Variation of Phytoestrogen Content and Major Agronomic Traits in Alfalfa (Medicago sativa L.) Populations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Design
2.2. Agronomic Data Collection
2.3. Weather Conditions at the Experimental Site
2.4. Field Sampling and Sample Preparation for Biochemical Analysis
2.5. Extraction Procedure
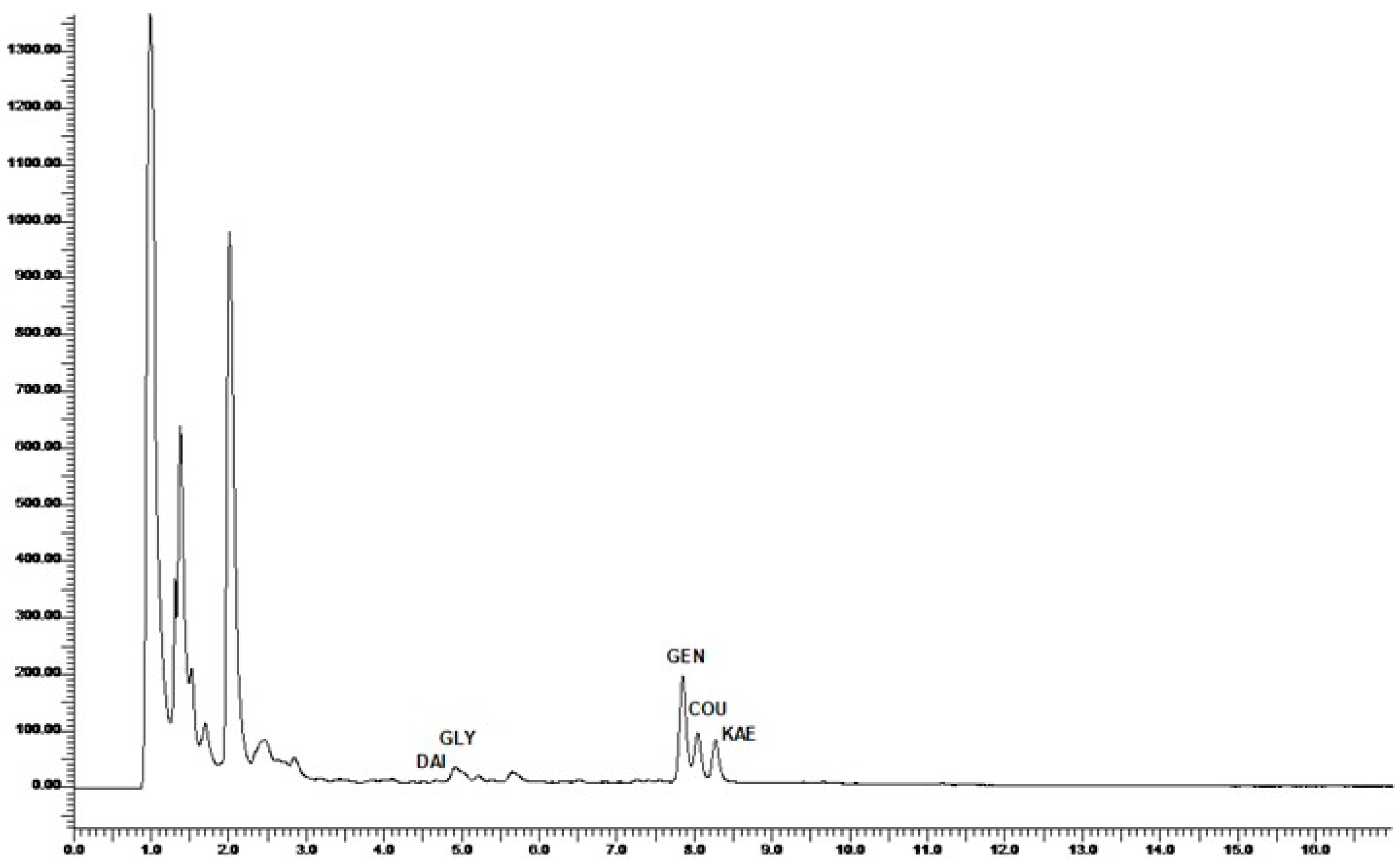
2.6. Chromatographic Identification and Quantification of Phytoestrogens
2.7. Statistical Analysis
3. Results and Discussion
3.1. Phytoestrogen Profiles
3.2. Variation of Phytoestrogens and Agronomic Traits
3.2.1. Agronomic Traits
3.2.2. Phytoestrogen Content
3.3. Cluster Analysis of Alfalfa Populations Based on Agronomic and Phytoestrogen Data
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Russelle, M.P. Environmental benefits of growing perennial legumes in cropping systems. Legume Perspect. 2014, 4, 11–12. [Google Scholar]
- Annicchiarico, P. Alfalfa forage yield and leaf/stem ratio: Narrow-sense heritability, genetic correlation, and parent selection procedures. Euphytica 2015, 205, 409–420. [Google Scholar] [CrossRef]
- Annicchiarico, P.; Barrett, B.; Brummer, E.C.; Julier, B.; Marshall, A.H. Achievements and challenges in improving temperate perennial forage legumes. Crit. Rev. Plant Sci. 2015, 34, 327–380. [Google Scholar] [CrossRef]
- Vasileva, V.; Kostov, O. Effect of mineral and organic fertilization on alfalfa forage and soil fertility. Emir. J. Food Agric. 2015, 27, 678–686. [Google Scholar] [CrossRef] [Green Version]
- Shi, S.; Nan, L.; Smith, K.F. The Current Status, Problems, and Prospects of Alfalfa (Medicago sativa L.) Breeding in China. Agronomy 2017, 7, 1. [Google Scholar] [CrossRef] [Green Version]
- Křížová, L.; Dadáková, K.; Kašparovská, J.; Kašparovský, T. Isoflavones. Molecules 2019, 24, 1076. [Google Scholar] [CrossRef] [Green Version]
- Lipovac, M.; Chedraui, P.; Gruenhut, C.; Gocan, A.; Kurz, C.; Neuber, B.; Imhof, M. The effect of red clover isoflavone supplementation over vasomotor and menopausal symptoms in postmenopausal women. Gynecol. Endocrinol. 2012, 28, 203–207. [Google Scholar] [CrossRef]
- Sirotkin, V.A.; Harrath, A.H. Phytoestrogens and their effects. Eur. J. Pharmacol. 2014, 741, 230–236. [Google Scholar] [CrossRef]
- Gatouillat, G.; Magid, A.A.; Bertin, E.; Okiemy-Akeli, M.G.; Morjani, H.; Lavaud, C.; Madoulet, C. Cytotoxicity and Apoptosis Induced by Alfalfa (Medicago sativa) Leaf Extracts in Sensitive and Multidrug-Resistant Tumor Cells. Nutr. Cancer 2014, 66, 483–491. [Google Scholar] [CrossRef]
- Nabatchian, F.; Aghoosi, S.M.H.; Mordadi, A.; Khodaverdi, F. Evaluation of the effect of alfalfa extract on breast cancer. J. Appl. Environ. Biol. Sci. 2015, 4, 288–294. [Google Scholar]
- Shakeri, F.; Taavoni, S.; Goushegir, A.; Haghani, H. Effectiveness of red clover in alleviating menopausal symptoms: A 12-week randomized, controlled trial. Climacteric 2015, 18, 568–573. [Google Scholar] [CrossRef] [PubMed]
- Abdi, F.; Alimoradi, A.; Hagi, P.; Mahdizad, F. Effects of phytoestrogens on bone mineral density during the menopause transition: A systematic review of randomized controlled trials. Climacteric 2016, 19, 535–545. [Google Scholar] [CrossRef]
- Chen, M.N.; Lin, C.C.; Liu, C.F. Efficacy of phytoestrogens for menopausal symptoms: A meta-analysis and systematic review. Climacteric 2016, 18, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Gopal, N.M.; Tejaswini, J.; Mantry, S.; Kumar, A. International standards of medicinal plants. Int. J. Innov. Pharm. Sci. Res. 2014, 2, 2498–2532. [Google Scholar]
- Aparicio-Fernandez, X.; Yousef, G.G.; Loarca-Pina, G.; de Mejia, E.; Lila, M.A. Characterization of polyphenolics in the seed coat of black jamapa bean (Phaseolus vulgaris L.). J. Agric. Food Chem. 2005, 53, 4615–4622. [Google Scholar] [CrossRef] [PubMed]
- Saviranta, N.M.M.; Anttonen, M.J.; von Wright, A.; Karjalainen, R.O. Red clover (Trifolium pratense L.) isoflavones: Determination of concentrations by plant stage, flower colour, plant part and cultivar. J. Sci. Food Agric. 2008, 88, 125–132. [Google Scholar] [CrossRef]
- Butkute, B.; Lemeziene, N.; Dabkeviviene, G.; Jakstas, V.; Vilcinskas, E.; Janulis, V. Source of variation of isoflavone concentrations in perennial clover species. Pharmacogn. Mag. 2014, 10, 181–188. [Google Scholar] [CrossRef] [Green Version]
- Moorby, J.M.; Fraser, M.D.; Theobald, V.J.; Wood, J.D.; Haresign, W. The effect of red clover formononetin concentration on live-weight gain, carcass characteristics and muscle equol concentration of finishing lambs. Anim. Sci. 2004, 79, 303–313. [Google Scholar] [CrossRef]
- Rodrigues, F.; Almeida, I.; Sarmento, B.; Amaral, M.H.; Oliveira, P.P.M.B. Study of the isoflavone content of different extracts of Medicago spp.as potential active ingredient. Ind. Crops Prod. 2014, 57, 110–115. [Google Scholar] [CrossRef]
- Mustonen, E.A.; Tuori, M.; Saastamoinen, I.; Taponen, J.; Wahala, K.; Saloniemi, H.; Vanhatalo, A. Equol in milk of dairy cows is derived from forage legumes such as red clover. Br. J. Nutr. 2009, 102, 1552–1556. [Google Scholar] [CrossRef] [Green Version]
- Kalač, P. Fresh and ensiled forages as a source of estrogenic equol in bovine milk: A review. Czech J. Anim. Sci. 2013, 58, 296–303. [Google Scholar] [CrossRef] [Green Version]
- Adler, S.A.; Purup, S.; Hansen-Moller, J.; Thuen, E.; Steinshamn, H. Phytoestrogens and Their Metabolites in Bulk-Tank Milk: Effects of Farm Management and Season. PLoS ONE 2015, 10, e0127187. [Google Scholar] [CrossRef] [Green Version]
- Mustonen, E. Red Clover Isoflavonoids in Feed, Plasma and Milk of Ruminants. Ph.D. Thesis, The Faculty of Veterinary Medicine, University of Helsinki, Helsinki, Finland, 2015. [Google Scholar]
- Daems, F.; Romnee, J.M.; Heuskin, S.; Froidmont, E.; Lognay, G. Analytical methods used to quantify isoflavones in cow’s milk: A review. Dairy Sci. Technol. 2016, 96, 261–283. [Google Scholar] [CrossRef] [PubMed]
- Kasparovska, J.; Pecinkova, M.; Dadakova, K.; Krizova, L.; Hadrova, S.; Lexa, M.; Lochman, J.; Kasparovsky, T. Effects of Isoflavone-Enriched Feed on the Rumen Microbiota in Dairy Cows. PLoS ONE 2016, 11, e0154642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sivesind, E.; Seguin, P. Effects of the environment, cultivar, maturity, and preservation method on red clover isoflavone concentration. J. Agric. Food Chem. 2005, 53, 6397–6402. [Google Scholar] [CrossRef] [PubMed]
- Moravcova, J.; Kleinova, T.; Loučka, R. The determination of coumestrol in alfalfa (Medicago sativa) by capillary electrophoresis. Rostlinná Vyroba 2002, 48, 224–229. [Google Scholar] [CrossRef] [Green Version]
- Visnevschi-Necrasov, T.; Faria, M.A.; Cunha, S.C.; Harris, J.; Meimberg, H.W.E.; Curto, M.A.C.; Pereira, M.G.; Oliveira, M.B.P.P.; Nunes, E. Isoflavone synthase (IFS) gene phylogeny in Trifolium species associated with plant isoflavone contents. Plant Syst. Evol. 2013, 299, 357–367. [Google Scholar] [CrossRef]
- Reed, K.F.M. Fertility of Herbivores Consuming Phytoestrogen-Containing Medicago and Trifolium Species. Agriculture 2016, 6, 35. [Google Scholar] [CrossRef] [Green Version]
- Hloucalova, P.; Skladanka, J.; Horky, J.; Klejdus, B.; Pelikan, J.; Knotova, K. Determination of phytoestrogen content in fresh-cut legume forage. Animals 2016, 6, 43. [Google Scholar] [CrossRef] [Green Version]
- Ramos, G.P.; Dias, P.M.B.; Morais, C.B.; Fröehlich, P.E.; Dall´Agnol, M.; Zuanazzi, J.A.S. LC determination of four isoflavone aglycones in red clover (Trifolium pratense L.). Chromatographia 2008, 67, 125–129. [Google Scholar] [CrossRef]
- IRRI. Statistical Tool for Agricultural Research (STAR) Version: 2.0.1; International Rice Research Institute: Los Baños, Philippines, 2013. [Google Scholar]
- Seguin, P.; Zheng, W.; Souleimanov, A. Alfalfa phytoestrogen content: Impact of plant maturity and herbage components. J. Agron. Crop Sci. 2004, 190, 211–217. [Google Scholar] [CrossRef]
- Fields, R.L.; Sedcole, J.R.; Barrell, G.K.; Moot, D.J. Prediction of coumestrol content in unirrigated lucerne crops using weather variables. N. Z. J. Agric. Res. 2019, 62, 528–542. [Google Scholar] [CrossRef]
- Seguin, P.; Zheng, W. Phytoestrogen content of alfalfa cultivars grown in eastern Canada. J. Sci. Food Agric. 2006, 86, 765–771. [Google Scholar] [CrossRef]
- Tucak, M.; Horvat, D.; Čupić, T.; Krizmanić, G.; Tomaš, V.; Ravlić, M.; Popović, S. Forage Legumes as Sources of Bioactive Phytoestrogens for Use in Pharmaceutics: A Review. Curr. Pharm. Biotechnol. 2018, 19, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.M.; Castilho, M.C.; Silveira, I.; Abreu, J.M. Liquid chromatographic validation of a quantitation method for phytoestrogens, biochanin-A, coumestrol, daidzein, formononetin, and genistein, in lucerne. J. Liq. Chromatogr. Relat. Technol. 2006, 29, 2875–2884. [Google Scholar] [CrossRef]
- Butkute, B.; Padarauskas, A.; Ceseviciene, J.; Pavilonis, A.; Taujenis, L.; Lemeziene, N. Perennial legumes as a source of ingredients for healthy food: Proximate, mineral and phytoestrogen composition and antibacterial activity. J. Food Sci. Technol. 2017, 54, 2661–2669. [Google Scholar] [CrossRef] [PubMed]
- Fields, R.L.; Barrell, G.K.; Gash, A.; Zhao, J.; Moot, D.J. Alfalfa Coumestrol Content in Response to Development Stage, Fungi, Aphids, and Cultivar. Agron. J. 2018, 110, 910–921. [Google Scholar] [CrossRef] [Green Version]
- Ramos, G.P.; Dias, P.M.B.; Morais, C.B.; Dall’Agnol, M.; Zuanazzi, J.A.S. Genetic variability of isoflavones in the USDA red clover core collection. Rev. Bras. Farmacogn. 2012, 22, 1241–1252. [Google Scholar] [CrossRef] [Green Version]
- Little, V.; Reed, K.F.M.; Smith, K.F. Variation for concentrations of various phytoestrogens and agronomic traits among a broad range of red clover (Trifolium pratense) cultivars and accessions. Agronomy 2017, 7, 34. [Google Scholar] [CrossRef]
- Tucak, M.; Popović, S.; Horvat, D.; Čupić, T.; Krizmanić, G.; Viljevac Vuletić, M.; Ravlić, M. The characterization of isoflavone content in the Croatian red clover collection. Poljoprivreda 2019, 25, 3–11. [Google Scholar] [CrossRef]
- Abidi, S.; Jabri, C.; Souissi, A.; Ferchichi, M.; Zoghlami-Khélil, A. Comparative analysis of antioxidant and secondary metabolites contents in eight populations of Medicago ciliaris L. J. Anim. Plant Sci. 2019, 41, 7042–7054. [Google Scholar] [CrossRef]
- Egan, L.M.; Hofmann, R.W.; Ghamkhar, K.; Hoyos-Villegas, V. Identification of Founding Accessions and Patterns of Relatedness and Inbreeding Derived from Historical Pedigree Data in a Red Clover Germplasm Collection in New Zealand. Crop Sci. 2019, 59, 2100–2108. [Google Scholar] [CrossRef] [Green Version]


| Month | Temperature (°C) | Precipitation (mm) | ||||
|---|---|---|---|---|---|---|
| 2014 | 2015 | LTA | 2014 | 2015 | LTA | |
| March | 9.5 | 7.5 | 6.4 | 39.4 | 50.5 | 40.5 |
| April | 13.2 | 12.1 | 11.2 | 81.3 | 12.9 | 51.0 |
| May | 16.1 | 17.8 | 16.7 | 161.4 | 113.4 | 59.2 |
| June | 20.5 | 20.8 | 19.9 | 91.0 | 17.1 | 82.0 |
| July | 21.8 | 24.6 | 21.3 | 66.4 | 25.6 | 66.3 |
| August | 20.8 | 23.7 | 20.8 | 54.3 | 105.8 | 61.9 |
| September | 17.0 | 17.9 | 16.5 | 68.9 | 41.1 | 51.0 |
| October | 13.3 | 11.1 | 11.1 | 87.9 | 142.1 | 55.9 |
| Mean/Total | 16.5 | 16.9 | 15.4 | 650.6 | 508.5 | 467.8 |
| P | GMY | DMC | PH | DAI | GLY | GEN | COU | KAE | TOT CON |
|---|---|---|---|---|---|---|---|---|---|
| Af1 | 67.96 | 20.27 | 64.24 | 53.20 | 218.51 | 600.24 | 445.90 | 531.62 | 1849.47 |
| Af2 | 63.61 | 20.06 | 62.64 | 57.26 | 296.91 | 617.61 | 538.56 | 598.58 | 2108.92 |
| Af3 | 64.58 | 20.64 | 60.74 | 64.27 | 390.37 | 770.58 | 543.98 | 621.14 | 2390.33 |
| Af4 | 62.90 | 20.30 | 62.55 | 77.50 | 296.78 | 790.56 | 631.28 | 681.16 | 2477.27 |
| Af5 | 64.85 | 19.56 | 62.47 | 79.37 | 377.96 | 748.27 | 579.90 | 644.71 | 2430.21 |
| Af6 | 62.13 | 20.21 | 64.23 | 43.61 | 252.61 | 836.50 | 614.61 | 560.86 | 2308.19 |
| Af7 | 63.20 | 19.92 | 61.37 | 54.46 | 264.26 | 737.49 | 625.42 | 760.30 | 2441.92 |
| Af8 | 65.82 | 19.56 | 64.45 | 60.84 | 399.93 | 797.20 | 672.21 | 706.92 | 2637.10 |
| Af9 | 65.56 | 19.26 | 67.89 | 67.98 | 315.59 | 668.26 | 547.02 | 560.26 | 2159.11 |
| Af10 | 66.52 | 19.78 | 64.68 | 61.42 | 352.01 | 715.39 | 576.51 | 558.84 | 2264.17 |
| Af11 | 65.39 | 19.53 | 65.03 | 65.60 | 343.11 | 709.09 | 584.57 | 619.46 | 2321.84 |
| Af12 | 69.45 | 19.07 | 62.35 | 77.68 | 330.90 | 594.30 | 509.12 | 574.01 | 2086.01 |
| Af13 | 66.24 | 19.97 | 63.87 | 74.47 | 428.00 | 627.97 | 577.38 | 576.15 | 2283.97 |
| Af14 | 68.88 | 18.87 | 65.23 | 72.21 | 325.36 | 773.01 | 595.47 | 612.87 | 2378.91 |
| Af15 | 62.62 | 19.79 | 63.95 | 67.15 | 315.58 | 727.64 | 511.83 | 523.46 | 2145.66 |
| Af16 | 64.46 | 19.59 | 62.78 | 78.44 | 299.74 | 573.42 | 566.54 | 709.56 | 2227.70 |
| Af17 | 65.24 | 19.38 | 62.90 | 72.96 | 304.93 | 540.56 | 552.50 | 617.56 | 2088.51 |
| Af18 | 71.93 | 19.69 | 65.48 | 71.28 | 434.38 | 678.19 | 498.52 | 554.14 | 2236.51 |
| Af19 | 70.68 | 19.38 | 64.59 | 83.27 | 440.99 | 663.49 | 686.53 | 602.55 | 2476.83 |
| Af20 | 71.66 | 19.80 | 67.33 | 66.51 | 352.59 | 602.83 | 503.72 | 575.04 | 2100.69 |
| Average | 66.18 | 19.73 | 63.94 | 67.47 | 337.00 | 688.63 | 568.08 | 609.46 | 2270.67 |
| MS y | 92,553.95 ** | 45.99 ** | 5771.2 ** | 381.75 ns | 46,501.26 * | 29,414.02 ** | 0.68ns | 9412.83 ns | 254,552.92 ns |
| MS p | 71.22 ** | 1.54 ** | 25.82 ** | 415.64 ** | 14,895.40 ** | 28,553.05 ** | 14,385.83 ** | 16,115.02 ** | 133,759.50 ** |
| MS y × p | 14.15 * | 0.92 ** | 15.82 ** | 713.00 ** | 19,698.71 ** | 7971.62 ** | 6529.52 ** | 7285.57 ** | 50,879.93 ** |
| LSD 0.05 p | 4.01 | 0.11 | 2.85 | 2.74 | 15.42 | 9.64 | 12.04 | 21.13 | 30.37 |
| LSD 0.01 p | 5.31 | 0.14 | 3.82 | 3.67 | 20.66 | 12.92 | 16.13 | 28.30 | 40.68 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tucak, M.; Čupić, T.; Horvat, D.; Popović, S.; Krizmanić, G.; Ravlić, M. Variation of Phytoestrogen Content and Major Agronomic Traits in Alfalfa (Medicago sativa L.) Populations. Agronomy 2020, 10, 87. https://doi.org/10.3390/agronomy10010087
Tucak M, Čupić T, Horvat D, Popović S, Krizmanić G, Ravlić M. Variation of Phytoestrogen Content and Major Agronomic Traits in Alfalfa (Medicago sativa L.) Populations. Agronomy. 2020; 10(1):87. https://doi.org/10.3390/agronomy10010087
Chicago/Turabian StyleTucak, Marijana, Tihomir Čupić, Daniela Horvat, Svetislav Popović, Goran Krizmanić, and Marija Ravlić. 2020. "Variation of Phytoestrogen Content and Major Agronomic Traits in Alfalfa (Medicago sativa L.) Populations" Agronomy 10, no. 1: 87. https://doi.org/10.3390/agronomy10010087
APA StyleTucak, M., Čupić, T., Horvat, D., Popović, S., Krizmanić, G., & Ravlić, M. (2020). Variation of Phytoestrogen Content and Major Agronomic Traits in Alfalfa (Medicago sativa L.) Populations. Agronomy, 10(1), 87. https://doi.org/10.3390/agronomy10010087





