Synergetic Effects of Zinc, Boron, Silicon, and Zeolite Nanoparticles on Confer Tolerance in Potato Plants Subjected to Salinity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Location
2.2. Plant Material and Harvest Dates
2.3. Soil Preparation
2.4. Organic Matter (Compost)
2.5. Irrigation System
2.6. Chemical Fertilizers
2.7. Nano-Zeolite
2.8. Nano Zinc, Boron, and Silicon
- -
- Recommended dose of chemical fertilizers (NPK) as control T0
- -
- Nano zeolite T1
- -
- Nano zinc T2
- -
- Nano boron T3
- -
- Nano silicon T4
- -
- Nano zinc, boron and silicon T5
- -
- Nano zeolite, zinc, boron and silicon T6
2.9. Data Recorded
- -
- Plant height [cm]
- -
- Shoot fresh and dry weight [g plant−1]
- -
- Relative water content [%]
- -
- Number of stems per plant
- -
- Number of tubers per plant
- -
- Photosynthetic rate [CO2 m-2 s−1]
- -
- Intercellular CO2 concentration [ppm]
- -
- Stomatal conductance [H2O m-2 s−1]
- -
- Tuber yield [t ha−1].
- -
- Water use efficiency [μmol mmol−1].
2.10. Endogenous Phytohormones
2.11. Free Proline
2.12. Starch Content (%)
2.13. Peroxidase (POD)
2.14. Polyphenol Oxidase (PPO)
2.15. Data Analysis
3. Results and Discussion
3.1. Impacts of Soil Salinity on Plant Growth Parameters and Relative Water Content
3.2. Impacts of Soil Salinity on Leaf Chlorophyll and Photosynthetic Parameters
3.3. Impacts of Soil Salinity on Endogenous Elements of Potato Plants
3.4. Impacts of Soil Salinity on Leaf Proline, Gibberellic Acid and Abscisic Acid Contents
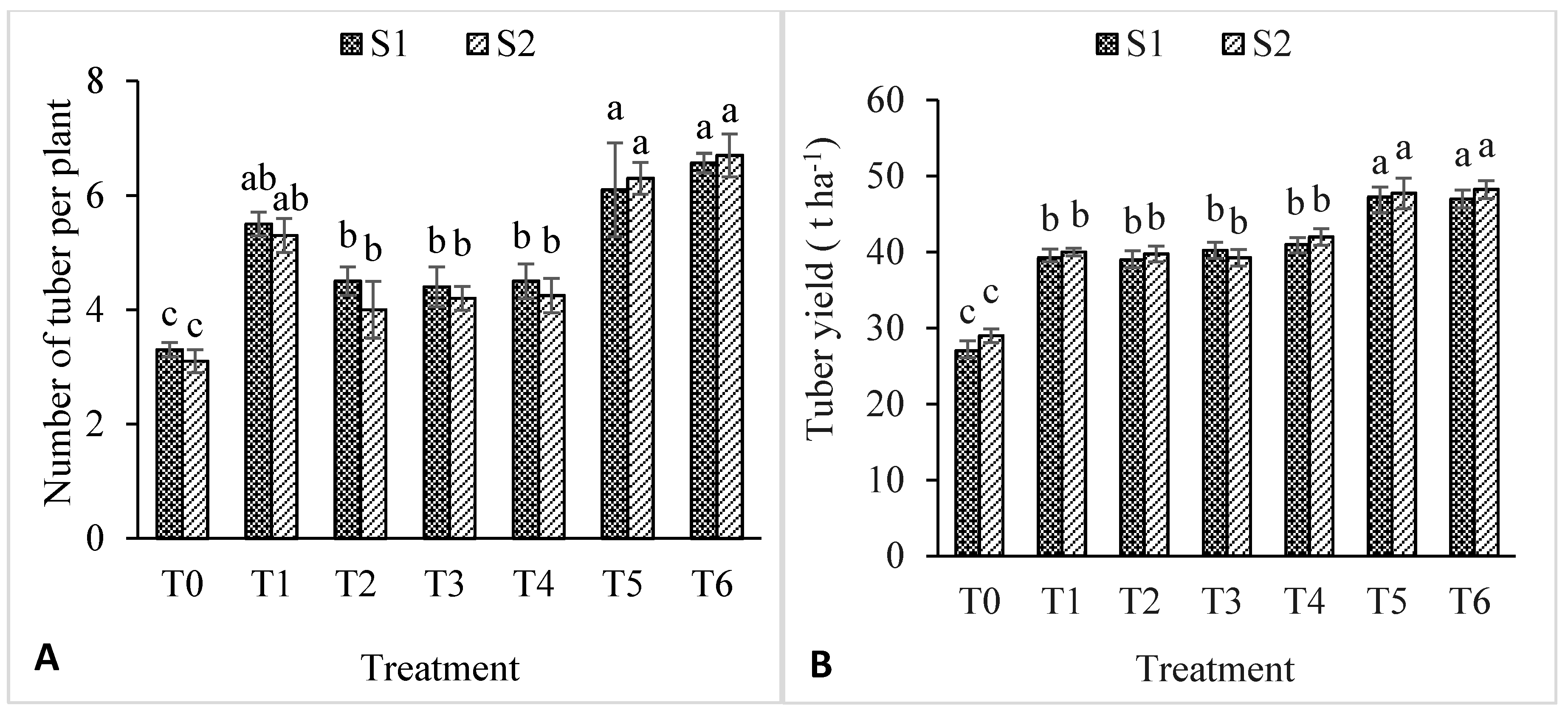
3.5. Impacts of Soil Salinity on Number and Yield of Potato Tubers
3.6. Impacts of Soil Salinity on Chemical Compositions of Potato Tubers
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Negrão, S.; Schmöckel, S.M.; Tester, M. Evaluating physiological responses of plants to salinity stress. Ann. Bot. 2017, 119, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- FAO (Food and Agriculture Organization). 2008. Available online: http://www.fao.org/land-water/en/ (accessed on 12 December 2019).
- Jha, D.; Shirley, N.; Tester, M.; Roy, S.J. Variation in salinity tolerance and shoot sodium accumulation in Arabidopsis ecotypes linked to differences in the natural expression levels of transporters involved in sodium transport. Plant Cell Environ. 2010, 33, 793–804. [Google Scholar] [CrossRef] [PubMed]
- Shabala, S.; Munns, R. salinity stress: Physiological constraints and adaptive mechanisms. In Plant Stress Physiology; Shabala, S., Ed.; CABI: Wallingford, UK, 2012; pp. 59–93. [Google Scholar] [CrossRef]
- Abdelaziz, M.E.; Abdeldaym, E.A. Effect of grafting and different EC levels of saline irrigation water on growth, yield and fruit quality of tomato (lycopersicon esculentum) in greenhouse. Plant Arch. 2019, 19, 3021–3027. [Google Scholar]
- Fatma, M.; Masood, A.; Per, T.S.; Rasheed, F.; Khan, N.A. Interplay between nitric oxide and sulfur assimilation in salt tolerance in plants. Crop J. 2016, 4, 153–161. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Zhang, H.; Zhang, X.; Tang, M. Arbuscular mycorrhizal symbiosis alleviates salt stress in black locust through improved photosynthesis, water status, and K+/Na+ 640 homeostasis. Front. Plant Sci. 2017, 8, 1739. [Google Scholar] [CrossRef]
- FAO (Food and Agriculture Organization). 2006 Buried Treasure: The Potato. Available online: http://www.fao.org/potato-2008/en/potato/utilization.html (accessed on 15 December 2012).
- Abdeldaym, E.A.; Traversa, A.; Cocozza, C.; Brunetti, G. Effects of a 2-year application of different residual biomasses on soil properties and potato yield. CLEAN Soil Air Water 2018, 46, 1800261. [Google Scholar] [CrossRef]
- Maas, E.V.; Grattan, S.R. Crop yields as affected by salinity. Agronomy 1999, 38, 55–110. [Google Scholar]
- Shannon, M.C.; Grieve, C.M. Tolerance of vegetable crops to salinity. Sci. Hortic. 1999, 78, 5–38. [Google Scholar] [CrossRef]
- Levy, D. The response of potatoes (Solunum tuberosum L.) to salinity: Plant growth and tuber yields in the arid desert of Israel. Ann. Appl. Biol. 1992, 120, 547–555. [Google Scholar] [CrossRef]
- Van Hoorn, J.W.; Katerji, N.; Hamdy, A.; Mastrorilli, M. Effect of saline water on soil salinity and on water stress, growth, and yield of wheat and potatoes. Agric. Water Manag. 1993, 23, 247–265. [Google Scholar] [CrossRef]
- Patell, R.M.; Prasher, S.O.; Donnelly, D.; Bonnell, R.B. Effect of initial soil salinity and subirrigation water salinity on potato tuber yield and size. Agric. Water Manag. 2001, 46, 231–239. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y.; Leonardi, C.; Bie, Z. Role of grafting in vegetable crops grown under saline conditions. Sci. Hortic. 2010, 127, 147–155. [Google Scholar] [CrossRef]
- Fan, P.; Chen, D.; He, Y.; Zhou, Q.; Tian, Y.; Gao, L. Alleviating salt stress in tomato seedlings using Arthrobacter and Bacillus megaterium isolated from the rhizosphere 667 of wild plants grown on saline;alkaline lands. Int. J. Phytoremediat. 2016, 18, 1113–1121. [Google Scholar] [CrossRef] [PubMed]
- Boukari, N.; Jelali, N.; Renaud, J.B.; Youssef, R.B.; Abdelly, C.; Hannoufa, A. Salicylic acid seed priming improves tolerance to salinity, iron deficiency and their combined effect in two ecotypes of Alfalfa. Environ. Exp. Bot. 2019, 167, 103820. [Google Scholar] [CrossRef]
- Yupeng, W.U.; Yufei, I.; Zhang, Y.; Yanmeng, B.I.; Zhenjun, S.U.N. Responses of saline soil properties and cotton growth to different organic amendments. Pedosphere 2018, 28, 521–529. [Google Scholar] [CrossRef]
- Chávez-garcía, E.; Siebe, C. Rehabilitation of a highly saline-sodic soil using a rubble 678 barrier and organic amendments. Soil Tillage Res. 2019, 189, 176–188. [Google Scholar] [CrossRef]
- El-sharkawy, M.S.; El-beshsbeshy, T.R.; Mahmoud, E.K.; Abdelkader, N.I.; Al-shal, R.M.; Missaoui, A.M. Response of alfalfa under salt stress to the application of 682 potassium sulfate nanoparticles. Am. J. Plant Sci. 2017, 8, 1751. [Google Scholar] [CrossRef] [Green Version]
- Mahmoud, A.W.M.; Abdelaziz, S.M.; El-mogy, M.M.; Abdeldaym, E.A. Effect of Foliar Zno and Feo Nanoparticles Application on Growth and Nutritional Quality of Red Radish and Assessment of Their Accumulation on Human Health. Agric. Pol’nohospod. 2019, 65, 16–29. [Google Scholar] [CrossRef] [Green Version]
- Sabaghnia, N.; Janmohammadi, M. Graphic analysis of nano-silicon by salinity stress interaction on germination properties of lentil using the biplot method. Agric. For. Poljopr. Sumar. 2014, 60, 29–40. [Google Scholar]
- Sabaghnia, N.; Janmohammadi, M. Effect of nano-silicon particles application on salinity tolerance in early growth of some lentil genotypes/Wpływ nanocząstek krzemionki na tolerancję zasolenia we wczesnym rozwoju niektórych genotypów soczewicy. Ann. UMCS Biol. 2015, 69, 39–55. [Google Scholar] [CrossRef] [Green Version]
- Siddiqui, M.H.; Al-Whaibi, M.H.; Firoz, M.; Al-khaishany, M.Y. Role of nanoparticles in plants. Nanotechnol. Plant Sci. 2015, 10, 19–35. [Google Scholar] [CrossRef]
- Wen, J.; Dong, H.; Zeng, G. Application of zeolite in removing salinity/sodicity from wastewater: A review of mechanisms, challenges and opportunities. J. Clean. Prod. 2018, 197 Pt 1, 1435–1446. [Google Scholar] [CrossRef]
- Arora, S.; Sharma, P.; Kumar, S.; Nayan, R.; Khanna, P.K.; Zaidi, M.G.H. Gold-nanoparticle induced enhancement in growth and seed yield of Brassica juncea. Plant Growth Regul. 2012, 66, 303–310. [Google Scholar] [CrossRef]
- Haghighi, M.; Afifipour, Z.; Mozafarian, M. The effect of n-si on tomato seed germination under salinity levels. J. Biol. Environ. Sci. 2012, 6, 87–90. [Google Scholar]
- Siddiqui, M.H.; Al-Whaibi, M.H.; Faisal, M.; Al sahli, A.A. Nano-silicon dioxide mitigates the adverse effects of salt stress on Cucurbita pepo L. Environ. Toxicol. Chem. 2014, 33, 2429–2437. [Google Scholar] [CrossRef] [PubMed]
- Hussein, M.M.; Alva, A.K. Effects of Zinc and Ascorbic Acid Application on the Growth and Photosynthetic Pigments of Millet Plants Grown under Different Salinity. Agric. Sci. 2014, 5, 1253–1260. [Google Scholar] [CrossRef] [Green Version]
- Hussein, M.M.; Abou-Baker, N.H. The contribution of nano-zinc to alleviate salinity stress on cotton plants. R. Soc. Open Sci. 2018, 5, 171809. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Ballesta, M.D.C.; Bastías, E.; Carvajal, M. Combined effect of boron and salinity on water transport. The role of aquaporins. Plant Signal. Behav. 2008, 3, 844–845. [Google Scholar] [CrossRef] [Green Version]
- Richards, L.S. Diagnosis and Improvement of Saline and Alkaline Soils; Handbook; U.S. Department of Agriculture: Washington, DC, USA, 1954; p. 60.
- Jackson, M.L. Soil Chemical Analysis; Text book; Printice-Hall of India, Privat Limited: New Delhi, India, 1973; Volume 144–197, p. 381. [Google Scholar]
- Page, A.I.; Miller, R.H.; Keeney, T.R. Methods of Soil Analysis Part 2; American Society of Agronomy Inc.: Madison, WI, USA, 1982; p. 595. [Google Scholar]
- Karmeli, D.; Keller, J. Trickle Irrigation Design; Rain-Bird Sprinkler Mfg. Co.: Glendora, CA, USA, 1975. [Google Scholar]
- Hassan, A.Z.A.; Mahmoud, A.W.M. Hydrothermal synthesis of nano crystals (AM) zeolite using variable temperature programs. J. Nanomater. Mol. Nanotechnol. 2015, 4. [Google Scholar] [CrossRef]
- Li, X.; Yang, Y.; Jia, L.; Chen, H.; Wei, X. Zinc-induced oxidative damage, antioxidant enzyme response and proline metabolism in roots and leaves of wheat 734 plants. Ecotoxicol. Environ. Saf. 2013, 89, 150–157. [Google Scholar] [CrossRef]
- Seifolazadeh, A.; Mohammadi, S. Synthesis and characterization of nano-boron powders prepared with mechano-chemical reaction between B2O3 and Mg powders. Bull. Mater. Sci. 2012, 39, 479–486. [Google Scholar] [CrossRef] [Green Version]
- Daneshvar, N.; Aber, S.; Sayed, D.; Khataee, M.S.; Rasoulifard, A.R. Preparation and investigation of photocatalytic properties of ZnO nanocrystals: Effect of operational parameters and kinetic study. Int. J. Nucl. Quantum Eng. 2007, 1, 24–29. [Google Scholar]
- Zhu, Y.; Gong, H. Beneficial effects of silicon on salt and drought tolerance in plants. Agron. Sustain. Dev. 2014, 34, 455–472. [Google Scholar] [CrossRef] [Green Version]
- Helrich, K. Official Methods of Analysis, 15th ed.; Association of Official Agricultural Chemist: Arlington, VA, USA, 1990; Volume 1, p. 673. [Google Scholar]
- Moran, R. Formulae for determination of chlorophyllous pigments extracted with N, N-dimethylformamide. Plant Physiol. 1982, 69, 1376–1381. [Google Scholar] [CrossRef] [Green Version]
- Fales, H.M.; Jaouni, T.M.; Babashak, J.F. Simple device for preparing ethereal diazomrthane without restoring to Codisitillation. Anal. Chem. 1973, 45, 2302–2303. [Google Scholar] [CrossRef]
- Furniss, B.S. Vogel’s Textbook of Practical Organic Chemistry; Pearson Education: Delhi, India, 1989. [Google Scholar]
- Bates, L.; Waldren, R.; Teare, I. Rapid determination of free proline for water-stress 758 studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Puter, J. Methods of Enzymatic Analysis; AcademicPrs: New York, NY, USA, 1974; Volume 2, p. 685. [Google Scholar]
- Malick, C.P.; Singh, M.B. Plant Enzymology and Histo-Enzymology; Kalyani Publication: New Delhi, India, 1980; p. 762. [Google Scholar]
- Reza, K.H.; Roosta, H.R. Evaluation of inter-specific hybrid of P. atlantica and P. vera L. cv.‘BadamiRiz-e-Zarand’as pistachio rootstock to salinity stress according to some growth indices and eco-physiological and biochemical parameters. J. Stress Physiol. Biochem. 2014, 10, 5–17. [Google Scholar]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [Green Version]
- Liang, Y.C.; Ding, R.X.; Liu, Q. Effects of silicon on salt tolerance of barley and its mechanism. Sci. Agric. Sin. 1999, 32, 75–83. [Google Scholar]
- Hajiboland, R.; Norouzi, F.; Poschenrieder, C. Growth, physiological, biochemical 781 and ionic responses of pistachio seedlings to mild and high salinity. Trees 2014, 28, 1065–1078. [Google Scholar] [CrossRef]
- Liang, Y.; Shen, Q.; Shen, Z.; Ma, T. Effects of silicon on salinity tolerance of two barley cultivars. J. Plant Nutr. 1996, 19, 173–183. [Google Scholar] [CrossRef]
- Bao-Shan, L.; Chun-Hui, L.; Li-Jun, F.; Shu-Chun, Q.; Min, Y. Effect of TMS 804 (nanostructured silicon dioxide) on growth of Changbai larch seedlings. J. For. Res. 2004, 15, 138–140. [Google Scholar] [CrossRef]
- Kalteh, M.; Alipour, Z.T.; Ashraf, S.; Marashi Aliabadi, M.; Falah Nosratabadi, A. Effect of silica nanoparticles on basil (Ocimumbasilicum) under salinity stress. J. Chem. Health Risks 2018, 4, 49–55. [Google Scholar]
- Romero-Aranda, M.R.; Jurado, O.; Cuartero, J. Silicon alleviates the deleterious salt effect on tomato plant growth by improving plant water status. J. Plant Physiol. 2006, 8, 847–855. [Google Scholar] [CrossRef] [PubMed]
- Tahir, M.A.; Aziz, T.; Farooq, M.; Sarwar, G. Silicon-induced changes in growth, ionic composition, water relations, chlorophyll contents and membrane permeability in two salt-stressed wheat genotypes. Arch. Agron. Soil Sci. 2012, 58, 247–256. [Google Scholar] [CrossRef]
- Raliya, R.; Tarafdar, J.C. ZnO nanoparticle biosynthesis and its effect on phosphorous-mobilizing enzyme secretion and gum contents in Clusterbean (Cyamopsis tetragonoloba L.). Agric. Res. 2013, 2, 48–57. [Google Scholar] [CrossRef] [Green Version]
- Raliya, R.; Tarafdar, J.C.; Biswas, P. Enhancing the mobilization of native phosphorus in the mung bean rhizosphere using ZnO nanoparticles synthesized by 785 soil fungi. J. Agric. Food Chem. 2016, 64, 3111–3118. [Google Scholar] [CrossRef]
- Noori, M.; Zendehdel, M.; Ahmadi, A. Using natural zeolite for the improvement of soil salinity and crop yield. Toxicol. Environ. Chem. 2006, 88, 77–84. [Google Scholar] [CrossRef]
- Al-Busaidi, A.; Yamamoto, T.; Inoue, M.; Eneji, A.E.; Mori YIrshad, M. Effects of zeolite on soil nutrients and growth of barley following irrigation with saline water. J. Plant Nutr. 2008, 31, 1159–1173. [Google Scholar] [CrossRef]
- Abou-Baker, N.H.; Ibrahim, E.A.; Abd-Eladl, M.M. Biozeolite for improving bean production under abiotic stress conditions. Bull. Transilv. Univ. Brasov. For. Wood Ind. Agric. Food Eng. Ser. II 2017, 10, 31–46. [Google Scholar]
- Bybordi, A.; Saadat, S.; Zargaripour, P. The effect of zeolite, selenium and silicon on qualitative and quantitative traits of onion grown under salinity conditions. Arch. Agron. Soil Sci. 2018, 64, 520–530. [Google Scholar] [CrossRef]
- Hussain, M.; Khan, M.A.; Khan, M.B.; Farooq, M.; Farooq, S. Boron application improves growth, yield and net economic return of rice. Rice Sci. 2012, 19, 259–262. [Google Scholar] [CrossRef]
- Wasaya, A.; Shahzad Shabir, M.; Hussain, M.; Ansar, M.; Aziz, A.; Hassan, W.; Ahmad, I. Foliar application of zinc and boron improved the productivity and net returns of maize grown under rainfed conditions of Pothwar plateau. J. Soil Sci. Plant Nutr. 2017, 17, 33–45. [Google Scholar] [CrossRef] [Green Version]
- Panhwar, Q.A.; Radziah, O.; Khanif, Y.M.; Naher, U.A. Application of boron and zinc in the tropical soils and its effect on maize (Zea mays) growth and soil microbial environment. Aust. J. Crop Sci. 2011, 5, 1649. [Google Scholar]
- Gong, H.J.; Randall, D.P.; Flowers, T.J. Silicon deposition in the root reduces sodium uptake in rice (Oryza sativa L.) seedlings by reducing bypass flow. Plant Cell Environ. 2006, 29, 1970–1979. [Google Scholar] [CrossRef] [PubMed]
- Abdi, G.; Salehi, H.; Eshghi, S. Effect of natural zeolite and paclobutrazol on reducing salt stress in Kentucky bluegrass (Poa pratensis L.). Hortic. Environ. Biotechnol. 2010, 51, 159–166. [Google Scholar]
- Eker, S.; Heybet, E.H.; Barut, H.; Erdem, H. Effects of zinc on growth and sodium, potassium and calcium concentrations of shoot in bread wheat under salt stress. Fresenius Environ. Bull. 2013, 22, 1622–1627. [Google Scholar]
- Ahmad, P.; John, R.; Sarwat, M.; Umar, S. Responses of proline, lipid peroxidation and antioxidative enzymes in two varieties of Pisum sativum L. under salt stress. Int. J. Plant Prod. 2012, 2, 353–366. [Google Scholar]
- Lawlor, D.W.; Cornic, G. Photosynthetic carbon assimilation and associated metabolism in relation to water deficits in higher plants. Plant Cell Environ. 2002, 25, 275–294. [Google Scholar] [CrossRef] [Green Version]
- Fadzilla, N.A.M.; Finch, R.P.; Burdon, R.H. Salinity, oxidative stress and antioxidant responses in shoot cultures of rice. J. Exp. Bot. 1997, 48, 325–331. [Google Scholar] [CrossRef]
- Steduto, P.; Albrizio, R.; Giorio, P.; Sorrentino, G. Gas-exchange response and stomatal and non-stomatal limitations to carbon assimilation of sunflower under salinity. Environ. Exp. Bot. 2000, 44, 243–255. [Google Scholar] [CrossRef]
- Song, A.; Li, P.; Fan, F.; Li, Z.; Liang, Y. The effect of silicon on photosynthesis and expression of its relevant genes in rice (Oryza sativa L.) under high-zinc stress. PLoS ONE 2014, 9, e113782. [Google Scholar] [CrossRef]
- Wang, N.; Yang, C.; Pan, Z.; Liu, Y.; Peng, S.A. Boron deficiency in woody plants: Various responses and tolerance mechanisms. Front. Plant Sci. 2015, 6, 916. [Google Scholar] [CrossRef] [Green Version]
- De Smedt, C.; Steppe, K.; Spanoghe, P. Beneficial effects of zeolites on plant photosynthesis. Adv. Mater. 2017, 2, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Yeo, A.R.; Flowers, S.A.; Rao, G.; Welfare, K.; Senanayake, N.; Flowers, T.J. Silicon reduces sodium uptake in rice (Oryza sativa L.) in saline conditions and this is accounted for by a reduction in the transpirational bypass flow. Plant Cell Environ. 1999, 22, 559–565. [Google Scholar] [CrossRef]
- Shah, S.H.; Houborg, R.; McCabe, M.F. Response of Chlorophyll, Carotenoid and SPAD-502 Measurement to Salinity and Nutrient Stress in Wheat (Triticum aestivum L.). Agronomy 2017, 7, 61. [Google Scholar] [CrossRef] [Green Version]
- Mateos-Naranjo, E.; Andrades-Moreno, L.; Davy, A.J. Silicon alleviates deleterious effects of high salinity on the halophytic grass Spartina densiflora. Plant Physiol. Biochem. 2013, 63, 115–121. [Google Scholar] [CrossRef]
- Siddiqui, M.H.; Al-Whaibi, M.H. Role of nano-SiO2 in germination of tomato (Lycopersicumesculentum seeds Mill.). Saudi J. Biol. Sci. 2014, 21, 13–17. [Google Scholar] [CrossRef] [Green Version]
- Xie, Y.; Li, B.; Zhang, Q.; Zhang, C.; Lu, K.; Tao, G. Effects of nano-TiO2 on photosynthetic characteristics of Indocalamus barbatus. J. Northeast. For. Univ. 2011, 39, 22–25. [Google Scholar]
- Soliman, A.S.; El-Feky, S.A.; Darwish, E. Alleviation of salt stress on Moringa peregrina using foliar application of nanofertilizers. J. Hortic. For. 2015, 7, 36–47. [Google Scholar] [CrossRef]
- Torabian, S.; Zahedi, M.; Khoshgoftar, A.H. Effects of foliar spray of two kinds of zinc oxide on the growth and ion concentration of sunflower cultivars under salt stress. J. Plant Nutr. 2016, 39, 172–180. [Google Scholar] [CrossRef]
- Bhat, F.A.; Ganai, B.A.; Uqab, B. Carbonic Anhydrase: Mechanism, Structure and Importance in Higher Plants. Asian J. Plant Sci. Res. 2017, 7, 17–23. [Google Scholar]
- Alpaslan, M.; Gunes, A. Interactive effects of boron and salinity stress on the growth, membrane permeability and mineral composition of tomato and cucumber plants. Plant Soil. 2001, 236, 123–128. [Google Scholar] [CrossRef]
- Edelstein, M.; Ben-Hur, M.; Cohen, R.; Burger, Y.; Ravina, I. Boron and salinity effects on grafted and non-grafted melon plants. Plant Soil. 2005, 269, 273–284. [Google Scholar] [CrossRef]
- Yermiyahu, U.; Ben-Gal, A.; Sarig, P.; Zipilevitch, E. Boron toxicity in grapevine (Vitis vinifera L.) in conjunction with salinity and rootstock effects. J. Hortic. Sci. Biotech. 2007, 82, 547–554. [Google Scholar] [CrossRef]
- Karimi, S.; Tavallali, V. Interactive effects of soil salinity and boron on growth, mineral composition and CO2 assimilation of pistachio seedlings. Acta Physiol. plant. 2017, 39, 242. [Google Scholar] [CrossRef]
- Kong, L.; Wang, M.; Bi, D. Selenium modulates the activities of antioxidant enzymes, osmotic homeostasis and promotes the growth of sorrel seedlings under salt stress. Plant Growth Regul. 2005, 45, 155–163. [Google Scholar] [CrossRef]
- Bybordi, A. Influence of zeolite, selenium and silicon upon some agronomic and physiologic characteristics of canola grown under salinity. Commun. Soil Sci. Plant Anal. 2016, 47, 832–850. [Google Scholar] [CrossRef]
- Bolaños, L.; Lukaszewski, K.; Bonilla, I.; Blevins, D. Why Boron? Plant Physiol. Biochem. 2004, 42, 907–912. [Google Scholar] [CrossRef]
- Mehrabanjoubani, P.; Abdolzadeh, A.; Sadeghipour, H.R.; Aghdasi, M. Impacts of silicon nutrition on growth and nutrient status of rice plants grown under varying zinc regimes. Exp. Plant Phys. 2015, 27, 19–29. [Google Scholar] [CrossRef]
- Saffaryazdi, A.; Lahouti, M.; Ganjeali, A.; Bayat, H. Impact of selenium supplementation on growth and selenium accumulation on spinach (Spinacia oleracea L.) plants. Not. Sci. Biol. 2012, 4, 95–100. [Google Scholar] [CrossRef] [Green Version]
- Mottaleb, S.A.; Darwish, E.; Mostafa, M.; Safwat, G. Phenotyping Root System Architecture of Cotton (Gossypium barbadense L.) Grown Under Salinity. Agric. Pol’nohospod. 2017, 63, 142–150. [Google Scholar] [CrossRef] [Green Version]
- Jaarsma, R.; De Boer, A.H. Salinity tolerance of two potato cultivars (Solanum tuberosum) correlates with differences in vacuolar transport activity. Front. Plant Sci. 2018, 9, 737. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Rosales, M.P.; Gálvez, F.J.; Huertas, R.; Aranda, M.N.; Baghour, M.; Cagnac, O.; Venema, K. Plant NHX cation/proton antiporters. Plant Signal Behav. 2009, 4, 265–276. [Google Scholar] [CrossRef] [Green Version]
- Krivosheeva, A.B.; Varlamova, T.V.; Yurieva, N.O.; Sobol’kova, G.I.; Kholodova, V.P.; Belyaev, D.V. Potato transformation with the HvNHX3 gene and the improvement of transformant salt tolerance. Russ. J. Plant Physiol. 2014, 61, 792–800. [Google Scholar] [CrossRef]
- Lee, S.K.; Sohn, E.Y.; Hamayun, M.; Yoon, J.Y.; Lee, I.J. Effect of silicon on growth and salinity stress of soybean plant grown under hydroponic system. Agrofor. Syst. 2010, 80, 333–340. [Google Scholar] [CrossRef]
- Kim, Y.H.; Khan, A.L.; Kim, D.H.; Lee, S.Y.; Kim, K.M.; Waqas, M.; Jung, H.Y.; Shin, J.H.; Kim, J.G.; Lee, I.J. Silicon mitigates heavy metal stress by regulating P-type heavy metal ATPases, Oryza sativa low silicon genes, and endogenous phytohormones. BMC Plant Biol. 2014, 14, 13. [Google Scholar] [CrossRef] [Green Version]
- Jha, G.; Choudhary, O.P.; Sharda, R. Comparative effects of saline water on yield and quality of potato under drip and furrow irrigation. Cogent Food Agric. 2017, 3, 1369345. [Google Scholar] [CrossRef]
- Nagaz, K.; Toumi, I.; Masmoudi, M.M.; Mechlia, N.B. Comparative effects of drip and furrow Irrigation with saline water on the yield and water use efficiency of potato (Solanum tuberosum L.) in arid conditions of Tunisia. J. Agric. 2008, 3, 272–277. [Google Scholar]
- Shahriaripour, R.; Pour, A.T.; Mozaffari, V.; Dashti, H.; Adhami, E. Effects of salinity and soil zinc application on growth and chemical composition of pistachio seedlings. J. Plant Nutr. 2010, 33, 1166–1179. [Google Scholar] [CrossRef]
- Liang, Y.; Nikolic, M.; Bélanger, R.; Gong, H.; Song, A. Silicon-Mediated Tolerance to Salt Stress. In Silicon in Agriculture; Springer: Dordrecht, The Netherlands, 2015; pp. 123–142. [Google Scholar]
- Subbaiah, L.V.; Prasad, T.N.; Krishna, T.G.; Sudhakar, P.; Reddy, B.R.; Pradeep, T. Novel effects of nano-particle delivery of zinc on growth, productivity, and zinc biofortification in maize (Zea mays L.). J. Agric. Food Chem. 2016, 64, 3778–3788. [Google Scholar] [CrossRef]
- Astafurova, T.P.; Burenina, A.A.; Suchkova, S.A.; Zotikova, A.B.P.; Kulizhskiy, S.P.; Morgalev, Y.N. Influence of ZnO and Pt nanoparticles on cucumber yielding capacity and fruit quality. Nano Hybrids Compos. 2017, 13, 142–148. [Google Scholar] [CrossRef]
- Wang, Q.; Liang, X.; Dong, Y.; Xu, L.; Zhang, X.; Kong, J.; Liu, S. Effects of exogenous salicylic acid and nitric oxide on physiological characteristics of perennial ryegrass under cadmium stress. J. Plant Growth Regul. 2013, 32, 721–731. [Google Scholar] [CrossRef]
- Hernández, J.A.; Corpas, F.J.; Gómez, M.; Del Rio, L.A.; Sevilla, F. Salt-Induced oxidative stress mediated by activated oxygen species in pea leaf mitochondria. Physiol. Plant. 1993, 89, 103–110. [Google Scholar] [CrossRef]
- Harinasut, P.; Poonsopa, D.; Roengmongkol, K.; Charoensataporn, R. Salinity effects on Antioxidant Enzymes in Mulberry Cultivar. Sci. Asia 2003, 29, 109–113. [Google Scholar] [CrossRef]
- Balakhnina, T.I.; Bulak, P.; Matichenkov, V.V.; Kosobryukhov, A.A.; Włodarczyk, T.M. The influence of Si-rich mineral zeolite on the growth processes and adaptive potential of barley plants under cadmium stress. Plant Growth Regul. 2015, 75, 557–565. [Google Scholar] [CrossRef]
- Prakash, M.G.; Chung, I.M. Determination of zinc oxide nanoparticles toxicity in root growth in wheat (Triticum aestivum L.) seedlings. Acta Biol. Hung. 2016, 67, 286–296. [Google Scholar] [CrossRef] [Green Version]
- Venkatachalam, P.; Priyanka, N.; Manikandan, K.; Ganeshbabu, I.; Indiraarulselvi, P.; Geetha, N.; Muralikrishna, K.; Bhattacharya, R.C.; Tiwari, M.; Sharma, N.; et al. Enhanced plant growth promoting role of phycomolecules coated zinc oxide nanoparticles with P supplementation in cotton (Gossypium hirsutum L.). Plant Physiol. Biochem. 2017, 110, 118–127. [Google Scholar] [CrossRef]
- Matichenkov, V.V.; Bocharnikova, E.A.; Ammosova, J.M. The influence of silicon fertilizers on the plants and soils. Agrochemistry 2001, 12, 30–37. [Google Scholar]
- Abdelaziz, M.E.; Abdelsattar, M.; Abdeldaym, E.A.; Atia, M.A.; Mahmoud, A.W.M.; Saad, M.M.; Hirt, H. Piriformospora indica alters Na+/k+ homeostasis, antioxidant enzymes and lenhx1 expression of greenhouse tomato Grown Under Salt Stress. Sci. Hortic. 2019, 256, 108532. [Google Scholar] [CrossRef]
- El-Mogy, M.M.; Salama, A.M.; Mohamed, H.F.Y.; Abdelgawad, K.F.; Abdeldaym, E.A. Responding of long green pepper plants to different sources of foliar potassium fertiliser. Agric. Pol’nohospod. 2019, 65, 59–76. [Google Scholar] [CrossRef]
- Abbas, G.; Chen, Y.; Khan, F.Y.; Feng, Y.; Palta, J.A.; Siddique, K.H.M. Salinity and Low Phosphorus Differentially Affect Shoot and Root Traits in Two Wheat Cultivars with Contrasting Tolerance to Salt. Agronomy 2018, 8, 155. [Google Scholar] [CrossRef] [Green Version]

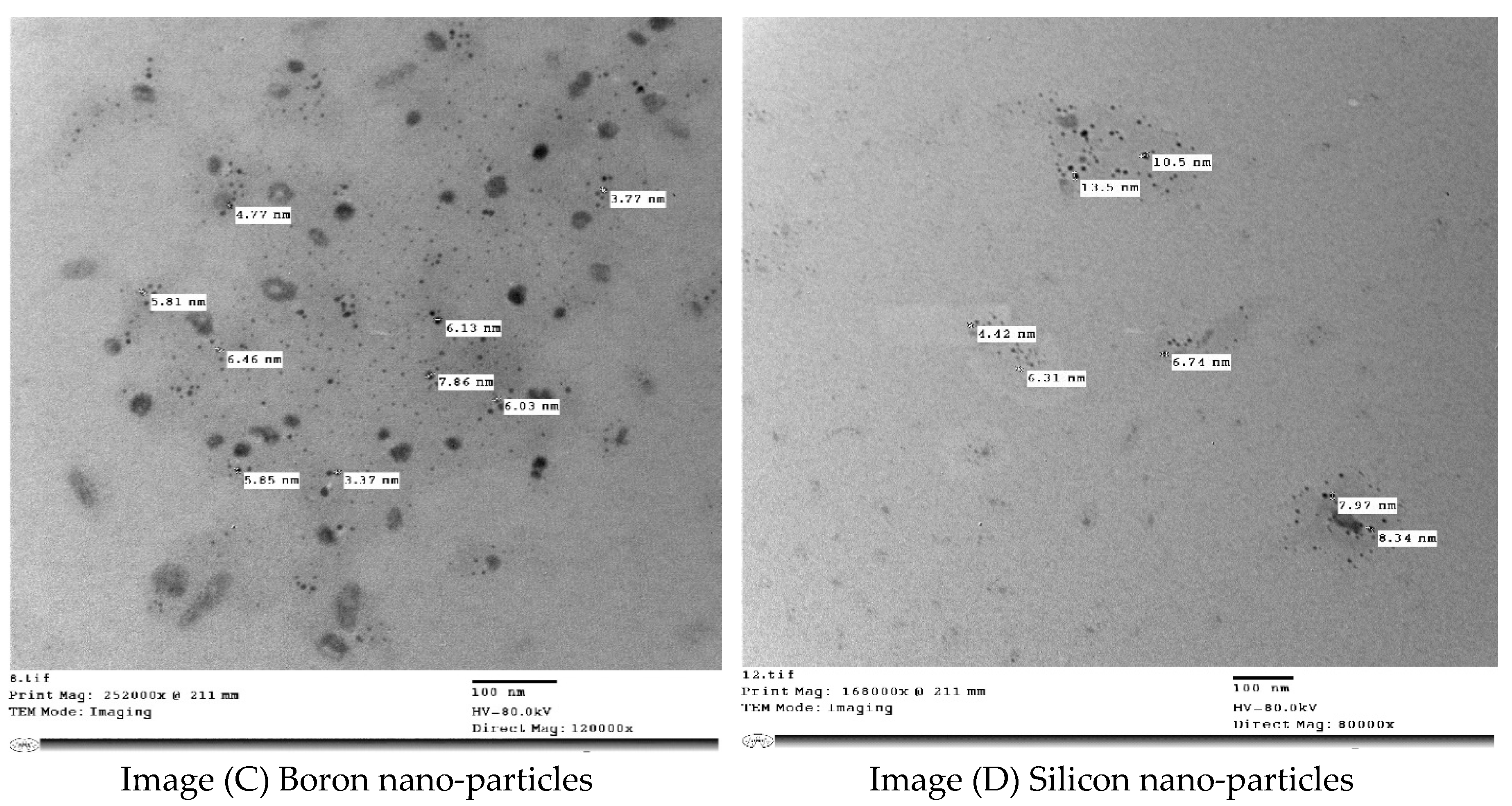

| Parameters | Soil Depth (cm) | |
|---|---|---|
| 0–30 | 30–60 | |
| Particle-size distribution [%] | ||
| Sand | 90.10 | 90.00 |
| Silt | 6.90 | 6.50 |
| Clay | 3.00 | 3.50 |
| Textural class | Sand | Sand |
| Saturation water content [cm3 cm−3] | 0.385 | 0.396 |
| Field capacity [cm3 cm−3] | 0.213 | 0.218 |
| Permanent wilting point [cm3 cm−3] | 0.057 | 0.057 |
| Available water [cm3.cm−3] | 0.156 | 0.161 |
| Bulk density [mg m−3] | 1.64 | 1.65 |
| Saturated hydraulic conductivity, [cm day−1] | 240.00 | 234.00 |
| Organic matter [%] | 0.31 | 0.25 |
| Calcium carbonates [%] | 4.80 | 3.71 |
| pH (1:1, soil: water suspension) | 7.70 | 7.81 |
| EC (1: 1, soil: water extract) [dS.m−1] | 4.02 | 4.13 |
| Soluble Cations, [Cmole(+) Kg−1 soil] | ||
| Ca2+ | 13.85 | 13.41 |
| Mg2+ | 12.15 | 10.59 |
| Na+ | 8.10 | 10.25 |
| K+ | 6.00 | 6.05 |
| Soluble Anions, [Cmole(–) Kg−1 soil] | ||
| CO32− | - | - |
| HCO3− | 11.92 | 9.75 |
| Cl− | 14.00 | 10.50 |
| SO42− | 15.08 | 21.30 |
| Available nutrients [mg Kg−1 soil] | ||
| N | 16.21 | 13.12 |
| P | 7.78 | 6.21 |
| K | 46.50 | 45.89 |
| Fe | 9.20 | 12.00 |
| Mn | 1.63 | 1.50 |
| Cu | 2.10 | 1.15 |
| Zn | 2.00 | 1.61 |
| B | 0.23 | 0.21 |
| Property | Value |
|---|---|
| Moisture content [%] | 25 |
| pH [1:5] | 7.5 |
| EC (1: 5 extract) [dS m−1] | 3.1 |
| Organic-C [%] | 33.11 |
| Organic matter [%] | 70 |
| Total-N [%] | 1.82 |
| Total-K [%] | 1.25 |
| C/N ratio | 14:1 |
| Total-P [%] | 1.29 |
| Fe [ppm] | 1019 |
| Mn [ppm] | 111 |
| Cu [ppm] | 180 |
| Zn [ppm] | 280 |
| Total content of Bacteria [CFU·g−1] | 2.5 × 107 |
| Phosphate dissolving Bacteria [CFU·g−1] | 2.5 × 106 |
| Weed seeds | 0 |
| Chemical Composition (%) | SiO2 | TiO2 | Al2O3 | Fe2O3 | FeO | MnO | MgO | CaO | Na2O | K2O | SrO | P2O3 | N |
| 45.50 | 2.81 | 13.30 | 5.40 | 8.31 | 0.51 | 6.30 | 9.52 | 2.83 | 0.87 | 0.22 | 0.67 | 2.70 | |
| Trace elements (ppm) | Ba | Co | Cr | Se | Cu | Zn | Zr | Nb | Ni | Rb | Y | ||
| 10 | 1.2 | 35 | 0.8 | 19 | 64 | 257 | 13 | 55 | 15 | 22 |
| Treatment | Plant Height (cm) | Shoot Fresh Weight (g) | Shoot Dry Weight (g) | Relative Water Content (%) | Number of Stems Per Plant | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| S1 | S2 | S1 | S2 | S1 | S2 | S1 | S2 | S1 | S2 | |
| T0 | 27.44 ± 1.78 c | 27.71 ± 4.13 d | 223.4 ± 14.5 d | 210.8 ± 16.3 e | 53.35 ± 4.95 c | 51.82 ± 3.3 c | 74.6 ± 5.73 b | 76.5 ± 6.32 b | 2.3 ± 0.65 b | 2.6 ± 0.36 b |
| T1 | 32.81 ± 5.48 b | 35.14 ± 2.93 b | 235.8 ± 12.01 c | 240.2 ± 13.9 c | 57.88 ± 5.88 b | 63.08 ± 6.7 a | 83.6 ± 4.41 a | 81.8 ± 7.17 a | 3.0 ± 0.41 a | 2.8 ± 0.26 b |
| T2 | 30.12 ± 1.85 b | 29.83 ± 3.53 c | 239.5 ± 10.31 b | 238.3 ± 18.1 d | 58.87 ± 6.04 b | 59.68 ± 7.3 b | 81.4 ± 6.8 a | 80.95 ± 3.66 a | 2.5 ± 0.22 b | 2.7 ± 0.25 b |
| T3 | 29.19 ± 3.12 c | 27.65 ± 2.78 d | 235.3 ± 19.65 c | 239.4 ± 20.7 c | 56.89 ± 4.6 b | 58.96 ± 3.92 b | 73.8 ± 7.90 b | 78.7 ± 9.42 b | 2.7 ± 0.52 b | 2.6 ± 0.44 b |
| T4 | 30.35 ± 4.1 b | 30.06 ± 4.45 c | 240.2 ± 11.23 b | 235.6 ± 23.5 c | 57.22 ± 1.99 b | 59.33 ± 6.07 b | 75.8 ± 6.45 b | 77.5 ± 8.41 b | 2.4 ± 0.38 b | 2.6 ± 0.34 b |
| T5 | 33.17 ± 5.37 b | 35.72 ± 2.36 b | 244.7 ± 21.23 b | 251.5 ± 13.2 b | 60.47 ± 2.24 b | 63.92 ± 4.5 a | 86.5 ± 7.2 a | 85.7 ± 5.31 a | 3.1 ± 0.35 a | 3.3 ± 0.42 a |
| T6 | 37.89 ± 2.82 a | 40.05 ± 3.91 a | 257.8 ± 6.31 a | 263.6 ± 9.31 a | 67.78 ± 3.4 a | 66.7 ± 3.10 a | 85.6 ± 5.21 a | 83.5 ± 4.50 a | 3.6 ± 0.43 a | 3.2 ± 0.56 a |
| Treatment | N (%) | P (%) | K (%) | |||||
| S1 | S2 | S1 | S2 | S1 | S2 | |||
| T0 | 2.1 ± 0.24 c | 1.8 ± 0.21 d | 0.17 ± 0.048 d | 0.19 ± 0.057 d | 3.41 ± 0.34 c | 3.39 ± 0.18 c | ||
| T1 | 2.9 ± 0.35 b | 2.8 ± 0.38 b | 0.26 ± 0.05 b | 0.28 ± 0.008 b | 6.01 ± 0.40 a | 6.03 ± 0.35 a | ||
| T2 | 2.9 ± 0.36 b | 2.8 ± 0.37 b | 0.28 ± 0.019 a | 0.31 ± 0.065 a | 5.58 ± 0.26 b | 6.00 ± 0.42 a | ||
| T3 | 2.7 ± 0.26 b | 2.8 ± 0.26 b | 0.27 ± 0.01 b | 0.29 ± 0.042 a | 5.89 ± 0.37 b | 6.06 ± 0.46 a | ||
| T4 | 2.6 ± 0.42 c | 2.5 ± 0.51 c | 0.25 ± 0.055 c | 0.26 ± 0.07 c | 5.88 ± 0.31 b | 5.79 ± 0.31 b | ||
| T5 | 3.1 ± 0.28 a | 3 ± 0.25 a | 0.26 ± 0.07 c | 0.28 ± 0.009 b | 6.04 ± 0.47 a | 6.00 ± 0.52 a | ||
| T6 | 3.5 ± 0.22 a | 3.4 ± 0.4 a | 0.24 ± 0.034 c | 0.25 ± 0.031 c | 6.11 ± 0.52 a | 6.09 ± 0.70 a | ||
| Treatment | Ca (%) | Na (%) | Zn (ppm) | B (ppm) | ||||
| S1 | S2 | S1 | S1 | S2 | S1 | S1 | S2 | |
| T0 | 1.42 ± 0.19 d | 1.54 ± 0.22 e | 3.42 ± 0.57 d | 3.81 ± 0.38 d | 26.4 ± 5.1 e | 25.1 ± 2.7f | 26.8 ± 3.1 d | 28.2 ± 6.9 d |
| T1 | 2.58 ± 0.38 b | 2.81 ± 0.25 b | 6.09 ± 0.42 a | 6.07 ± 0.36 a | 74.5 ± 9.9 c | 77.3 ± 5.27 c | 45.4 ± 5.22 a | 42.3 ± 4.30 b |
| T2 | 2.57 ± 0.29 b | 2.21 ± 0.23 d | 5.46 ± 0.62 b | 5.51 ± 0.59 b | 85.2 ± 9.7 b | 82.7 ± 9.5b | 41.8 ± 4.09 b | 43.5 ± 3.44 b |
| T3 | 2.59 ± 0.20 b | 2.77 ± 0.49 c | 5.50 ± 0.48 b | 5.49 ± 0.62 b | 59.3 ± 6.9 d | 56.6 ± 6.10 e | 37.5 ± 3.29 c | 40.2 ± 2.1 b |
| T4 | 2.54 ± 0.35 c | 2.71 ± 0.30 c | 4.78 ± 0.55 c | 4.81 ± 0.68 c | 72.5 ± 7.8 c | 70.4 ± 12.4 c | 47.2 ± 6.46 a | 45.6 ± 5.22 a |
| T5 | 2.64 ± 0.52 b | 2.89 ± 0.66 b | 6.11 ± 0.71 a | 6.19 ± 0.68 a | 83.4 ± 4.1 b | 81.6 ± 9.41 b | 47.6 ± 4.5 a | 46.7 ± 3.65 a |
| T6 | 2.85 ± 0.43 a | 3.01 ± 0.57 a | 6.54 ± 0.80 a | 6.38 ± 0.81 a | 102.1 ± 15.4 a | 99.3 ± 14.1a | 49.3 ± 6.1 a | 48.2 ± 5.41 a |
| Treatment | Proline Content (mg g−1) Leaves | GA3 (µg g−1 F W/Leaves) | ABA (µg g−1 F W Leaves) | |||
|---|---|---|---|---|---|---|
| S1 | S2 | S1 | S2 | S1 | S2 | |
| T0 | 4.12 ± 0.81 e | 3.74 ± 0.4 e | 6.65 ± 0.71 e | 6.92 ± 0.58 e | 5.881 ± 0.74 a | 7.982 ± 0.91 a |
| T1 | 6.44 ± 1.04 b | 6.42 ± 1.8 b | 10.32 ± 2.07 c | 9.92 ± 1.95 b | 4.221 ± 0.82 b | 4.131 ± 0.76 c |
| T2 | 5.03 ± 0.095 d | 4.84 ± 0.99 d | 10.41 ± 1.89 c | 10.37 ± 2.21 b | 4.232 ± 0.77 b | 4.201 ± 0.91 c |
| T3 | 5.37 ± 0.85 c | 5.28 ± 1.01 c | 8.97 ± ± 1.71 d | 9.12 ± 2.01 c | 4.155 ± 0.83 b | 4.193 ± 0.53 c |
| T4 | 5.09 ± 0.93 d | 5.1 ± 0.98 c | 8.93 ± 1.80 d | 9.07 ± 1.75 c | 4.311 ± 0.79 b | 4.352 ± 0.98 c |
| T5 | 7.24 ± 1.31 a | 7.53 ± 1.17 a | 12.68 ± 2.28 b | 13.22 ± 2.80 a | 3.115 ± 0.69 c | 2.742 ± 0.36 d |
| T6 | 6.52 ± 1.16 b | 6.33 ± 1.13 b | 13.53 ± 3.43 a | 13.62 ± 4.01 a | 2.053 ± 0.38 d | 2.016 ± 0.29 d |
| Treatment | Starch Content (%) | Carbohydrates (%) | Protein Content (%) | POD (Units mg−1Protein) | PPO (Units mg−1 Protein) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| S1 | S2 | S1 | S2 | S1 | S2 | S1 | S2 | S1 | S2 | |
| T0 | 80.4 ± 10.3 a | 81.6 ± 11.3 a | 72.14 ± 6.8 b | 71.58 ± 4.8 b | 0.83 ± 0.08 d | 0.72 ± 0.07 e | 0.010 ± 0.0005 e | 0.008 ± 0.0001f | 6.81 ± 0.92 d | 7.01 ± 0.52 d |
| T1 | 67.8 ± 7.4 c | 61.5 ± 6.9 c | 75.22 ± 5.7 b | 74.48 ± 5.2 b | 2.20 ± 0.47 b | 2.79 ± 0.61 b | 0.037 ± 0.0018 b | 0.035 ± 0.0006 c | 7.28 ± 0.72 b | 7.27 ± 0.87 b |
| T2 | 70.3 ± 9.2 b | 70.1 ± 8.5 b | 74.66 ± 7.2 b | 75.06 ± 6.6 b | 2.04 ± 0.21 b | 2.045 ± 0.30 c | 0.032 ± 0.0009 b | 0.031 ± 0.0003 c | 7.19 ± 0.83 b | 7.22 ± 0.80 b |
| T3 | 68.5 ± 5.1 c | 68.3 ± 7.6 b | 72.69 ± 6.5 b | 73.15 ± 6.9 b | 2.1 ± 0.56 b | 2.21 ± 0.37 b | 0.040 ± 0.0005 a | 0.042 ± 0.0081 b | 7.15 ± 1.01 c | 7.19 ± 1.4 c |
| T4 | 69.4 ± 8.7 c | 68.7 ± 6.39 b | 73.91 ± 8.4 b | 73.96 ± 5.6 b | 1.91 ± 0.19 c | 1.75 ± 0.14 d | 0.027 ± 0.0015 c | 0.025 ± 0.0001 d | 7.14 ± 0.99 c | 7.18 ± 0.57 c |
| T5 | 58.7 ± 6.69 d | 54.9 ± 7.3 d | 78.51 ± 7.5 a | 77.89 ± 6.3 a | 2.58 ± 0.15 a | 2.64 ± 0.51 a | 0.040 ± 0.0026 a | 0.048 ± 0.0009 a | 7.52 ± 1.01 a | 7.45 ± 1.8 a |
| T6 | 56.3 ± 5.9 d | 53.2 ± 4.9 d | 81.36 ± 9.1 a | 85.72 ± 7.7 a | 3.21 ± 0.54 a | 2.89 ± 0.88 a | 0.044 ± 0.00088 a | 0.051 ± 0.0006 a | 7.48 ± 1.3 a | 7.50 ± 2.01 a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahmoud, A.W.M.; Abdeldaym, E.A.; Abdelaziz, S.M.; El-Sawy, M.B.I.; Mottaleb, S.A. Synergetic Effects of Zinc, Boron, Silicon, and Zeolite Nanoparticles on Confer Tolerance in Potato Plants Subjected to Salinity. Agronomy 2020, 10, 19. https://doi.org/10.3390/agronomy10010019
Mahmoud AWM, Abdeldaym EA, Abdelaziz SM, El-Sawy MBI, Mottaleb SA. Synergetic Effects of Zinc, Boron, Silicon, and Zeolite Nanoparticles on Confer Tolerance in Potato Plants Subjected to Salinity. Agronomy. 2020; 10(1):19. https://doi.org/10.3390/agronomy10010019
Chicago/Turabian StyleMahmoud, Abdel Wahab M., Emad A. Abdeldaym, Suzy M. Abdelaziz, Mohamed B. I. El-Sawy, and Shady A. Mottaleb. 2020. "Synergetic Effects of Zinc, Boron, Silicon, and Zeolite Nanoparticles on Confer Tolerance in Potato Plants Subjected to Salinity" Agronomy 10, no. 1: 19. https://doi.org/10.3390/agronomy10010019
APA StyleMahmoud, A. W. M., Abdeldaym, E. A., Abdelaziz, S. M., El-Sawy, M. B. I., & Mottaleb, S. A. (2020). Synergetic Effects of Zinc, Boron, Silicon, and Zeolite Nanoparticles on Confer Tolerance in Potato Plants Subjected to Salinity. Agronomy, 10(1), 19. https://doi.org/10.3390/agronomy10010019






