Evolution of NH3 Concentrations in Weaner Pig Buildings Based on Setpoint Temperature
Abstract
:1. Introduction
2. Materials and Methods
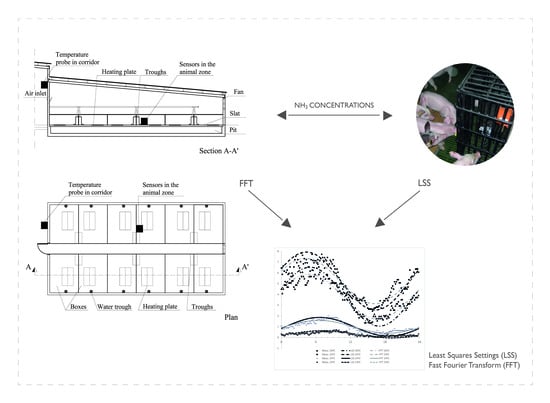
2.1. Experimental Test
- NH3 concentration in the animal-occupied zone (): ST–IAM IP66 electrochemical detector (Murco Ltd, Dublin, Ireland) with splash guard, 0–100 ppm detection range, 5% accuracy, temperature correction and auto-zero factory calibration before installation, implemented with a particulate filter made of wire cloth with 0.168 mm aperture width and 0.110 mm wire diameter.
- Relative humidity () and temperature () in the animal-occupied zone: temperature/relative humidity sensor, model S-THB-M008 sensor (Onset Computer Corporation, Bourne, MA, USA), with 40–75 °C temperature measurement range, ± 0.21 °C accuracy over the range 0–50 °C, and 0%–100% relative humidity range, and ± 2.50% accuracy from 10% to 90% RH.
- Fresh air temperature at air inlets (): negative temperature coefficient type sensors, model 107 sensor (Campbell Scientific Ltd., Loughborough, United Kingdom), with −35–50 °C measurement range and a thermistor interchangeability error of < ± 0.20 °C over the range 0–50 °C.
- Average temperatures measured with temperature probe model 107 were stored in a CR-10X datalogger (Campbell Scientific Ltd., Loughborough, United Kingdom).
- Average , , , and the voltage and intensity supplied to the fan were stored in one HOBO H-22 datalogger (Onset Computer Corporation, Bourne, MA, USA).
2.2. Mathematical Analysis
3. Results
4. Discussion
5. Conclusions
- NH3 concentration in the animal-occupied zone varies with the temperature setpoint defined for the climate control system. At night, when air temperature is lower, the ventilation rate decreases, which causes an increase in NH3 concentration. The increase in outdoor temperature during the daytime causes an increase in the ventilation rate and, consequently, in the rate of gas removal.
- The daily sine wave for NH3 concentrations provides a reliable pattern at every setpoint temperature, with R2 values between 0.93 and 0.71 for the two methods used, LSS and FFT. The FFT method showed a better sine fit, with RMSE values below 0.55 ppm as compared to 0.70 ppm in the LSS method. This occurs because the trend component of the series is neglected and only the seasonal component is considered. With the decrease in setpoint temperature, the amplitude of the wave diminishes and, generally, the sine wave is delayed. These sine waves were obtained by using inexpensive electrochemical sensors that could be easily incorporated in livestock farms. Our results show that these sensors, if maintained properly, can accurately represent the daily evolution of NH3 concentration.
- The use of sine wave equations to estimate NH3 concentrations can be beneficial for farmers, insofar as sine wave equations provide a reliable pattern for real-time estimation of NH3 concentration and can be included as a parameter in control strategies considering daytime. In addition, sine wave equations can be implemented in many conventional controllers because of their simplicity. Sine wave equations based on setpoint temperatures could be useful for real-time environmental control, which would substantially improve animal welfare.
Author Contributions
Funding
Conflicts of Interest
References
- Bjerg, B.; Norton, T.; Banhazi, T.; Zhang, G.; Bartzanas, T.; Liberati, P.; Cascone, G.; Lee, B.; Marucci, A. Modelling of ammonia emissions from naturally ventilated livestock buildings. Part 1: Ammonia release modelling. Biosyst. Eng. 2013, 116, 232–245. [Google Scholar] [CrossRef]
- Blanes–Vidal, V.; Nadimi, E.S.; Ellermann, T.; Andersen, H.V.; Løfstrøm, P. Perceived annoyance from environmental odors and association with atmospheric ammonia levels in non–urban residential communities: A cross–sectional study. Environ. Health 2012, 11, 11–27. [Google Scholar] [CrossRef] [Green Version]
- Saha, C.K.; Zhang, G.; Kai, P.; Bjerg, B. Effects of a partial pit ventilation system on indoor air quality and ammonia emission from a fattening pig room. Biosyst. Eng. 2010, 105, 279–287. [Google Scholar] [CrossRef]
- Ye, Z.; Zhang, G.; Li, B.; Strøm, J.S.; Dahl, P.J. Ammonia emissions affected by airflow in a model pig house: Effects of ventilation rate, floor slat opening, and headspace height in a manure storage pit. Trans. ASABE 2008, 51, 2113–2122. [Google Scholar] [CrossRef]
- Ni, J.Q.; Heber, A.J.; Lim, T.T. Ammonia and hydrogen sulphide in swine production. In Air Quality and Livestock Farming; CRC Press: Florida, FA, USA, 2018; pp. 69–88. [Google Scholar]
- Zhang, G.; Strøm, J.S.; Li, B.; Rom, H.B.; Morsing, S.; Dahl, P.; Wang, C. Emission of ammonia and other contaminant gases from naturally ventilated dairy cattle buildings. Biosyst. Eng. 2005, 92, 355–364. [Google Scholar] [CrossRef]
- Cheng, Z.; O’Connor, E.A.; Jia, Q.; Demmers, T.G.M.; Wathes, C.M.; Wathes, D.C. Chronic ammonia exposure does not influence hepatic gene expression in growing pigs. Animal 2014, 8, 331–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Done, S.H.; Chennells, D.J.; Gresham, A.C.J.; Williamson, S.; Hunt, B.; Taylor, L.L.; Bland, V.; Jones, P.; Armstrong, D.; White, R.P.; et al. Clinical and pathological responses of weaned pigs to atmospheric ammonia and dust. Vet. Rec. 2005, 157, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Drummond, J.G.; Curtis, S.E.; Simon, J.; Norton, H.W. Effects of aerial ammonia on growth and health of young pigs. J. Anim. Sci. 1980, 50, 1085–1091. [Google Scholar] [CrossRef] [Green Version]
- Hamilton, T.D.C.; Roe, J.M.; Hayes, C.M.; Webster, A.J.F. Effects of ammonia inhalation and acetic acid pretreatment on colonization kinetics of toxigenic Pasteurella multocida within upper respiratory tracts of swine. J. Clin. Microbiol. 1998, 36, 1260–1265. [Google Scholar] [CrossRef] [Green Version]
- O’Connor, E.A.; Parker, M.O.; McLeman, M.A.; Demmers, T.G.; Lowe, J.C.; Cui, L.; Davey, E.L.; Owen, R.C.; Wathes, C.M.; Abeyesinghe, S.M. The impact of chronic environmental stressors on growing pigs, Sus scrofa (Part 1): Stress physiology, production and play behaviour. Animal 2010, 4, 1899–1909. [Google Scholar] [CrossRef] [Green Version]
- Parker, M.O.; O’Connor, E.A.; McLeman, M.A.; Demmers, T.G.M.; Lowe, J.C.; Owen, R.C.; Davey, E.L.; Wathes, C.M.; Abeyesinghe, S.M. The impact of chronic environmental stressors on growing pigs, Sus scrofa (Part 2): Social behaviour. Animal 2010, 4, 1910–1921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von Borell, E.; Özpinar, A.; Eslinger, K.M.; Schnitz, A.L.; Zhao, Y.; Mitloehner, F.M. Acute and prolonged effects of ammonia on hematological variables, stress responses, performance, and behavior of nursery pigs. J. Swine Health Prod. 2007, 15, 137–145. [Google Scholar]
- Wathes, C.M.; Demmers, T.G.M.; Teer, N.; White, R.P.; Taylor, L.L.; Bland, V.; Jones, P.; Armstrong, D.; Greshan, A.C.J.; Hartung, J.; et al. Production responses of weaned pigs after chronic exposure to airborne dust and ammonia. Anim. Sci. 2004, 78, 87–97. [Google Scholar] [CrossRef]
- COUNCIL DIRECTIVE of 19 November 1991 laying down minimum standards for the protection of pigs (91/630/EEC). Off. J. Eur. Comm. 1991, L340, 33–38.
- International Commission of Agricultural and Biosystems Engineering. CIGR 4th Report of working group climatization of animal houses: Heat and moisture production. In The International Commission of Agricultural Engineering, Section II; Research Centre Bygholm: Horsens, Denmark; Danish Institute of Agricultural Sciences: Tjele, Denmark, 2002. [Google Scholar]
- Bottcher, R.; Mathis, S.; Roberts, J. Monitoring air quality with instruments. In Proceedings of the North Carolina Healthy Hogs Seminar; North Carolina Cooperative Extension Service: Raleigh, NC, USA, 2001; p. 10. [Google Scholar]
- Cargill, C.; Murphy, T.; Banhazi, T. Hygiene and air quality in intensive housing facilities in Australia. Animal Prod. Aust. 2002, 24, 387–393. [Google Scholar]
- Donham, K.; Thorne, P.; Breuer, G.; Powers, W.; Marquez, S.; Reynolds, S. Exposure limits related to air quality and risk assessment. In Iowa Concentrated Animal Feeding Operations Air Quality Study; Iowa State University and The University of Iowa Study Group: Ames, IA, USA, 2002; p. 164. [Google Scholar]
- Krupa, S.V. Effects of atmospheric ammonia (NH3) on terrestrial vegetation: A review. Environ. Pollut. 2003, 124, 179–221. [Google Scholar] [CrossRef]
- Schauberger, G.; Piringer, M.; Mikovits, C.; Zollitsch, W.; Hörtenhuber, S.J.; Baumgartner, J.; Niebuhr, K.; Anders, I.; Andre, K.; Hennig–Pauka, I.; et al. Impact of global warming on the odour and ammonia emissions of livestock buildings used for fattening pigs. Biosyst. Eng. 2018, 175, 106–114. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Han, W.; Tang, A.; Shen, J.; Cui, Z.; Vitousek, P.; Erisman, J.W.; Goulding, K.; Christie, P.; et al. Enhanced nitrogen deposition over China. Nature 2013, 494, 459–462. [Google Scholar] [CrossRef]
- Clark, C.M.; Tilman, D. Loss of plant species after chronic low–level nitrogen deposition to prairie grasslands. Nature 2008, 451, 712–715. [Google Scholar] [CrossRef]
- Backes, A.; Aulinger, A.; Bieser, J.; Matthias, V.; Quante, M. Ammonia emissions in Europe, part I: Development of a dynamical ammonia emission inventory. Atmos. Environ. 2016, 131, 55–66. [Google Scholar] [CrossRef] [Green Version]
- Backes, A.M.; Aulinger, A.; Bieser, J.; Matthias, V.; Quante, M. Ammonia emissions in Europe, part II: How ammonia emission abatement strategies affect secondary aerosols. Atmos. Environ. 2016, 126, 153–161. [Google Scholar] [CrossRef] [Green Version]
- Hendriks, C.; Kranenburg, R.; Kuenen, J.; van Gijlswijk, R.; Kruit, R.W.; Segers, A.; van der Gon, H.D.; Schaap, M. The origin of ambient particulate matter concentrations in the Netherlands. Atmos. Environ. 2013, 69, 289–303. [Google Scholar] [CrossRef]
- Geels, C.; Andersen, H.V.; Ambelas, S.C.; Christensen, J.H.; Ellermann, T.; Løfstrøm, P.; Gyldenkærne, S.; Brandt, J.; Hansen, J.B.; Frohn, L.M.; et al. Improved modelling of atmospheric ammonia over Denmark using the coupled modelling system DAMOS. Biogeosciences 2012, 9, 2625–2647. [Google Scholar] [CrossRef] [Green Version]
- Kryza, M.; Dore, A.J.; Błaś, M.; Sobik, M. Modelling deposition and air concentration of reduced nitrogen in Poland and sensitivity to variability in annual meteorology. J. Environ. Manag. 2011, 92, 1225–1236. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Zheng, K.; Liu, X.; Meng, L.; Huaitalla, R.M.; Shen, J.; Hartung, E.; Gallmann, E.; Roelcke, M.; Zhang, F. Atmospheric NH3 dynamics at a typical pig farm in China and their implications. Atmos. Pollut. Res. 2014, 5, 455–463. [Google Scholar] [CrossRef] [Green Version]
- Webb, J.; Menzi, H.; Pain, B.F.; Misselbrook, T.H.; Dämmgen, U.; Hendriks, H.; Döhler, H. Managing ammonia emissions from livestock production in Europe. Environ. Pollut. 2005, 135, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Schauberger, G.; Piringer, M.; Petz, E. Diurnal and annual variation of odour emission from animal houses: A model calculation for fattening pigs. J. Agric. Eng. Res. 1999, 74, 251–259. [Google Scholar] [CrossRef]
- Wang, K.; Wei, B.; Zhu, S.; Ye, Z. Ammonia and odor emitted from deep litter and fully slatted floor systems for growing–finishing pigs. Biosyst. Eng. 2011, 109, 203–210. [Google Scholar] [CrossRef]
- Heber, A.J.; Tao, P.C.; Ni, J.Q.; Lim, T.T.; Schmidt, A.M. Air emissions from two swine finishing building with flushing: Ammonia characteristics. In Livestock Environment VII; American Society of Agricultural and Biological Engineers: Saint Joseph, MI, USA, 2005; pp. 436–443. [Google Scholar]
- Koerkamp, P.G.; Metz, J.H.M.; Uenk, G.H.; Phillips, V.R.; Holden, M.R.; Sneath, R.W.; Short, J.L.; White, R.P.; Hartung, J.; Seedorf, J.; et al. Concentrations and emissions of ammonia in livestock buildings in Northern Europe. J. Agric. Eng. Res. 1998, 70, 79–95. [Google Scholar] [CrossRef]
- Zong, C.; Li, H.; Zhang, G. Ammonia and greenhouse gas emissions from fattening pig house with two types of partial pit ventilation systems. Agric. Ecosyst. Environ. 2015, 208, 94–105. [Google Scholar] [CrossRef]
- Raynor, P.C.; Engelman, S.; Murphy, D.; Ramachandran, G.; Bender, J.B.; Alexander, B.H. Effects of gestation pens versus stalls and wet versus dry feed on air contaminants in swine production. J. Agromed. 2018, 23, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Banhazi, T.; Seedorf, J.; Rutley, D.; Pitchford, W. Identification of risk factors for sub-optimal house conditions in Australian piggeries: Part 2. Airborne Pollutants. J. Agric. Saf. Health 2008, 14, 21–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banhazi, T.M. Seasonal, diurnal and spatial variations of environmental variables in Australian livestock buildings. Aust. J. Multi Discip. Eng. 2013, 10, 60–69. [Google Scholar] [CrossRef]
- Le Dividich, J.; Herpin, P. Effects of climatic conditions on the performance, metabolism and health–status of weaned piglets: A review. Livestock Prod. Sci. 1994, 38, 79–90. [Google Scholar] [CrossRef]
- Müirhead, M.R.; Alexander, T.J.L. Managing health and disease. In Managing Pig Health and de Treatment of Disease: A Reference for the Farm; 5M Enterprises: Sheffield, UK, 1997; pp. 55–104. [Google Scholar]
- Rinaldo, D.; Le Dividich, J. Assessment of optimal temperature for performance and chemical body composition of growing pigs. Livest. Prod. Sci. 1991, 29, 61–75. [Google Scholar] [CrossRef]
- Van Wagenberg, A.V.; Metz, J.H.M.; den Hartog, L.A. Methods for evaluation of the thermal environment in the animal–occupied zone for weaned piglets. Trans. ASABE 2005, 48, 2323–2332. [Google Scholar] [CrossRef] [Green Version]
- Park, J.H.; Peters, T.M.; Altmaier, R.; Sawvel, R.A.; Renée Anthony, T. Simulation of air quality and cost to ventilate swine farrowing facilities in winter. Comput. Electron. Agric. 2013, 98, 136–145. [Google Scholar] [CrossRef] [Green Version]
- Van Ransbeeck, N.; Van Langenhove, H.; Van Weyenberg, S.; Maes, D.; Demeyer, P. Typical indoor concentrations and emission rates of particulate matter at building level: A case study to setup a measuring strategy for pig fattening facilities. Biosyst. Eng. 2012, 111, 280–289. [Google Scholar] [CrossRef]
- Hamon, L.; Andrès, Y.; Dumont, E. Aerial pollutants in swine buildings: A review of their characterization and methods to reduce them. Environ. Sci. Technol. 2012, 46, 12287–12301. [Google Scholar] [CrossRef]
- Blanes–Vidal, V.; Hansen, M.N.; Pedersen, S.; Rom, H.B. Emissions of ammonia, methane and nitrous oxide from pig houses and slurry: Effects of rooting material, animal activity and ventilation flow. Agric. Ecosyst. Environ. 2008, 124, 237–244. [Google Scholar] [CrossRef]
- Calvet, S.; Cambra–López, M.; Estellés, F.; Torres, A.G. Characterization of the indoor environment and gas emissions in rabbit farms. World Rabbit. Sci. 2011, 19, 49–61. [Google Scholar] [CrossRef]
- Calvet, S.; Cambra–López, M.; Estellés, F.; Torres, A.G. Characterization of gas emissions from a Mediterranean broiler farm. Poult. Sci. 2011, 90, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Philippe, F.X.; Cabaraux, J.F.; Nicks, B. Ammonia emissions from pig houses: Influencing factors and mitigation techniques. Agric. Ecosyst. Environ. 2011, 141, 245–260. [Google Scholar] [CrossRef]
- Saha, C.K.; Ammon, C.; Berg, W.; Fiedler, M.; Loebsin, C.; Sanftleben, P.; Brunsch, R.; Amon, T. Seasonal and diel variations of ammonia and methane emissions from a naturally ventilated dairy building and the associated factors influencing emissions. Sci. Total Environ. 2014, 468, 53–62. [Google Scholar] [CrossRef]
- Takai, H.; Nimmermark, S.; Banhazi, T.; Norton, T.; Jacobson, L.D.; Calvet, S.; Hassouna, M.; Bjerg, B.; Zhang, G.Q.; Pedersen, S.; et al. Airborne pollutant emissions from naturally ventilated buildings: Proposed research directions. Biosyst. Eng. 2013, 116, 214–220. [Google Scholar] [CrossRef] [Green Version]
- García-Ramos, F.J.; Aguirre, A.J.; Barreiro, P.; Horcas, E.; Boné, A.; Vidal, M. Applicability of Ammonia Sensors for Controlling Environmental Parameters in Accommodations for Lamb Fattening. J. Sens. 2018, 8. [Google Scholar] [CrossRef] [Green Version]
- MeteoGalicia. Available online: https://www.meteogalicia.gal/observacion/estacions/estacions.action?request_locale=gl# (accessed on 8 January 2019).
- Andersson, M. Performance of bedding materials in affecting ammonia emissions from pig manure. J. Agric. Eng. Res. 1996, 65, 213–222. [Google Scholar] [CrossRef]





| Setpoint Temperatures (ST) (°C) | ||||
|---|---|---|---|---|
| 26 | 25 | 24 | 23 | |
| Onset date | 2 March | 8 March | 19 March | 27 March |
| End date | 6 March | 17 March | 25 March | 7 April |
| No. of days | 5 | 10 | 7 | 12 |
| Fan air inlet section (m2) | 0.0707 | 0.0962 | 0.1256 | 0.1963 |
| ST (°C) | CNH3 (ppm) | RHaz (%) AVE | Taz (°C) | Tac (°C) AVE | Tao (°C) AVE | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| AVE | SD | MAX | MIN | AVE | MAX | MIN | ||||
| 26 | 3.79 | 2.48 | 6.84 | 1.38 | 58 | 28.07 | 29.43 | 26.85 | 14.51 | 11.74 |
| 25 | 5.24 | 2.55 | 7.82 | 2.45 | 57 | 27.88 | 28.62 | 25.72 | 10.74 | 8.33 |
| 24 | 1.00 | 0.78 | 2.00 | 0.25 | 59 | 26.56 | 28.02 | 24.94 | 10.97 | 10.69 |
| 23 | 0.30 | 0.48 | 0.72 | 0.05 | 61 | 24.56 | 26.33 | 22.87 | 11.05 | 10.88 |
| ST (°C) | Method | A (ppm) | B (ppm) | φ (Rad) | Wave Onset Time |
|---|---|---|---|---|---|
| 26 | LSS FFT | 2.73 2.08 | 3.79 | 0.26 0.33 | 23:00 22:44 |
| 25 | LSS FFT | 2.69 2.10 | 5.24 | 0.44 0.47 | 22:19 22:13 |
| 24 | LSS FFT | 0.87 0.60 | 1.00 | −0.17 −0.14 | 00:39 00:31 |
| 23 | LSS FFT | 0.33 0.22 | 0.30 | −0.31 −0.32 | 01:11 01:12 |
| ST (°C) | Method | R2 | SDE (ppm) | RMSE (ppm) |
|---|---|---|---|---|
| 26 | LSS FFT | 0.93 0.92 | 0.64 0.42 | 0.64 0.42 |
| 25 | LSS FFT | 0.88 0.88 | 0.70 0.55 | 0.70 0.55 |
| 24 | LSS FFT | 0.84 0.84 | 0.26 0.19 | 0.26 0.19 |
| 23 | LSS FFT | 0.71 0.71 | 0.13 0.09 | 0.13 0.09 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodriguez, M.R.; Losada, E.; Besteiro, R.; Arango, T.; Velo, R.; Ortega, J.A.; Fernandez, M.D. Evolution of NH3 Concentrations in Weaner Pig Buildings Based on Setpoint Temperature. Agronomy 2020, 10, 107. https://doi.org/10.3390/agronomy10010107
Rodriguez MR, Losada E, Besteiro R, Arango T, Velo R, Ortega JA, Fernandez MD. Evolution of NH3 Concentrations in Weaner Pig Buildings Based on Setpoint Temperature. Agronomy. 2020; 10(1):107. https://doi.org/10.3390/agronomy10010107
Chicago/Turabian StyleRodriguez, Manuel R., Eugenio Losada, Roberto Besteiro, Tamara Arango, Ramon Velo, Juan A. Ortega, and Maria D. Fernandez. 2020. "Evolution of NH3 Concentrations in Weaner Pig Buildings Based on Setpoint Temperature" Agronomy 10, no. 1: 107. https://doi.org/10.3390/agronomy10010107