Effect of High Tunnel Coverings on Antioxidants of Breaker and Light Red Tomatoes at Harvest and during Ripening
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Design
UV-A and UV-B Measurement
2.2. Tomato Sampling for Nutritional Analysis
2.3. Standards, Reagents, and Equipment
2.4. Extraction and Analysis of AsA
2.5. Extraction and Analysis of Lycopene and β–Carotene
2.6. Extraction for Antioxidant Capacity and Phenolic Compounds
2.7. Analysis of Antioxidant Capacity
2.8. Analysis of Phenolic Compounds
2.9. Statistical Analysis
3. Results
3.1. Antioxidant Capacity (FRAP)
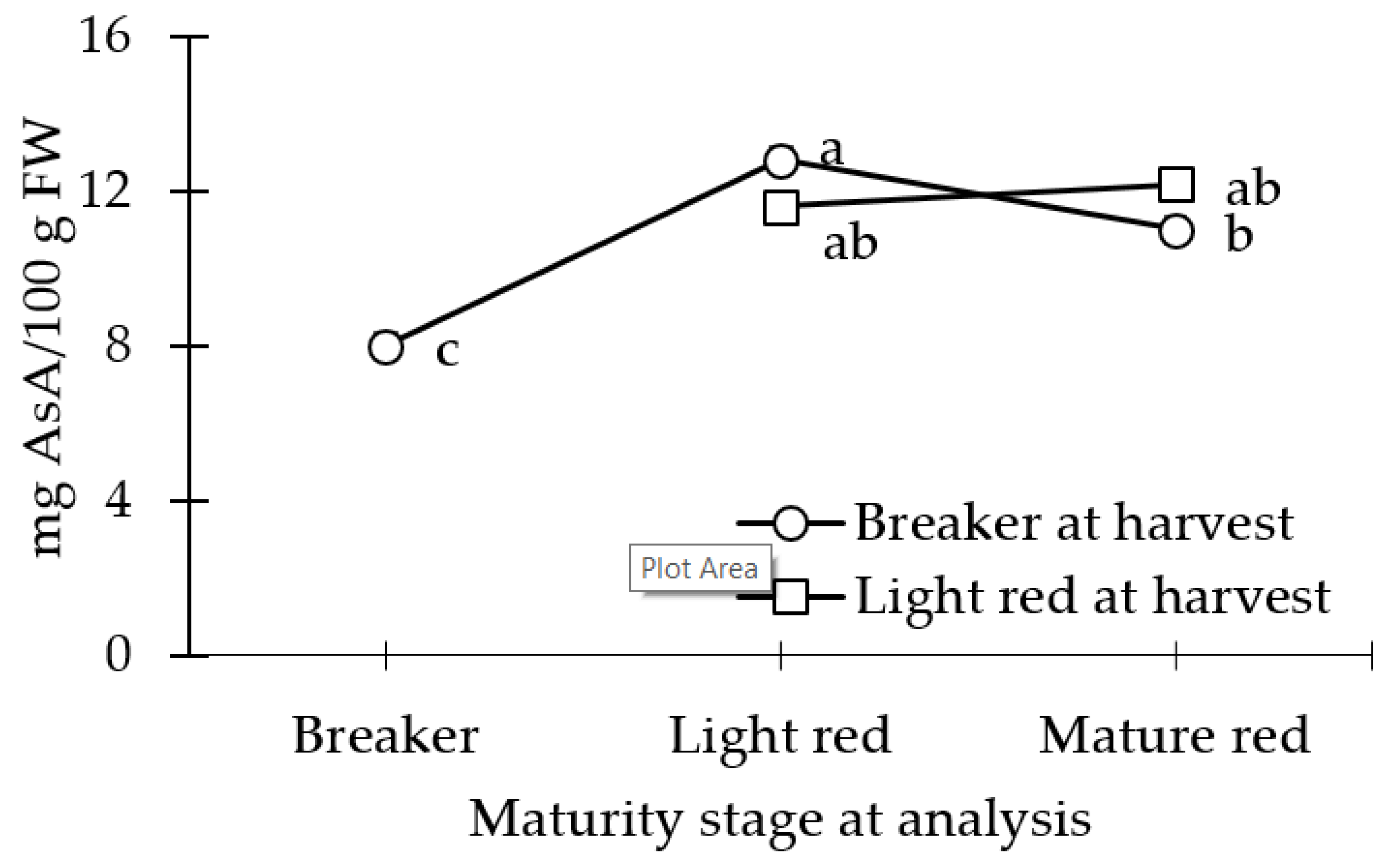
3.2. AsA Concentration
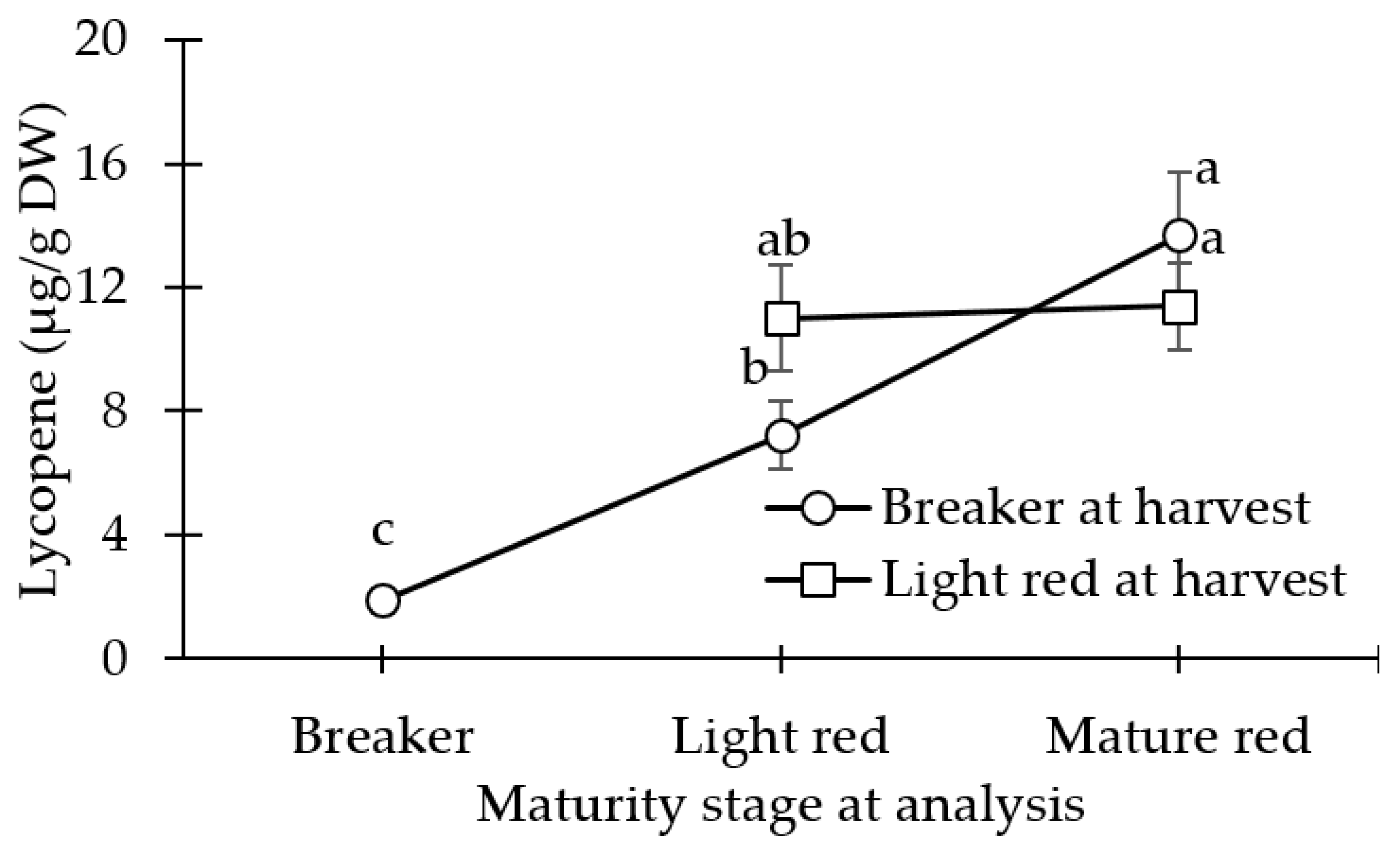
3.3. Carotenoid (Lycopene and β-carotene) Concentration
3.4. Phenolic Compound Concentration
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Carey, E.E.; Jett, L.; Lamont, W.J.; Nennich, T.T.; Orzolek, M.D.; Williams, K.A. Horticultural Crop Production in High Tunnels in the United States: A Snapshot. HortTechnology 2009, 19, 37–43. [Google Scholar] [CrossRef]
- Ilić, Z.S.; Milenković, L.; Šunic, L.; Fallik, E. Effect of coloured shade-nets on plant leaf parameters and tomato fruit quality. J. Sci. Food Agric. 2014, 95, 2660–2667. [Google Scholar] [CrossRef] [PubMed]
- Cowan, J.S.; Miles, C.A.; Andrews, P.K.; Inglis, D.A. Biodegradable mulch performed comparably to polyethylene in high tunnel tomato (Solanum lycopersicum L.) production. J. Sci. Food Agric. 2014, 94, 1854–1864. [Google Scholar] [CrossRef] [PubMed]
- Flaishman, M.A.; Peles, Y.; Dahan, Y.; Milo-Cochavi, S.; Frieman, A.; Naor, A. Differential response of cell-cycle and cell-expansion regulators to heat stress in apple (Malus domestica) fruitlets. Plant Sci. 2015, 233, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Wilkens, R.T.; Spoerke, J.M.; Stamp, N.E. Differential Responses of Growth and Two Soluble Phenolics of Tomato to Resource Availability. Ecology 1996, 77, 247–258. [Google Scholar] [CrossRef]
- Stamp, N. Out of The Quagmire of Plant Defense Hypotheses. Q. Rev. Biol. 2003, 78, 23–55. [Google Scholar] [CrossRef]
- Stewart, A.J.; Bozonnet, S.; Mullen, W.; Jenkins, G.I.; Lean, M.E.J.; Crozier, A. Occurrence of Flavonols in Tomatoes and Tomato-Based Products. J. Agric. Food Chem. 2000, 48, 2663–2669. [Google Scholar] [CrossRef]
- George, B.; Kaur, C.; Khurdiya, D.; Kapoor, H. Antioxidants in tomato (Lycopersium esculentum) as a function of genotype. Food Chem. 2004, 84, 45–51. [Google Scholar] [CrossRef]
- Gautier, H.; Diakou-Verdin, V.; Bénard, C.; Reich, M.; Buret, M.; Bourgaud, F.; Poëssel, J.L.; Caris-Veyrat, C.; Génard, M. How Does Tomato Quality (Sugar, Acid, and Nutritional Quality) Vary with Ripening Stage, Temperature, and Irradiance? J. Agric. Food Chem. 2008, 56, 1241–1250. [Google Scholar] [CrossRef]
- Oh, M.-M.; Carey, E.E.; Rajashekar, C.B. Antioxidant phytochemicals in lettuce grown in high tunnels and open field. Hortic. Environ. Biotechnol. 2011, 52, 133–139. [Google Scholar] [CrossRef]
- Romani, A.; Pinelli, P.; Galardi, C.; Sani, G.; Cimato, A.; Heimler, D. Polyphenols in greenhouse and open-air-grown lettuce. Food Chem. 2002, 79, 337–342. [Google Scholar] [CrossRef]
- Selahle, K.M.; Sivakumar, D.; Jifon, J.; Soundy, P. Postharvest responses of red and yellow sweet peppers grown under photo-selective nets. Food Chem. 2015, 173, 951–956. [Google Scholar] [CrossRef] [PubMed]
- Tsormpatsidis, E.; Henbest, R.; Davis, F.J.; Battey, N.; Hadley, P.; Wagstaffe, A. UV irradiance as a major influence on growth, development and secondary products of commercial importance in Lollo Rosso lettuce ‘Revolution’ grown under polyethylene films. Environ. Exp. Bot. 2008, 63, 232–239. [Google Scholar] [CrossRef]
- Zhao, X.; Carey, E.E.; Young, J.E.; Wang, W.; Iwamoto, T. Influences of Organic Fertilization, High Tunnel Environment, and Postharvest Storage on Phenolic Compounds in Lettuce. HortScience 2007, 42, 71–76. [Google Scholar] [CrossRef]
- Agati, G.; Tattini, M. Multiple functional roles of flavonoids in photoprotection. New Phytol. 2010, 186, 786–793. [Google Scholar] [CrossRef]
- Selahle, M.K.; Sivakumar, D.; Soundy, P. Effect of photo-selective nettings on post-harvest quality and bioactive compounds in selected tomato cultivars. J. Sci. Food Agric. 2014, 94, 2187–2195. [Google Scholar] [CrossRef] [PubMed]
- Petropoulos, S.; Fernandes, Â.; Katsoulas, N.; Barros, L.; Ferreira, I.C. The effect of covering material on the yield, quality and chemical composition of greenhouse grown tomato fruit. J. Sci. Food Agric. 2018, 99, 3057–3068. [Google Scholar] [CrossRef]
- Ahmad, P.; Jaleel, C.A.; Salem, M.A.; Nabi, G.; Sharma, S. Roles of enzymatic and nonenzymatic antioxidants in plants during abiotic stress. Crit. Rev. Biotechnol. 2010, 30, 161–175. [Google Scholar] [CrossRef]
- Antonious, G.F.; Bomford, M.; Vincelli, P. Screening Brassica species for glucosinolate content. J. Environ. Sci. Health Part B 2009, 44, 311–316. [Google Scholar] [CrossRef]
- Luthria, D.L.; Mukhopadhyay, S.; Krizek, D.T. Content of total phenolics and phenolic acids in tomato (Lycopersicon esculentum Mill.) fruits as influenced by cultivar and solar UV radiation. J. Food Compos. Anal. 2006, 19, 771–777. [Google Scholar] [CrossRef]
- Oh, M.-M.; Rajashekar, C.B. Antioxidant content of edible sprouts: Effects of environmental shocks. J. Sci. Food Agric. 2009, 89, 2221–2227. [Google Scholar] [CrossRef]
- Shalata, A.; Mittova, V.; Volokita, M.; Guy, M.; Tal, M. Response of the cultivated tomato and its wild salt-tolerant relative Lycopersiconpennellii to salt-dependent oxidative stress: The root antioxidative system. Physiol. Plant. 2001, 112, 487–494. [Google Scholar] [CrossRef] [PubMed]
- El-Gizawy, A.; Abdallah, M.; Gomaa, H.; Mohamed, S. Effect of different shading levels on tomato plants. 2. yield and fruit quality. Acta Hortic. 1993, 323, 349–354. [Google Scholar] [CrossRef]
- Giuntini, D.; Graziani, G.; Lercari, B.; Fogliano, V.; Soldatini, G.F.; Ranieri, A. Changes in Carotenoid and Ascorbic Acid Contents in Fruits of Different Tomato Genotypes Related to the Depletion of UV-B Radiation. J. Agric. Food Chem. 2005, 53, 3174–3181. [Google Scholar] [CrossRef] [PubMed]
- García-Valverde, V.; Navarro-González, I.; García-Alonso, J.; Periago, M.J. Antioxidant Bioactive Compounds in Selected Industrial Processing and Fresh Consumption Tomato Cultivars. Food Bioprocess Technol. 2011, 6, 391–402. [Google Scholar] [CrossRef]
- Gautier, H.; Rocci, A.; Buret, M.; Grasselly, D.; Dumas, Y.; Causse, M. Effect of photoselective filters on the physical and chemical traits of vine-ripened tomato fruits. Can. J. Plant Sci. 2005, 85, 439–446. [Google Scholar] [CrossRef]
- Krizek, D.T.; Kramer, G.F.; Upadhyaya, A.; Mirecki, R.M. UV-B response of cucumber seedlings grown under metal halide and high pressure sodium/deluxe lamps. Physiol. Plant. 1993, 88, 350–358. [Google Scholar] [CrossRef]
- Kubasek, W.L.; Shirley, B.W.; McKillop, A.; Goodman, H.M.; Briggs, W.; Ausubel, F.M. Regulation of flavonoid biosynthetic genes in germinating Arabidopsis seedlings. Plant Cell 1992, 4, 1229–1236. [Google Scholar] [CrossRef]
- Jones, D. Phenylalanine ammonia-lyase: Regulation of its induction, and its role in plant development. Phytochemistry 1984, 23, 1349–1359. [Google Scholar] [CrossRef]
- Yaginuma, S.; Shiraishi, T.; Ohya, H.; Igarashi, K. Polyphenol Increases in Safflower and Cucumber Seedlings Exposed to Strong Visible Light with Limited Water. Biosci. Biotechnol. Biochem. 2002, 66, 65–72. [Google Scholar] [CrossRef]
- Winkel-Shirley, B. Flavonoid Biosynthesis. A Colorful Model for Genetics, Biochemistry, Cell Biology, and Biotechnology. Plant Physiol. 2001, 126, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Fuglevand, G.; Jackson, J.A.; Jenkins, G.I. UV-B, UV-A, and blue light signal transduction pathways interact synergistically to regulate chalcone synthase gene expression in Arabidopsis. Plant Cell 1996, 8, 2347–2357. [Google Scholar] [PubMed]
- Li, T.; Bi, G.; Lecompte, J.; Barickman, T.C.; Evans, B.B. Effect of Colored Shadecloth on the Quality and Yield of Lettuce and Snapdragon. HortTechnology 2017, 27, 860–867. [Google Scholar] [CrossRef]
- Sytar, O.; Zivcak, M.; Brückova, K.; Brestic, M.; Hemmerich, I.; Rauh, C.; Simko, I. Shift in accumulation of flavonoids and phenolic acids in lettuce attributable to changes in ultraviolet radiation and temperature. Sci. Hortic. 2018, 239, 193–204. [Google Scholar] [CrossRef]
- Woolley, A.; Sumpter, S.; Lee, M.; Xu, J.; Barry, S.; Wang, W.; Rajashekar, C.B. Accumulation of Mineral Nutrients and Phytochemicals in Lettuce and Tomato Grown in High Tunnel and Open Field. Am. J. Plant Sci. 2019, 10, 125–138. [Google Scholar] [CrossRef]
- Tomás-Barberán, A.F.; Espín, J.C. Phenolic compounds and related enzymes as determinants of quality in fruits and vegetables. J. Sci. Food Agric. 2001, 81, 853–876. [Google Scholar] [CrossRef]
- Krizek, D.T.; Mirecki, R.M.; Britz, S.J. Inhibitory effects of ambient levels of solar UV-A and UV-B radiation on growth of cucumber. Physiol. Plant. 1997, 100, 886–893. [Google Scholar] [CrossRef]
- Papaioannou, C.; Katsoulas, N.; Maletsika, P.; Siomos, A.; Kittas, C. Effects of a UV-absorbing greenhouse covering film on tomato yield and quality. Span. J. Agric. Res. 2012, 10, 959–966. [Google Scholar] [CrossRef]
- Dannehl, D.; Huber, C.; Rocksch, T.; Huyskens-Keil, S.; Schmidt, U. Interactions between changing climate conditions in a semi-closed greenhouse and plant development, fruit yield, and health-promoting plant compounds of tomatoes. Sci. Hortic. 2012, 138, 235–243. [Google Scholar] [CrossRef]
- Zhao, X.; Iwamoto, T.; Carey, E.E. Antioxidant capacity of leafy vegetables as affected by high tunnel environment, fertilisation and growth stage. J. Sci. Food Agric. 2007, 87, 2692–2699. [Google Scholar] [CrossRef]
- Becker, C.; Kläring, H.-P.; Kroh, L.W.; Krumbein, A. Temporary reduction of radiation does not permanently reduce flavonoid glycosides and phenolic acids in red lettuce. Plant Physiol. Biochem. 2013, 72, 154–160. [Google Scholar] [CrossRef]
- Hipol, R.L.B.; Dionisio-Sese, M.L. Impact of light variation on the antioxidant properties of red lettuce. Electron. J. Biol. 2014, 10, 28–34. [Google Scholar]
- Gierson, D.; Kader, A.A. Fruit Ripening and Quality. In The Tomato Crop; Atherton, J.G., Rudich, J., Eds.; Chapman and Hall: London, UK, 1986; pp. 241–280. [Google Scholar] [CrossRef]
- Dannehl, D.; Huyskens-Keil, S.; Eichholz, I.; Ulrichs, C.; Schmidt, U. Effects of direct-electric-current on secondary plant compounds and antioxidant activity in harvested tomato fruits (Solanum lycopersicon L.). Food Chem. 2011, 126, 157–165. [Google Scholar] [CrossRef]
- Poiroux-Gonord, F.; Bidel, L.P.R.; Fanciullino, A.-L.; Gautier, H.; Lauri-Lopez, F.; Urban, L. Health Benefits of Vitamins and Secondary Metabolites of Fruits and Vegetables and Prospects To Increase Their Concentrations by Agronomic Approaches. J. Agric. Food Chem. 2010, 58, 12065–12082. [Google Scholar] [CrossRef] [PubMed]
- Betancourt, L.A.; Stevens, M.A.; Kader, A.A. Accumulation and loss of sugars and reduced ascorbic. J. Am. Soc. Hort. Sci. 1977, 102, 721–723. [Google Scholar]
- Vinha, A.F.; Barreira, S.V.P.; Castro, A.; Costa, A.S.G.; Oliveira, M.B.P.P. Influence of the Storage Conditions on the Physicochemical Properties, Antioxidant Activity and Microbial Flora of Different Tomato (Lycopersicon esculentum L.) Cultivars. J. Agric. Sci. 2013, 5, 118. [Google Scholar] [CrossRef]
- Tyapkina, D.Y.; Kochieva, E.; Slugina, M.A. Vitamin C in fleshy fruits: Biosynthesis, recycling, genes, and enzymes. Vavilov J. Genet. Breed. 2019, 23, 270–280. [Google Scholar] [CrossRef]
- Pietta, P.-G. Flavonoids as Antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef]
- Reimers, K.J.; Keast, D.R. Tomato Consumption in the United States and Its Relationship to the US Department of Agriculture Food Pattern. Nutr. Today 2016, 51, 198–205. [Google Scholar] [CrossRef]
- Institute of Medicine (US) Panel on Dietary Antioxidants and Related Compounds. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids; The National Academies Press: Washington, DC, USA, 2000; pp. 95–185. [Google Scholar]
- Andrés, F.L.C.; Perla, A.G. Comparison of color indexes for tomato ripening Comparação dos índices de cor para maturação do tomate. Hortic. Bras. 2004, 22, 534–537. [Google Scholar]
- U.S. Department of Agriculture. United States Standards for Grades of Fresh Tomatoes; Fresh Products Branch: Washington, DC, USA, 1997. [Google Scholar]
- Verheul, M.J.; Slimestad, R.; Tjøstheim, I.H. From Producer to Consumer: Greenhouse Tomato Quality As Affected by Variety, Maturity Stage at Harvest, Transport Conditions, and Supermarket Storage. J. Agric. Food Chem. 2015, 63, 5026–5034. [Google Scholar] [CrossRef]
- Nunes, N.; Emond, J.P.; Rauth, M.; Dea, S.; Chau, K.V. Environmental conditions encountered during typical consumer retail display affect fruit and vegetable quality and waste. Postharvest Biol. Technol. 2009, 51, 232–241. [Google Scholar] [CrossRef]
- Alarcón-Flores, M.I.; Romero-González, R.; Vidal, J.L.M.; Frenich, A.G. Multiclass determination of phytochemicals in vegetables and fruits by ultra high performance liquid chromatography coupled to tandem mass spectrometry. Food Chem. 2013, 141, 1120–1129. [Google Scholar] [CrossRef] [PubMed]
- Klimczak, I.; Gliszczyńska-Świgło, A. Comparison of UPLC and HPLC methods for determination of vitamin C. Food Chem. 2015, 175, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Nagata, M.; Yamashita, I. Simple Method for Simultaneous Determination of Chlorophyll and Carotenoids in Tomato Fruit. Nippon. Shokuhin Kogyo Gakkaishi 1992, 39, 925–928. [Google Scholar] [CrossRef]
- Vallverdú-Queralt, A.; Oms-Oliu, G.; Odriozola-Serrano, I.; Lamuela-Raventós, R.M.; Martín-Belloso, O.; Elez-Martínez, P. Metabolite profiling of phenolic and carotenoid contents in tomatoes after moderate-intensity pulsed electric field treatments. Food Chem. 2013, 136, 199–205. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Vallverdú-Queralt, A.; Oliu, G.O.; Odriozola-Serrano, I.; Lamuela-Raventós, R.M.; Martín-Belloso, O.; Elez-Martínez, P. Effects of Pulsed Electric Fields on the Bioactive Compound Content and Antioxidant Capacity of Tomato Fruit. J. Agric. Food Chem. 2012, 60, 3126–3134. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-H.; Kim, H.-R.; Kim, H.-J.; Lee, I.-S.; Kozukue, N.; Levin, C.E.; Friedman, M. Free Amino Acid and Phenolic Contents and Antioxidative and Cancer Cell-Inhibiting Activities of Extracts of 11 Greenhouse-Grown Tomato Varieties and 13 Tomato-Based Foods. J. Agric. Food Chem. 2011, 59, 12801–12814. [Google Scholar] [CrossRef]
- Gude, K. Altering Light with High Tunnel Coverings to Improve Health-Promoting Phytochemicals of Lettuce and Tomato. Ph.D. Thesis, Kansas State University, Manhattan, KS, USA, 2020. Available online: https://krex.k-state.edu/dspace/handle/2097/40741 (accessed on 16 October 2020).
- Lee, S.K.; Kader, A.A. Preharvest and postharvest factors influencing vitamin C content of horticultural crops. Postharvest Biol. Technol. 2000, 20, 207–220. [Google Scholar] [CrossRef]
- Boo, Y.C.; Jung, J. Water Deficit—Induced Oxidative Stress and Antioxidative Defenses in Rice Plants. J. Plant Physiol. 1999, 155, 255–261. [Google Scholar] [CrossRef]
- Yamamoto, H.Y.; Bassi, R. Carotenoids: Localization and Function. In Toxic Plant Proteins; Springer Science and Business Media LLC.: Berlin/Heidelberg, Germany, 2005; Volume 4, pp. 539–563. [Google Scholar]
- Loomis, W. Growth-differentiation balance vs. carbohydrate-nitrogen ratio. Proc. Am. Soc. Hortic. Sci. 1932, 29, 240–245. [Google Scholar]
- Loomis, W.E. Growth Correlation. In Growth and Differentiation in Plant; Loomis, W.E., Ed.; The Iowa State College Press: Ames, IA, USA, 1953; pp. 197–252. [Google Scholar]
- Becker, C.; Urlić, B.; Špika, M.J.; Kläring, H.-P.; Krumbein, A.; Baldermann, S.; Ban, S.G.; Perica, S.; Schwarz, D. Nitrogen Limited Red and Green Leaf Lettuce Accumulate Flavonoid Glycosides, Caffeic Acid Derivatives, and Sucrose while Losing Chlorophylls, Β-Carotene and Xanthophylls. PLoS ONE 2015, 10, e0142867. [Google Scholar] [CrossRef]
- Tilahun, S.; Park, D.S.; Solomon, T.; Choi, H.R.; Jeong, C.S. Maturity stages affect nutritional quality and storability of tomato cultivars. CyTA J. Food 2019, 17, 87–95. [Google Scholar] [CrossRef]
- Tinyane, P.P.; Sivakumar, D.; Soundy, P. Influence of photo-selective netting on fruit quality parameters and bioactive compounds in selected tomato cultivars. Sci. Hortic. 2013, 161, 340–349. [Google Scholar] [CrossRef]
- Alarcón-Flores, M.I.; Romero-González, R.; Vidal, J.L.M.; Frenich, A.G. Multiclass Determination of Phenolic Compounds in Different Varieties of Tomato and Lettuce by Ultra High Performance Liquid Chromatography Coupled to Tandem Mass Spectrometry. Int. J. Food Prop. 2015, 19, 494–507. [Google Scholar] [CrossRef]
- Vallverdú-Queralt, A.; Medina-Remón, A.; Martínez-Huélamo, M.; Jáuregui, O.; Andrés-Lacueva, C.; Lamuela-Raventós, R.M. Phenolic Profile and Hydrophilic Antioxidant Capacity as Chemotaxonomic Markers of Tomato Varieties. J. Agric. Food Chem. 2011, 59, 3994–4001. [Google Scholar] [CrossRef] [PubMed]
- Minoggio, M.; Bramati, L.; Simonetti, P.; Gardana, C.; Iemoli, L.; Santangelo, E.; Mauri, P.; Spigno, P.; Soressi, G.; Pietta, P. Polyphenol Pattern and Antioxidant Activity of Different Tomato Lines and Cultivars. Ann. Nutr. Metab. 2003, 47, 64–69. [Google Scholar] [CrossRef]
- Slimestad, R.; Verheul, M. Review of flavonoids and other phenolics from fruits of different tomato (Lycopersicon esculentum Mill.) cultivars. J. Sci. Food Agric. 2009, 89, 1255–1270. [Google Scholar] [CrossRef]
- Hallmann, E. The influence of organic and conventional cultivation systems on the nutritional value and content of bioactive compounds in selected tomato types. J. Sci. Food Agric. 2012, 92, 2840–2848. [Google Scholar] [CrossRef]
- Crozier, A.; Lean, M.E.; McDonald, M.S.; Black, C. Quantitative Analysis of the Flavonoid Content of Commercial Tomatoes, Onions, Lettuce, and Celery. J. Agric. Food Chem. 1997, 45, 590–595. [Google Scholar] [CrossRef]
- Takahashi, A.; Takeda, K.; Ohnishi, T. Light-Induced Anthocyanin Reduces the Extent of Damage to DNA in UV-Irradiated Centaurea cyanus Cells in Culture. Plant Cell Physiol. 1991, 32, 541–547. [Google Scholar] [CrossRef]
- Cheng, J.-C.; Dai, F.; Zhou, B.; Yang, L.; Liu, Z.-L. Antioxidant activity of hydroxycinnamic acid derivatives in human low density lipoprotein: Mechanism and structure–activity relationship. Food Chem. 2007, 104, 132–139. [Google Scholar] [CrossRef]
- Liu, W.; Ren, M.-L.; Liu, T.; Du, Y.-L.; Zhou, T.; Liu, X.-M.; Liu, J.; Hussain, S.; Yang, W. Effect of shade stress on lignin biosynthesis in soybean stems. J. Integr. Agric. 2018, 17, 1594–1604. [Google Scholar] [CrossRef]
- Slimestad, R.; Verheul, M.J. Content of Chalconaringenin and Chlorogenic Acid in Cherry Tomatoes Is Strongly Reduced during Postharvest Ripening. J. Agric. Food Chem. 2005, 53, 7251–7256. [Google Scholar] [CrossRef]
- Islam, S.; Matsui, T.; Yoshida, Y. Effect of carbon dioxide enrichment on physico-chemical and enzymatic changes in tomato fruits at various stages of maturity. Sci. Hortic. 1996, 65, 137–149. [Google Scholar] [CrossRef]
- Tsaniklidis, G.; Delis, C.; Nikoloudakis, N.; Katinakis, P.; Aivalakis, G. Low temperature storage affects the ascorbic acid metabolism of cherry tomato fruits. Plant Physiol. Biochem. 2014, 84, 149–157. [Google Scholar] [CrossRef]
- Fenech, M.; Amaya, I.; Valpuesta, V.; Botella, J.R. Vitamin C Content in Fruits: Biosynthesis and Regulation. Front. Plant Sci. 2019, 9, 2006. [Google Scholar] [CrossRef]
- Bramley, P.M. Regulation of carotenoid formation during tomato fruit ripening and development. J. Exp. Bot. 2002, 53, 2107–2113. [Google Scholar] [CrossRef] [PubMed]
- Kader, A. Maturity, ripening, and quality relationships of fruit-vegetables. Acta Hortic. 1996, 249–256. [Google Scholar] [CrossRef]
- Buta, J.G.; Spaulding, D.W. Endogenous Levels of Phenolics in Tomato Fruit during Growth and Maturation. J. Plant Growth Regul. 1997, 16, 43–46. [Google Scholar] [CrossRef]




| Maturity | L* | a* | b* | Chroma | Hue° |
|---|---|---|---|---|---|
| Breaker | 61.10 (±1.5) | −4.79 (±1.5) | 27.00 (±1.5) | 27.92 (±1.5) | 100.11 (±1.5) |
| Light Red | 47.78 (±1.5) | 22.34 (±1.5) | 32.68 (±1.5) | 39.75 (±1.5) | 55.87 (±1.5) |
| Mature Red | 44.84 (±1.5) | 27.94 (±1.5) | 31.17 (±1.5) | 40.79 (±1.5) | 48.98 (±1.5) |
| Maturity Stage at Harvest | Maturity Stage at Analysis | Code | Redness at Analysis (*a) |
|---|---|---|---|
| Breaker | Breaker | BR | −4.79 (± 1.5) |
| Light Red | BR_LR | 22.34 (± 1.5) | |
| Mature Red | BR_MR | 27.94 (± 1.5) | |
| Light Red | Light Red | LR | 22.34 (± 1.5) |
| Mature Red | LR_MR | 27.94 (± 1.5) |
| Covering | UV-A (w/m2) | UV-B (w/m2) |
|---|---|---|
| Movable | 28.8 | 1.9 |
| Standard | 4.7 | 0.3 |
| Block | 6.8 | 0.1 |
| Clear | 17.5 | 1.2 |
| Diffuse | 2.2 | 0.1 |
| Shade | 2.0 | 0.1 |
| Antioxidant | Covering 2 | Maturity Stage 3 | Maturity Stage × Covering |
|---|---|---|---|
| FRAP (µmol TE/100 g DW) | <0.05 | <0.001 | ns |
| Ascorbic Acid (mg AsA/100 g FW) | <0.001 | <0.001 | ns |
| Lycopene (μg/g DW) | ns | <0.001 | ns |
| β-carotene (μg/g DW) | ns | ns | ns |
| Isoquercetin (mg/kg DW) | ns | ns | ns |
| Rutin (mg/kg DW) | ns | <0.001 | ns |
| Quercetin (mg/kg DW) | ns | <0.001 | ns |
| Chlorogenic acid(mg/kg DW) | <0.01 | <0.001 | <0.01 |
| Ferulic acid (mg/kg DW) | <0.01 | <0.01 | <0.05 |
| Coverings 1 | BR 2 | BR_LR | BR_MR | LR | LR_MR | POC (BR_MR and LR_MR) |
|---|---|---|---|---|---|---|
| Standard | 52 (5.4) | 37 (5.8) | 14 (2.8) | 42 (6.8) | 20 (2.3) | ns 3 |
| Movable | 45 (4.9) | 29 (4.2) | 7 (1.4) | 52 (7.7) | 18 (2) | ** |
| Diffuse | 41 (4.2) | 23 (3.3) | 17 (3.2) | 64 (9.5) | 23 (2.5) | ns |
| Clear | 67 (6.9) | 32 (4.7) | 21 (4.1) | 58 (7.7) | 25 (2.8) | ns |
| Block | 56 (5.8) | 29 (4.7) | 18 (3.4) | 62 (8.2) | 32 (3.5) | * |
| Shade | 60 (6.8) | 41 (6.3) | 15 (3.2) | 46 (6) | 27 (3.2) | * |
| Mean | 54 | 32 | 15 | 54 | 24 | |
| p-value | <0.05 | ns | <0.01 | ns | <0.01 |
| Coverings 1 | BR 2 | BR_LR | BR_MR | LR | LR_MR | POC (BR_MR and LR_MR) |
|---|---|---|---|---|---|---|
| Standard | 3.2 (0.7) | 2.3 (0.7) | 3.6 (2.8) | 2.7 (0.7) | 1.4 (0.4) | * 3 |
| Movable | 2.2 (0.5) | 2.7 (0.8) | 1.7 (1.4) | 1 (0.3) | 1.1 (0.3) | ns |
| Diffuse | 1.9 (0.4) | 2.4 (0.7) | 2.1 (3.2) | 1.8 (0.4) | 1.4 (0.4) | ns |
| Clear | 1.1 (0.2) | 2.6 (0.8) | 3.5 (4.1) | 2.6 (0.6) | 3.3 (0.9) | ns |
| Block | 1.4 (0.3) | 2.1 (0.7) | 3.6 (0.9) | 2.9 (0.6) | 2.9 (0.8) | ns |
| Shade | 1.6 (0.4) | 5 (1.5) | 6.3 (1.8) | 2.2 (0.5) | 1.6 (0.5) | ** |
| p-value | <0.05 | ns | <0.05 | <0.05 | <0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gude, K.M.; Rajashekar, C.B.; Cunningham, B.; Kang, Q.; Wang, W.; Lee, M.; Rivard, C.L.; Pliakoni, E.D. Effect of High Tunnel Coverings on Antioxidants of Breaker and Light Red Tomatoes at Harvest and during Ripening. Agronomy 2020, 10, 1639. https://doi.org/10.3390/agronomy10111639
Gude KM, Rajashekar CB, Cunningham B, Kang Q, Wang W, Lee M, Rivard CL, Pliakoni ED. Effect of High Tunnel Coverings on Antioxidants of Breaker and Light Red Tomatoes at Harvest and during Ripening. Agronomy. 2020; 10(11):1639. https://doi.org/10.3390/agronomy10111639
Chicago/Turabian StyleGude, Kelly M., Channa B. Rajashekar, Brianna Cunningham, Qing Kang, Weiqun Wang, Myungjin Lee, Cary L. Rivard, and Eleni D. Pliakoni. 2020. "Effect of High Tunnel Coverings on Antioxidants of Breaker and Light Red Tomatoes at Harvest and during Ripening" Agronomy 10, no. 11: 1639. https://doi.org/10.3390/agronomy10111639
APA StyleGude, K. M., Rajashekar, C. B., Cunningham, B., Kang, Q., Wang, W., Lee, M., Rivard, C. L., & Pliakoni, E. D. (2020). Effect of High Tunnel Coverings on Antioxidants of Breaker and Light Red Tomatoes at Harvest and during Ripening. Agronomy, 10(11), 1639. https://doi.org/10.3390/agronomy10111639








