
3.1. Characterization of CTS–g–GMA
3.2. Characterization of PDEAEMA
3.3. Characterization of CTS–g–GMA–PDEAEMA
3.4. CO2 Regeneration Study
3.5. Ni(II) Adsorption Equilibrium Studies
3.6. Ni(II) Adsorption Modelling
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Sashiwa, H.; Aiba, S. Chemically modified chitin and chitosan as biomaterials. Prog. Polym. Sci. 2004, 29, 887–908. [Google Scholar] [CrossRef]
- Benvenuti, T.; Krapf, R.S.; Rodrigues, M.A.S.; Bernardes, A.M.; Zoppas-Ferreira, J. Recovery of nickel and water from nickel electroplating wastewater by electrodialysis. Sep. Purif. Technol. 2014, 129, 106–112. [Google Scholar] [CrossRef]
- Hammack, R.W.; Edenborn, H.M. The removal of nickel from mine waters using bacterial sulfate reduction. Appl. Microbiol. Biotechnol. 1992, 37, 674–678. [Google Scholar] [CrossRef]
- Dermentzis, K.; Christoforidis, A.; Valsamidou, E. Removal of nickel, copper, zinc and chromium from synthetic and industrial wastewater by electrocoagulation. Int. J. Environ. Sci. 2011, 1, 697–710. [Google Scholar]
- Kurniawan, T.A.; Chan, G.Y.S.; Lo, W.; Babel, S. Physico-chemical treatment techniques for wastewater laden with heavy metals. Chem. Eng. J. 2006, 118, 83–98. [Google Scholar] [CrossRef]
- Peng, C.; Liu, Y.; Bi, J.; Xu, H.; Ahmed, A. Recovery of copper and water from copper-electroplating wastewater by the combination process of electrolysis and electrodialysis. J. Hazard. Mater. 2011, 189, 814–820. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Qin, S.; Davidson, J.; Li, W.; He, Y.; Zhou, H.S. Recovery of nickel from aqueous solutions by complexation-ultrafiltration process with sodium polyacrylate and polyethylenimine. J. Hazard. Mater. 2013, 244, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Liu, G.; Fan, Z.; Yang, X.; Wang, J.; Wang, S. Experimental study on treatment of electroplating wastewater by nanofiltration. J. Membr. Sci. 2007, 305, 185–195. [Google Scholar] [CrossRef]
- Coman, V.; Robotin, B.; Ilea, P. Nickel recovery/removal from industrial wastes: A review. Resour. Conserv. Recycl. 2013, 73, 229–238. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Chan, G.Y.S.; Lo, W.; Babel, S. Comparisons of low-cost adsorbents for treating wastewaters laden with heavy metals. Sci. Total Environ. 2006, 366, 409–426. [Google Scholar] [CrossRef]
- Mahmoud, A.; Hoadley, A.F. An evaluation of a hybrid ion exchange electrodialysis process in the recovery of heavy metals from simulated dilute industrial wastewater. Water Res. 2012, 46, 3364–3376. [Google Scholar] [CrossRef] [PubMed]
- Miretzky, P.; Cirelli, A.F. Hg(II) removal from water by chitosan and chitosan derivatives: A review. J. Hazard. Mater. 2009, 167, 10–23. [Google Scholar] [CrossRef] [PubMed]
- Rhazi, M.; Desbrieres, J.; Tolaimate, A.; Rinaudo, M.; Vottero, P.; Alagui, A.; El Meray, M. Influence of the nature of the metal ions on the complexation with chitosan.: Application to the treatment of liquid waste. Eur. Polym. J. 2002, 38, 1523–1530. [Google Scholar] [CrossRef]
- Wang, J.; Chen, C. Chitosan-based biosorbents: Modification and application for biosorption of heavy metals and radionuclides. Bioresour. Technol. 2014, 160, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Glasing, J.; Champagne, P.; Cunningham, M. Graft modification of chitosan, cellulose and alginate using reversible deactivation radical polymerization (RDRP). Curr. Opin. Green Sustain. Chem. 2016, 2, 15–21. [Google Scholar] [CrossRef]
- Garcia-Valdez, O.; Champagne, P.; Cunningham, M.F. Graft Modification of Natural Polysaccharides via Reversible Deactivation Radical Polymerization. Prog. Polym. Sci. 2017. [Google Scholar] [CrossRef]
- Garcia-Valdez, O.; George, S.; Champagne-Hartley, R.; Saldivar-Guerra, E.; Champagne, P.; Cunningham, M.F. Chitosan Modification via Nitroxide-Mediated Polymerization and grafting to Approach in Homogenous Media. Polymer 2015, 67, 139–147. [Google Scholar] [CrossRef]
- Garcia-Valdez, O.; Champagne-Hartley, R.; Saldivar-Guerra, E.; Champagne, P.; Cunningham, M.F. Modification of chitosan with polystyrene and poly(n-butyl acrylate) via nitroxide-mediated polymerization and grafting from approach in homogeneous media. Polym. Chem. 2015, 6, 2827–2836. [Google Scholar] [CrossRef]
- Tsai, B.; Garcia-Valdez, O.; Champagne, P.; Cunningham, M.F. Poly(Poly(Ethylene Glycol) Methyl Ether Methacrylate) Grafted Chitosan for Dye Removal from Water. Processes 2017, 5, 12. [Google Scholar] [CrossRef]
- Reddy, A.R.; Reddy, K.H. Heavy metal ion uptake properties of polystyrene-supported chelating polymer resins. Proc. Indian Acad. Sci. 2003, 115, 155–160. [Google Scholar] [CrossRef]
- Darabi, A.; Jessop, P.G.; Cunningham, M.F. CO2-responsive polymeric materials: synthesis, self-assembly, and functional applications. Chem. Soc. Rev. 2016, 45, 4391–4436. [Google Scholar] [CrossRef] [PubMed]
- Pinaud, J.; Kowal, E.; Cunningham, M.; Jessop, P. 2-(Diethyl)aminoethyl Methacrylate as a CO2-Switchable Comonomer for the Preparation of Readily Coagulated and Redispersed Polymer Latexes. ACS Macro Lett. 2012, 1, 1103–1107. [Google Scholar] [CrossRef]
- Darabi, A.; Rezaee Shirin-Abadi, A.; Pinaud, J.; Jessop, P.G.; Cunningham, M.F. Nitroxide-mediated surfactant-free emulsion copolymerization of methyl methacrylate and styrene using poly(2-(diethyl)aminoethyl methacrylate-co-styrene) as a stimuli-responsive macroalkoxyamine. Polym. Chem. 2014, 5, 6163–6170. [Google Scholar] [CrossRef]
- Vinas, J.; Chagneux, N.; Gigmes, D.; Trimaille, T.; Favier, A.; Bertin, D. SG1-based alkoxyamine bearing a N-succinimidyl ester: A versatile tool for advanced polymer synthesis. Polymer 2008, 49, 3639–3647. [Google Scholar] [CrossRef]
- Champagne-Hartley, P.A. Combined Passive System for the Treatment of Acid Mine Drainage. Ph.D. Thesis, Ottawa-Carleton Institute for Civil Engineering, Ottawa, ON, Canada, 2001. [Google Scholar]
- Popuri, R.; Vijaya, Y.; Boddu, V.M.; Abburi, K. Adsorptive removal of copper and nickel ions from water using chitosan coated PVC beads. Bioresour. Technol. 2009, 100, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Eldridg, D.S.; Crawford, R.J.; Harding, I.H. The role of metal ion-ligand interactions during divalent metal ion adsorption. J. Colloid Interface Sci. 2015, 454, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Ikhsan, J.; Johnson, B.B.; Wells, J.D. A comparative study of the adsorption of transition metals on kaolinite. J. Colloid Interface Sci. 1999, 217, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.A.; Leckie, J.O. Effect of adsorbed complexing ligands on trace metal uptake by hydrous oxides. Environ. Sci. Technol. 1978, 12, 1309–1315. [Google Scholar] [CrossRef]
- Awual, M.R. Assessing of lead (III) capturing from contaminated wastewater using ligand doped conjugate adsorbent. Chem. Eng. J. 2016, 289, 65–73. [Google Scholar] [CrossRef]
- Cheung, W.H.; Ng, J.; McKay, G. Kinetic analysis of the sorption of copper(II) ions on chitosan. J. Chem. Technol. Biotechnol. 2003, 78, 562–571. [Google Scholar] [CrossRef]
- Roosen, J.; Spooren, J.; Binnemans, K. Adsorption performance of functionalized chitosan–silica hybrid materials toward rare earths. J. Mater. Chem. A 2014, 2, 19415–19426. [Google Scholar] [CrossRef]
- Huang, C.; Chung, Y.C.; Ming, R.L. Adsorption of Cu(II) and Ni(II) by pelletized biopolymer. J. Hazard. Mater. 1996, 45, 265–277. [Google Scholar] [CrossRef]
- Kalyani, S.; Priya, J.A.; Rao, P.S.; Krishnaiah, A. Removal of Copper and Nickel from Aqueous Solutions Using Chitosan Coated on Perlite as Biosorbent. Sep. Sci. Technol. 2005, 40, 1483–1495. [Google Scholar] [CrossRef]
- Inoue, K.; Yoshizuka, K.; Ohto, K. Adsorptive separation of some metal ions by complexing agent types of chemically modified chitosan. Anal. Chim. Acta 1999, 388, 209–218. [Google Scholar] [CrossRef]
- Tan, S.; Wang, Y.; Peng, C.; Tang, Y. Synthesis and adsorption properties for metal ions of crosslinked chitosan acetate crown ethers. J. Appl. Polym. Sci. 1999, 71, 2069–2074. [Google Scholar] [CrossRef]
- Eser, A.; Tirtom, V.N.; Aydemir, T.; Becerik, S.; Dincer, A. Removal of nickel(II) ions by histidine modified chitosan beads. Chem. Eng. J. 2012, 210, 590–596. [Google Scholar] [CrossRef]









| Adsorbent | pH | Langmuir Isotherm | Freundlich Isotherm | ||
|---|---|---|---|---|---|
| Theoretical (mg/g) | (L/g) | (L/g) | |||
| CTS | 5.80–5.87 | 66.2 | 1.91 × 10−2 | 5.55 | 0.744 |
| CTS-g-GMA-PDEAEMA | 5.80–5.87 | 51.3 | 7.57 × 10−3 | 1.29 | 0.112 |
| CTS-g-GMA-PDEAEMA with initial CO2 sparging alone | 4.56–4.83 | 59.9 | 1.52 × 10−2 | 5.45 | 0.736 |
| Regenerated CTS-g-GMA-PDEAEMA | 5.80–5.87 | 149.3 | 3.25 × 10−3 | 1.92 | 0.283 |
| Adsorbent | Equilibrium Adsorption Capacity (qe, mg/g) | pH |
|---|---|---|
| CTS [34] | 2.4 | 5.0 |
| CTS coated PVC beads [27] | 120.5 | 5.0 |
| CTS coated perlite [35] | 114.9 | 5.0 |
| CTS–g–EDTA [36] | 123.3 | 2.0 |
| CTS–g–Acetate crown ether [37] | 0.7 | 5.6 |
| CTS–g–Diacetate crown ether [37] | 4.1 | 5.6 |
| Epichlorohydrine cross-linked CTS [37] | 6.4 | 5.6 |
| Epichlorohydrine cross-linked CTS–g–Histidine [38] | 140.8 | 5.0 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madill, E.A.W.; Garcia-Valdez, O.; Champagne, P.; Cunningham, M.F. CO2-Responsive Graft Modified Chitosan for Heavy Metal (Nickel) Recovery. Polymers 2017, 9, 394. https://doi.org/10.3390/polym9090394
Madill EAW, Garcia-Valdez O, Champagne P, Cunningham MF. CO2-Responsive Graft Modified Chitosan for Heavy Metal (Nickel) Recovery. Polymers. 2017; 9(9):394. https://doi.org/10.3390/polym9090394
Chicago/Turabian StyleMadill, Evan A. W., Omar Garcia-Valdez, Pascale Champagne, and Michael F. Cunningham. 2017. "CO2-Responsive Graft Modified Chitosan for Heavy Metal (Nickel) Recovery" Polymers 9, no. 9: 394. https://doi.org/10.3390/polym9090394
APA StyleMadill, E. A. W., Garcia-Valdez, O., Champagne, P., & Cunningham, M. F. (2017). CO2-Responsive Graft Modified Chitosan for Heavy Metal (Nickel) Recovery. Polymers, 9(9), 394. https://doi.org/10.3390/polym9090394