Effects of the Irrigation with Treated Wastewaters on the Proximate Composition, Mineral, and Polyphenolic Profile of the Globe Artichoke Heads [Cynara cardunculus (L.)]
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description and Field Experimental Setup
2.2. Water and Plant Sampling
2.3. Qualitative Parameters Analysis of Artichoke Heads
2.3.1. Proximate Composition and Mineral Profile Analysis
2.3.2. HPLC Analysis of Polyphenolic Profile
2.4. Statistical Analysis
3. Results
3.1. Weather Conditions
3.2. Proximate Composition and Mineral Profile of the Artichoke Heads
3.3. Polyphenolic Profile of the Artichoke Heads
3.4. Multivariate Analysis on Qualitative Parameters
4. Discussion
4.1. Proximate Composition and Mineral Profile of the Artichoke Heads
4.2. Phenolic Profile of the Artichoke Heads
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Giuliani, M.M.; Nardella, E.; Gagliardi, A.; Gatta, G. Deficit irrigation and partial root-zone drying techniques in processing tomato cultivated under Mediterranean conditions. Sustainability 2017, 9, 2197. [Google Scholar] [CrossRef]
- Hassanein, R.A.; Hashem, H.A.; Hashem El-Deep, M.; Shouman, A. Soil contamination with heavy metal and its effect on growth, yield and physiological responses of vegetable crop plants (turnip and lettuce). J. Stress Physiol. Biochem. 2013, 9, 145–162. [Google Scholar]
- Gatta, G.; Libutti, A.; Beneduce, L.; Gagliardi, A.; Disciglio, G.; Lonigro, A.; Tarantino, E. Reuse of treated municipal wastewater for globe artichoke irrigation: Assessment of effects on morpho-quantitative parameters and microbial safety of yield. Sci. Hortic. 2016, 213, 55–65. [Google Scholar] [CrossRef]
- Benitez, E.; Romero, M.; Gomez, M.; Gallardolaro, F.; Nogales, R. Biosolid and biosolid ash as sources of heavy metals in plant-soil system. Water Air Soil Poll. 2001, 132, 75–87. [Google Scholar] [CrossRef]
- Abdallah, A.; Bader, A. Comparison between the irrigation qualities of conventional tertiary and UF + RO advanced treated wastewaters. J. Agric. Sci. 2011, 2, 526–532. [Google Scholar] [CrossRef]
- Kocak, S.; Tokusoglu, O.; Aycan, S. Some heavy metal and trace essential element detection in canned vegetable foodstuffs by differential pulse polarography (DPP). Electron. J. Environ. Agric. Food Chem. 2005, 4, 871–878. [Google Scholar]
- Gatta, G.; Gagliardi, A.; Disciglio, G.; Lonigro, A.; Francavilla, M.; Tarantino, E.; Giuliani, M.M. Irrigation with treated municipal wastewater on artichoke crop: Assessment of soil and yield heavy metal content and human risk. Water 2018, 10, 225. [Google Scholar] [CrossRef]
- Nazar, R.; Iqbal, N.; Masood, A.; Khan, M.I.R.; Syeed, S.; Khan, N.A. Cadmium toxicity in plants and role of mineral nutrients in its alleviation. Am. J. Plant Sci. 2012, 3, 1476–1489. [Google Scholar] [CrossRef]
- Rattan, R.K.; Datta, S.P.; Chhonkar, P.K.; Suribabu, K.; Singh, A.K. Long-term impact of irrigation with sewage effluents on heavy metal content in soils, crops and groundwater: A case study. Agric. Ecosyst. Environ. 2005, 109, 310–322. [Google Scholar] [CrossRef]
- Scoccianti, V.; Crinelli, R.; Tirillini, B.; Mancinelli, V.; Speranza, A. Uptake and toxicity of Cr (III) in celery seedlings. Chemosphere 2006, 64, 1695–1703. [Google Scholar] [CrossRef]
- Nada, E.; Ben, A.F.; Rhouma, A.; Ben, R.B.; Mezghani, I.; Boukhris, M. Cadmium-induced growth inhibition and alteration of biochemical parameters in almond seedlings grown in solution culture. Acta Physiol. Plant. 2007, 29, 57–62. [Google Scholar] [CrossRef]
- Lonigro, A.; Catalano, M.; Rubino, P. Impiego in agricoltura di acque reflue urbane depurate nel rispetto della sostenibilità ambientale. Ital. J. Agron. 2007, 2, 217–259. [Google Scholar] [CrossRef]
- Pandino, G.; Lombardo, S.; Mauromicale, G. Mineral profile in globe artichoke as affected by genotype, head part and environment. J. Sci. Food Agric. 2011, 91, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Pandino, G.; Courts, F.L.; Lombardo, S.; Mauromicale, G.; Williamson, G. Caffeoylquinic acids and flavonoids in the immature inflorescence of globe artichoke, wild cardoon and cultivated cardoon. J Agric. Food Chem. 2010, 58, 1026–1031. [Google Scholar] [CrossRef] [PubMed]
- Lattanzio, V.; Kroon, P.A.; Linsalata, V.; Cardinali, A. Globe artichoke: A functional food and source of nutraceutical ingredients. J. Funct. Foods 2009, 1, 131–144. [Google Scholar] [CrossRef]
- Ierna, A.; Mauro, R.P.; Mauromicale, G. Improved yield and nutrient efficiency in two globe artichoke genotypes by balancing nitrogen and phosphorus supply. Agron. Sustain. Dev. 2012, 32, 773–780. [Google Scholar] [CrossRef]
- FAO 2016. Food and Agriculture Organization of the United Nations. Available online: http://www.faostat.fao.org (accessed on 14 October 2018).
- Bonasia, A.; Conversa, G.; Lazzizera, C.; Gambacorta, G.; Elia, A. Morphological and qualitative characterisation of globe artichoke head from new seed-propagated cultivars. J. Sci. Food Agric. 2010, 90, 2689–2693. [Google Scholar] [CrossRef]
- Ceccarelli, N.; Curadi, M.; Picciarelli, P.; Martelloni, L.; Sbrana, C.; Giovannetti, M. Globe artichoke as a functional food. Med. J. Nutr. Metab. 2010, 3, 197–201. [Google Scholar] [CrossRef]
- Pandino, G.; Lombardo, S.; Mauromicale, G.; Williamson, G. Profile of polyphenols and phenolics acids in bracts and receptacles of globe artichoke (Cynara cardunculus var scolymus) germplasm. J. Food. Compos. Anal. 2011, 24, 148–153. [Google Scholar] [CrossRef]
- Lombardo, S.; Pandino, G.; Ierna, A.; Mauromicale, G. Variation of polyphenols in a germplasm collection of globe artichoke. Food Res. Int. 2012, 46, 544–551. [Google Scholar] [CrossRef]
- Nicoletto, C.; Santagata, S.; Tosini, F.; Sambo, P. Qualitative and healthy traits of different Italian typical artichoke genotypes. J. Food Res. 2013, 11, 108–113. [Google Scholar] [CrossRef]
- Schütz, K.; Kammerer, D.; Carle, R.; Schieber, A. Identification and quantification of caffeoylquinic acids and flavonoide from artichoke (Cynara scolymus L.) heads, juice, and pomace by HPLC-DAD-ESI/MSn. J. Agric. Food Chem. 2004, 52, 4090–4096. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, S.; Pandino, G.; Mauromicale, G.; Knodler, M.; Carle, R.; Schieber, A. Influence of genotype, harvest time and plant part on polyphenolic composition of globe artichoke [Cynara cardunculus L. var. scolymus (L.) Fiori]. Food Chem. 2010, 119, 1175–1181. [Google Scholar] [CrossRef]
- Pavlica, S.; Gebhardt, R. Protective effects of ellagic and chlorogenic acids against oxidative stress in PC12 cells. Free Radic. Res. 2005, 39, 1377–1390. [Google Scholar] [CrossRef] [PubMed]
- Pandino, G.; Lombardo, S.; Lo Monaco, A.; Mauromicale, G. Choice of time of harvest influences the polyphenol profile of globe artichoke. J. Funct. Foods 2013, 5, 1822–1828. [Google Scholar] [CrossRef]
- Pollice, A.; Lopez, A.; Laera, G.; Rubino, P.; Lonigro, A. Tertiary filtered municipal wastewater as alternative water source in agriculture: A field investigation in Southern Italy. Sci. Total Environ. 2004, 324, 201–210. [Google Scholar] [CrossRef]
- Beneduce, L.; Gatta, G.; Bevilacqua, A.; Troiano, E.; Spano, G. Impact of the reusing of food manufacturing wastewater for irrigation in a closed system on the microbiological quality of the food crops. Int. J. Food Microbiol. 2017, 260, 51–58. [Google Scholar] [CrossRef]
- Available online: www.consorzio.fg.it (accessed on 15 November 2014).
- AOAC. Official Method of Analysis; No. 934.06; Association of Official Analytical Chemists: Washington, DC, USA, 1990. [Google Scholar]
- Dolciotti, I.; Mambelli, S.; Grand, S.; Venturi, G. Comparison of two Sorghum genotypes for sugar and fiber production. Ind. Crops Prod. 1998, 7, 265–272. [Google Scholar] [CrossRef]
- De Clerk, J. Course de Brasserie, 2nd ed.; Universitè Catholique de Louvain: Louvain-la-Neuve, Belgium, 1963. [Google Scholar]
- Renna, M.; Gonnella, M.; Giannino, D.; Santamaria, P. Quality evaluation of cook-chilled chicory stems (Cichorium intybus L., Catalogna group) by conventional and sous vide cooking methods. J. Sci. Food Agric. 2014, 94, 656–665. [Google Scholar] [CrossRef]
- US-EPA. Risk Assessment Guidance for Superfund; EPA/540/1-89/002, OSWER; USEPA: Washington, DC, USA, 1989. [Google Scholar]
- Rouphael, Y.; Colla, G.; Graziani, G.; Ritieni, A.; Cardarelli, M.; De Pascale, S. Phenolic composition, antioxidant activity and mineral profile in two seed-propagated artichoke cultivars as affected by microbial inoculants and planting time. Food Chem. 2017, 234, 10–19. [Google Scholar] [CrossRef]
- Pandino, G.; Lombardo, S.; Mauromicale, G.; Williamson, G. Phenolic acids and flavonoids in leaf and floral stem of cultivated and wild L. genotypes. Food Chem. 2011, 126, 417–422. [Google Scholar] [CrossRef]
- Cox, D.R.; Box, G.E.P. An analysis of transformations (with discussion). J. R. Stat. Soc. 1964, 26, 211–250. [Google Scholar]
- Rencher, A.C. Interpretation of canonical discriminant functions, canonical variates, and principal components. Am. Stat. 1992, 46, 217–225. [Google Scholar]
- Zhao, H.; Guo, B.; Wei, Y.; Zhang, B.; Sun, S.; Zhang, L.; Junhui, Y. Determining the geographic origin of wheat using multielement analysis and multivariate statistics. J. Agric. Food Chem. 2011, 59, 4397–4402. [Google Scholar] [CrossRef] [PubMed]
- Mauro, R.P.; Occhipinti, A.; Longo, A.M.G.; Mauromicale, G. Effects of shading on chlorophyll content, chlorophyll fluorescence an photosynthesis of subterranean clover. J. Agron. Crop Sci. 2011, 197, 57–66. [Google Scholar] [CrossRef]
- Saleh, S.A.; Zaki, M.F.; Tantawy, A.S.; Salama, Y.A.M. Response of artichoke productivity to different proportions of nitrogen and potassium fertilizers. Int. J. Chem. Tech. Res. 2016, 9, 25–33. [Google Scholar]
- Ehaliotis, C.; Massas, I.; Pavlou, G. Efficient urea-N and KNO-N uptake by vegetable plants using fertigation. Agron. Sustain. Dev. 2010, 30, 763–768. [Google Scholar] [CrossRef][Green Version]
- Spiertz, H.J. Nitrogen, Sustainable Agriculture and Food Security: A Review. Agron. Sustain. Dev. 2010, 30, 43–55. [Google Scholar] [CrossRef]
- Mehraj, U.; Abidi, I.; Ahmad, M.; Gul-Zaffar, Z.A.; Dar Rather, M.A.; Lone, A.A. Stability analysis for physiological traits, grain yield and its attributing parameters in oats (Avena sativa L.) in the Kashmir Valley. Electron. J. Plant Breed 2017, 8, 59–62. [Google Scholar] [CrossRef]
- Romani, A.; Pinelli, P.; Cantini, C.; Cimato, A.; Heimler, D. Characterization of Violetto di Toscana, a typical Italian variety of artichoke (Cynara scolymus L.). Food Chem. 2006, 95, 221–225. [Google Scholar] [CrossRef]
- Spanu, E.; Deligios, P.A.; Azara, E.; Delogu, G.; Ledda, L. Effect of alternative cropping systems on globe artichoke qualitative traits. J. Sci. Food Agric. 2017, 98, 1079–1087. [Google Scholar] [CrossRef] [PubMed]
- Available online: http://eur-lex.europa.eu/legal-content (accessed on 25 January 2017).
- FAO/WHO. Food Additives and Contaminants. In Joint Codex Alimentarius Commission, FAO/WHO Food Standards Program; ALINORM 01/12A; FAO/WHO: Rome, Italy, 2001. [Google Scholar]
- Ackah, M.; Kwablah Anim, A.; Tabuaa Gyamfi, E.; Zakaria, N.; Hanson, J.; Tulasi, D.; Enti-Brown, E.; Saah-Nyarko, S.; Owusu Bentil, N.; Osei, J. Uptake of heavy metals by some edible vegetables irrigated using wastewater: A preliminary study in Accra, Ghana. Environ. Monit. Assess. 2014, 186, 621–634. [Google Scholar] [CrossRef] [PubMed]
- Al-Lahham, O.; El Assi, N.M.; Fayyad, M. Translocation of heavy metals to tomato (Solanum lycopersicom L.) fruit irrigated with treated wastewater. Sci. Hortic. 2007, 113, 250–254. [Google Scholar] [CrossRef]
- Lombardo, S.; Pandino, G.; Mauromicale, G. The nutraceutical response of two globe artichoke cultivars to contrasting NPK fertilizer regimes. Food Res. Int. 2015, 76, 852–859. [Google Scholar] [CrossRef]
- Tomas-Barberan, F.A.; Espin, J.K. Phenolic compounds and related enzymes as determinants of quality in fruits and vegetables. J. Sci. Food Agric. 2001, 81, 853–876. [Google Scholar] [CrossRef]
- Ma, D.; Sun, D.; Li, Y.; Wang, C.; Xiea, Y.; Guo, T. Effect of nitrogen fertilization and irrigation on phenolic content, phenolic acid composition, and antioxidant activity of winter wheat grain. J. Sci. Food Agric. 2015, 95, 1039–1046. [Google Scholar] [CrossRef]
- Heimler, D.; Romani, A.; Ieri, F. Plant polyphenol content, soil fertilization and agricultural management: A review. Eur. Food Res. Technol. 2017, 243, 1107–1115. [Google Scholar] [CrossRef]
- Stefanelli, D.; Goodwin, I.; Jones, R. Minimal nitrogen and water use in horticulture: Effects on quality and content of selected nutrients. Food Res. Int. 2010, 43, 1833–1843. [Google Scholar] [CrossRef]
- Delgado, R.; Martín, P.; del Álamo, M.; González, M.R. Changes in the phenolic composition of grape berries during ripening in relation to vineyard nitrogen and potassium fertilisation rates. J. Sci. Food Agric. 2004, 84, 623–630. [Google Scholar] [CrossRef]
- Roberfroid, M.B. A European consensus of scientific concepts of functional foods. Nutrition 2000, 16, 689–691. [Google Scholar] [CrossRef]
- Brown, J.E.; Rice-Evans, C.A. Luteolin-rich artichoke extract protects low density lipoprotein from oxidation in vitro. Free Radic. Res. 1998, 29, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Speroni, E.; Cervellati, R.; Govoni, P.; Guizzardi, S.; Renzulli, C.; Guerra, M.C. Efficacy of different Cynara scolymus preparations on liver complaints. J. Ethnopharmacol. 2003, 86, 203–211. [Google Scholar] [CrossRef]
- Wang, M.; Simon, J.E.; Aviles, I.F.; He, K.; Zheng, Q.Y.; Tadmor, Y. Analysis of antioxidative phenolic compounds in artichoke (Cynara scolymus L.). J. Agric. Food Chem. 2003, 51, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Schutz, K.; Carle, R.; Schieber, A. Taraxacum—A review on its phytochemical and pharmacological profile. Taraxacum A Rev. Its Phytochem. Pharmacol. Profile 2006, 107, 313–323. [Google Scholar] [CrossRef] [PubMed]
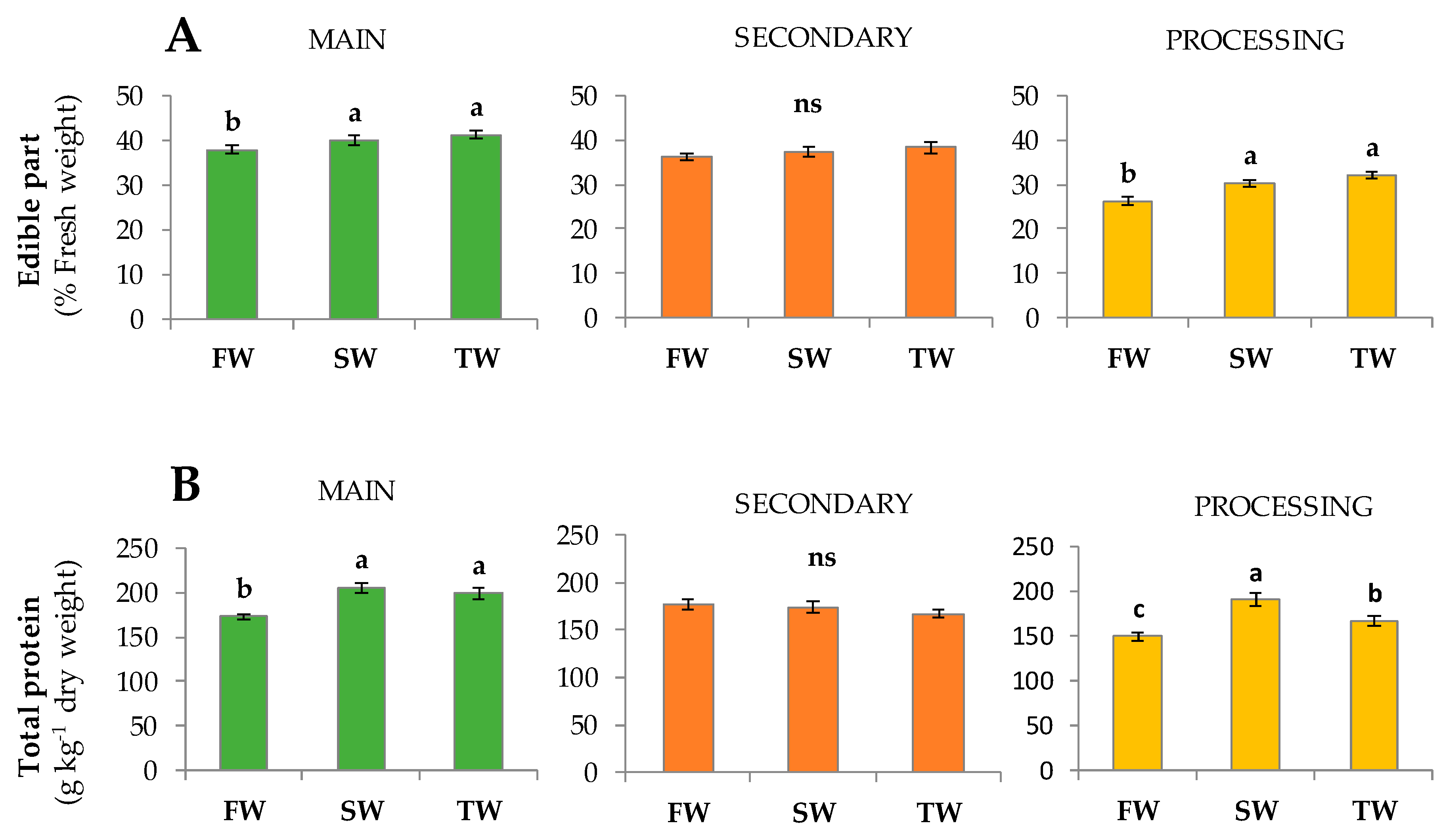

| Yield (Head Class) | Irrigation Treatment | Volume Applied (m3 ha−1) | Supplied with Irrigation Water (kg ha−1) | Supplied with Fertigation (kg ha−1) | Total Amount Supplied (kg ha−1) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Nitrogen | Phosphorus | Potassium | Nitrogen | Phosphorus | Potassium | Nitrogen | Phosphorus | Potassium | |||
| Early yield | FW | 1850 | 2.01 | 0.28 | 14.99 | 180 | 70 | 80 | 182.01 | 70.28 | 94.99 |
| (Main) | SW | 1850 | 55.78 | 10.27 | 48.75 | 180 | 70 | 80 | 235.78 | 80.27 | 128.75 |
| TW | 1850 | 38.94 | 11.29 | 48.84 | 180 | 70 | 80 | 218.94 | 81.29 | 128.84 | |
| Mid-yield | FW | 350 | 0.38 | 0.05 | 2.48 | − | − | − | 0.38 | 0.05 | 2.48 |
| (Secondary) | SW | 350 | 10.55 | 1.94 | 9.22 | 10.55 | 1.94 | 9.22 | |||
| TW | 350 | 7.37 | 2.14 | 9.24 | 7.37 | 2.14 | 9.24 | ||||
| Late yield | FW | 950 | 1.03 | 0.14 | 7.70 | − | − | − | 1.03 | 0.14 | 7.70 |
| (Processing) | SW | 950 | 28.64 | 5.27 | 25.03 | 28.64 | 5.27 | 25.03 | |||
| TW | 950 | 20.00 | 5.80 | 25.08 | 20.00 | 5.80 | 25.08 | ||||
| Total | FW | 3150 | 3.42 | 0.47 | 25.17 | 180 | 70 | 80 | 183.42 | 70.47 | 105.17 |
| SW | 3150 | 94.97 | 17.48 | 83.00 | 180 | 70 | 80 | 274.97 | 87.48 | 163.00 | |
| TW | 3150 | 66.31 | 19.22 | 83.16 | 180 | 70 | 80 | 246.31 | 89.23 | 163.16 | |
| IT † | Sodium (g kg−1 d.w.) | Potassium (g kg−1 d.w.) | ||||
| Main | Secondary | Processing | Main | Secondary | Processing | |
| FW | 2.0 ± 0.2 b | 1.6 ± 0.2 a | 2.4 ± 0.2 b | 13.8 ± 0.4 c | 15.4 ± 1.6 a | 14.5 ± 0.5 b |
| SW | 3.3 ± 0.2 a | 2.0 ± 0.1 a | 3.7 ± 0.2 a | 15.2 ± 0.3 b | 17.4 ± 0.6 a | 19.7 ± 1.1 a |
| TW | 3.8 ± 0.1 a | 1.6 ± 0.2 a | 4.0 ± 0.3 a | 18.3 ± 0.6 a | 18.6 ± 1.0 a | 19.1 ± 1.0 a |
| Magnesium (g kg−1 d.w.) | Calcium (g kg−1 d.w.) | |||||
| Main | Secondary | Processing | Main | Secondary | Processing | |
| FW | 1.4 ± 0.1 b | 2.1 ± 0.2 a | 2.4 ± 0.2 c | 2.7 ± 0.1 a | 2.4 ± 0.1 a | 2.4 ± 0.1 a |
| SW | 1.5 ± 0.1 ab | 2.4 ± 0.3 a | 2.6 ± 0.1 b | 2.7 ± 0.2 a | 2.5 ± 0.2 a | 2.4 ± 0.2 a |
| TW | 1.6 ± 0.1 a | 2.5 ± 0.2 a | 2.7 ± 0.2 a | 2.6 ± 0.2 a | 2.4 ± 0.2 a | 2.4 ± 0.2 a |
| IT † | Copper(mg kg−1 d.w.) | Iron (mg kg−1 d.w.) | ||||
| Main | Secondary | Processing | Main | Secondary | Processing | |
| FW | 9.6 ± 0.5 b | 9.6 ± 1.4 a | 8.2 ± 0.6 c | 49.3 ± 1.4 c | 55.0 ± 4.6 a | 45.2 ± 2.5 c |
| SW | 14.4 ± 1.3 a | 13.4 ± 0.6 a | 13.4 ± 1.3 a | 77.5 ± 4.3 a | 67.4 ± 3.4 a | 71.1 ± 3.3 a |
| TW | 13.4 ± 1.0 a | 12.0 ± 0.8 a | 11.3 ± 0.7 b | 65.8 ± 2.6 b | 59.8 ± 2.0 a | 60.8 ± 2.5 b |
| Nickel (mg kg−1 d.w.) | Zinc (mg kg−1 d.w.) | |||||
| Main | Secondary | Processing | Main | Secondary | Processing | |
| FW | 1.5 ± 0.2 b | 1.7 ± 0.1 a | 1.1 ± 0.1 b | 53.6 ± 2.0 c | 50.9 ± 5.9 a | 41.9 ± 4.0 c |
| SW | 2.3 ± 0.2 a | 2.0 ± 0.2 a | 2.1 ± 0.2 a | 99.5 ± 4.7 a | 67.6 ± 1.5 a | 64.7 ± 4.4 a |
| TW | 2.1 ± 0.1 ab | 1.9 ± 0.2 a | 1.4 ± 0.1 b | 77.7 ± 2.1 b | 60.0 ± 3.7 a | 53.4 ± 2.2 b |
| Chloride(g kg−1 d.w.) | Nitrate (g kg−1 d.w.) | |||||
| Main | Secondary | Processing | Main | Secondary | Processing | |
| FW | 5.2 ± 0.1 c | 6.3 ± 0.2 a | 5.8 ± 293.2 b | 0.32 ± 0.03 c | 0.38 ± 0.02 a | 0.36 ± 0.02 b |
| SW | 8.7 ± 0.4 a | 7.2 ± 0.3 a | 7.3 ± 239.0 a | 0.64 ± 0.05 a | 0.43 ± 0.03 a | 0.47 ± 0.03 a |
| TW | 6.8 ± 0.2 b | 7.1 ± 0.4 a | 7.5 ± 395.7 a | 0.46 ± 0.02 b | 0.43 ± 0.03 a | 0.38 ± 0.03 b |
| Phosphate (g kg−1 d.w.) | Sulphate (g kg−1 d.w.) | |||||
| Main | Secondary | Processing | Main | Secondary | Processing | |
| FW | 17.6 ± 1.4 b | 15.6 ± 1.0 a | 13.4 ± 0.05 a | 4.5 ± 0.3 a | 4.6 ± 0.2 a | 4.5 ± 0.3 a |
| SW | 24.4 ± 1.2 a | 17.2 ± 1.3 a | 13.8 ± 0.2 a | 4.9 ± 0.2 a | 4.9 ± 0.3 a | 4.9 ± 0.4 a |
| TW | 25.3 ± 1.9 a | 15.8 ± 0.8 a | 14.0 ± 0.2 a | 5.0 ± 0.4 a | 5.1 ± 0.3 a | 4.4 ± 0.3 a |
| 5-CQA | 1,3-diCQA | 1,5-diCQA | 3,4-diCQA | CafA | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Main | Secondary | Processing | Main | Secondary | Processing | Main | Secondary | Processing | Main | Secondary | Processing | Main | Secondary | Processing | |
| Growing season (GS)† | |||||||||||||||
| GS1 | 314.4 ± 12.8 a | 445.8 ± 12.0 a | 684.1 ± 12.0 a | 0.9 ± 0.1 a | 1.3 ± 0.4 a | 1.8 ± 0.4 a | 237.1 ± 10.8 a | 322.4 ± 10.8 a | 396.7 ± 10.8 a | 8.7 ± 0.8 a | 13.9 ± 0.8 a | 18.5 ± 0.8 a | 2.5 ± 0.1 b | 2.6 ± 0.3 a | 3.1 ± 0.2 a |
| GS2 | 276.3 ± 11.2 b | 364.1 ± 11.8 b | 594.9 ± 11.8 b | 0.7 ± 0.2 b | 1.1 ± 0.5 a | 1.6 ± 0.5 a | 195.0 ± 10.8 b | 265.7 ± 10.8 b | 338.5 ± 10.8 b | 7.2 ± 0.8 b | 10.7 ± 0.8 b | 15.0 ± 0.8 b | 2.7 ± 0.1 a | 2.2 ± 0.3 a | 2.5 ± 0.1 b |
| Irrigation treatment (IT)‡ | |||||||||||||||
| FW | 281.5 ± 10.5 b | 406.8 ± 11.4 a | 557.1 ± 11.4 b | 0.7 ± 0.1 b | 1.3 ± 0.6 a | 1.5 ± 0.6 b | 176.1 ± 10.8 c | 283.0 ± 10.8 a | 289.8 ± 10.8 b | 6.8 ± 0.8 b | 11.7 ± 0.8 a | 14.0 ± 0.8 b | 1.7 ± 0.3 b | 2.4 ± 0.2 a | 2.4 ± 0.1 c |
| SW | 319.2 ± 11.0 a | 402.8 ± 10.6 a | 713.2 ± 10.6 a | 1.1 ± 0.1 a | 1.2 ± 0.4 a | 2.0 ± 0.4 a | 261.1 ± 10.8 a | 293.0 ± 10.8 a | 412.8 ± 10.8 a | 10.0 ± 0.8 a | 13.4 ± 0.8 a | 18.2 ± 0.8 a | 2.3 ± 0.1 a | 2.5 ± 0.2 a | 3.2 ± 0.2 a |
| TW | 285.4 ± 11.3 b | 405.1 ± 10.8 a | 648.3 ± 10.8 a | 0.7 ± 0.1 b | 1.1 ± 0.5 a | 1.6 ± 0.5 b | 211.0 ± 10.8 b | 306.3 ± 10.8 a | 400.1 ± 10.8 a | 7.0 ± 0.8 b | 11.8 ± 0.8 a | 17.8 ± 0.8 a | 2.7 ± 0.2 a | 2.3 ± 0.3 a | 2.7 ± 0.1 b |
| Irrigation Treatment † | Api-glc | ||
|---|---|---|---|
| Main | Secondary | Processing | |
| FW | 26.1 ± 0.5 b | 34.2 ± 1.4 a | 34.0 ± 1.4 c |
| SW | 34.9 ± 1.0 a | 34.9 ± 0.6 a | 43.9 ± 0.6 a |
| TW | 31.5 ± 1.3 a | 32.6 ± 0.8 a | 37.9 ± 0.8 b |
| Lut-glc | |||
| Main | Secondary | Processing | |
| FW | 2.2 ± 0.5 b | 3.4 ± 0.4 a | 3.1 ± 0.2 b |
| SW | 3.1 ± 0.2 a | 3.1 ± 0.2 a | 3.5 ± 0.3 a |
| TW | 3.0 ± 0.3 a | 3.2 ± 0.3 a | 3.7 ± 0.2 a |
| Lut-glr | |||
| Main | Secondary | Processing | |
| FW | 6.5 ± 0.4 b | 10.7 ± 0.5 a | 12.7 ± 0.3 b |
| SW | 10.1 ± 0.2 a | 10.0 ± 0.4 a | 15.3 ± 0.4 a |
| TW | 8.3 ± 0.3 ab | 10.9 ± 0.3 a | 12.6 ± 0.5 b |
| TFA | |||
| Main | Secondary | Processing | |
| FW | 22.6 ± 1.4 ab | 25.5 ± 2.3 a | 25.5 ± 2.3 b |
| SW | 27.2 ± 1.2 a | 30.5 ± 4.4 a | 31.5 ± 2.4 a |
| TW | 21.6 ± 2.3 b | 27.4 ± 1.5 a | 26.1 ± 1.5 b |
| Parameter Selected | F Ratio Value | Prob F † | Parameter Selected | F Ratio Value | Prob F † |
|---|---|---|---|---|---|
| Irrigation treatment | Heads classes | ||||
| Chloride | 3.9 | 0.03 | Dry matter | 3.9 | 0.03 |
| Potassium | 10.0 | 0.0003 | Edible part | 44.7 | <0.0001 |
| Copper | 3.1 | 0.05 | Sodium | 35.2 | <0.0001 |
| Zinc | 10.1 | 0.00027 | Magnesium | 8.5 | <0.0008 |
| TFA | 19.8 | <0.0001 | 5-CQA | 5.8 | 0.005 |
| - | - | - | TFA | 5.6 | 0.007 |
| - | - | - | 1,5-diCQA | 3.2 | 0.05 |
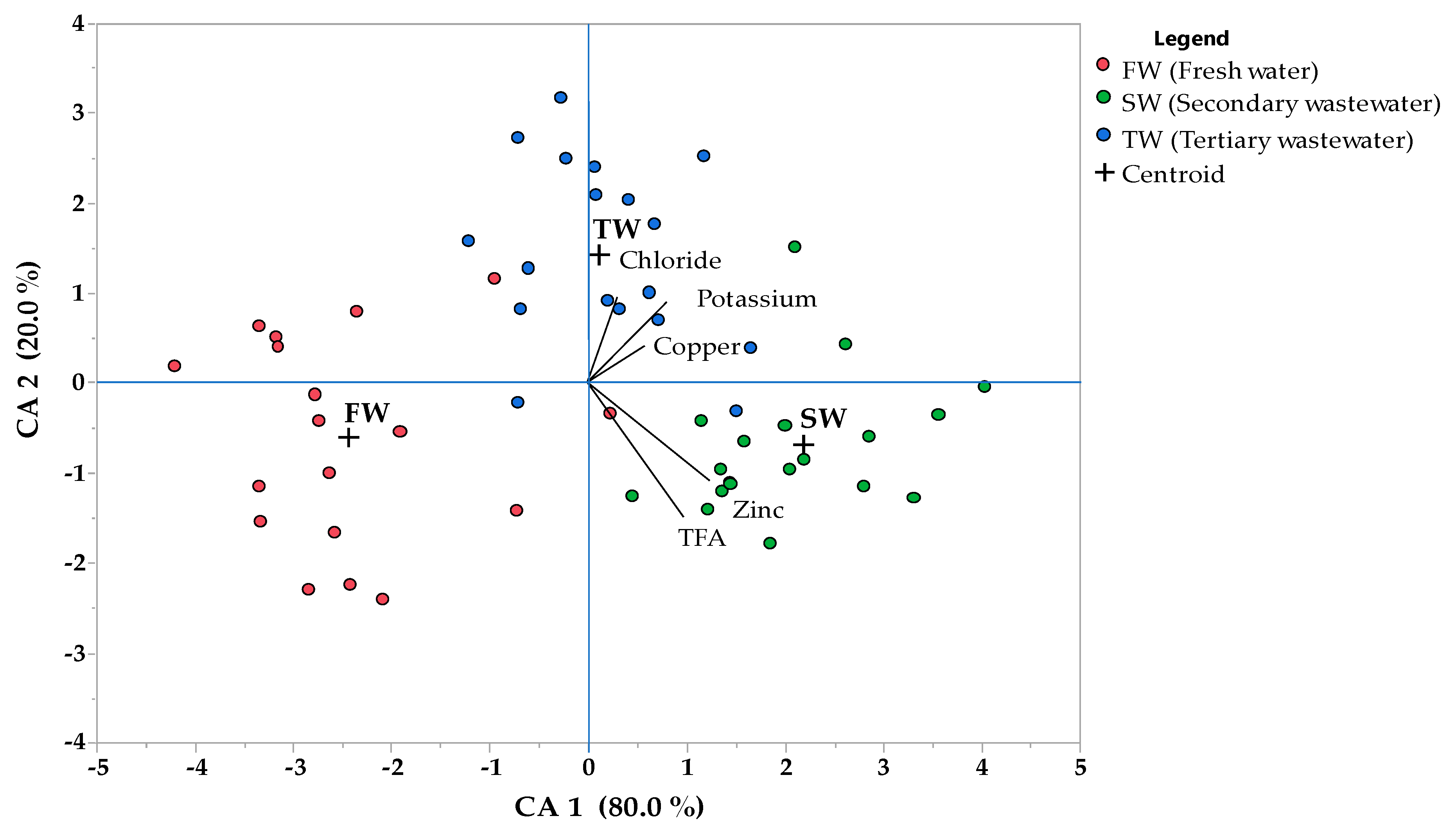
| Parameters | Standardized Coefficients | Pearson’s Correlation Coefficients | ||
|---|---|---|---|---|
| Irrigation treatment | CA1 | CA2 | CA1 | CA2 |
| Chloride | 0.198 | 0.635 | 0.18 ns | 0.80 *** |
| Potassium | 0.532 | 0.600 | 0.47 ** | 0.62 ** |
| Copper | 0.381 | 0.276 | 0.76 *** | 0.11 ns |
| Zinc | 0.827 | −0.721 | 0.75 *** | −0.62 ** |
| TFA | 0.647 | −0.996 | 0.53 ** | −0.49 ** |
| % variance explained | 80.0 | 20.0 | ||
| Head class | ||||
| Dry matter | 0.366 | 0.263 | 0.38 * | 0.23 ns |
| Edible part | −1.328 | 0.497 | −0.89 *** | 0.10 ns |
| Sodium | 0.396 | −1.119 | 0.42 ** | −0.76 *** |
| Magnesium | 0.197 | 0.619 | 0.29 ns | 0.45 ** |
| 5-CQA | 0.585 | 0.003 | 0.92 *** | 0.01 ns |
| TFA | 0.521 | 0.281 | 0.46 ** | 0.29 ns |
| 1,5-diCQA | 0.468 | 0.265 | 0.70 *** | 0.20 ns |
| % variance explained | 88.5 | 11.5 | ||
| Head Classes | NO3-N | PO4-P | SO4−2 | Cl− | K | Na | Ca | Mg | Fe | Cu | Zn | Ni |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Main | 0.85 *** | 0.54 ** | 0.18 ns | 0.72 *** | 0.59 ** | 0.80 *** | −0.33 ns | −0.02 ns | 0.6 *** | 0.85 *** | 0.82 *** | 0.83 *** |
| Secondary | 0.38 * | 0.12ns | 0.34 * | 0.51 ** | 0.43 * | 0.31 * | 0.18 ns | −0.12 ns | 0.43 * | 0.37 * | 0.59 ** | 0.19 ns |
| Processing | 0.55 ** | 0.11ns | −0.09 ns | 0.70 *** | 0.73 *** | 0.80 *** | −0.04 ns | 0.03 ns | 0.63 ** | 0.56 ** | 0.57 ** | 0.77 ** |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gagliardi, A.; Giuliani, M.M.; Carucci, F.; Francavilla, M.; Gatta, G. Effects of the Irrigation with Treated Wastewaters on the Proximate Composition, Mineral, and Polyphenolic Profile of the Globe Artichoke Heads [Cynara cardunculus (L.)]. Agronomy 2020, 10, 53. https://doi.org/10.3390/agronomy10010053
Gagliardi A, Giuliani MM, Carucci F, Francavilla M, Gatta G. Effects of the Irrigation with Treated Wastewaters on the Proximate Composition, Mineral, and Polyphenolic Profile of the Globe Artichoke Heads [Cynara cardunculus (L.)]. Agronomy. 2020; 10(1):53. https://doi.org/10.3390/agronomy10010053
Chicago/Turabian StyleGagliardi, Anna, Marcella Michela Giuliani, Federica Carucci, Matteo Francavilla, and Giuseppe Gatta. 2020. "Effects of the Irrigation with Treated Wastewaters on the Proximate Composition, Mineral, and Polyphenolic Profile of the Globe Artichoke Heads [Cynara cardunculus (L.)]" Agronomy 10, no. 1: 53. https://doi.org/10.3390/agronomy10010053
APA StyleGagliardi, A., Giuliani, M. M., Carucci, F., Francavilla, M., & Gatta, G. (2020). Effects of the Irrigation with Treated Wastewaters on the Proximate Composition, Mineral, and Polyphenolic Profile of the Globe Artichoke Heads [Cynara cardunculus (L.)]. Agronomy, 10(1), 53. https://doi.org/10.3390/agronomy10010053






