Long-Term Fertilization Affects Soil Microbiota, Improves Yield and Benefits Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design, Soil Sampling and Crop Yield
2.2. Soil Physicochemical Properties
2.3. Soil Microbial Biomass and Enzyme Activities
2.4. Soil DNA Extraction and Sequencing
2.5. Statistical Analyses
3. Results
3.1. Crop Yield
3.2. Soil Properties
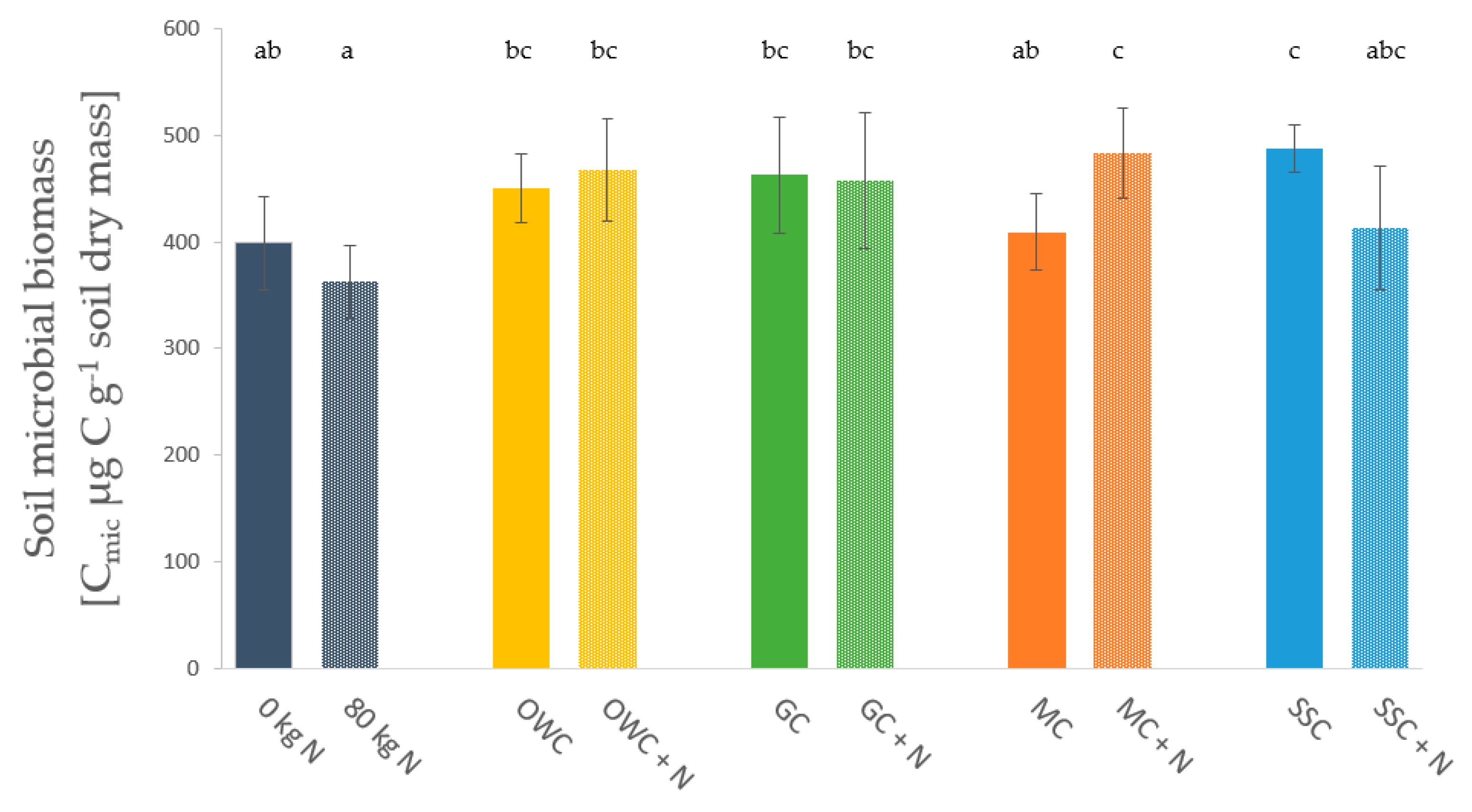
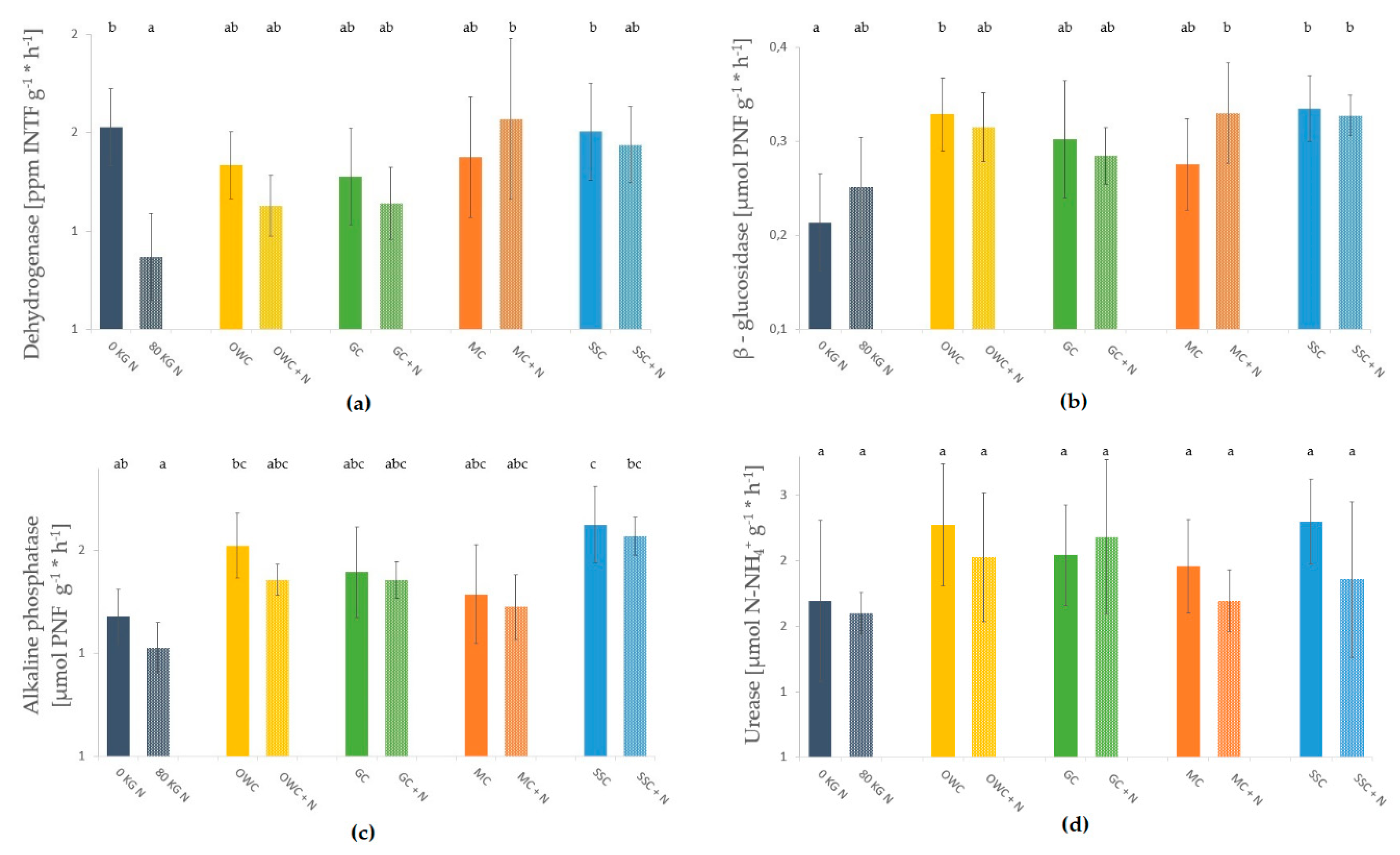
3.3. Soil Microbial Biomass and Enzyme Activities
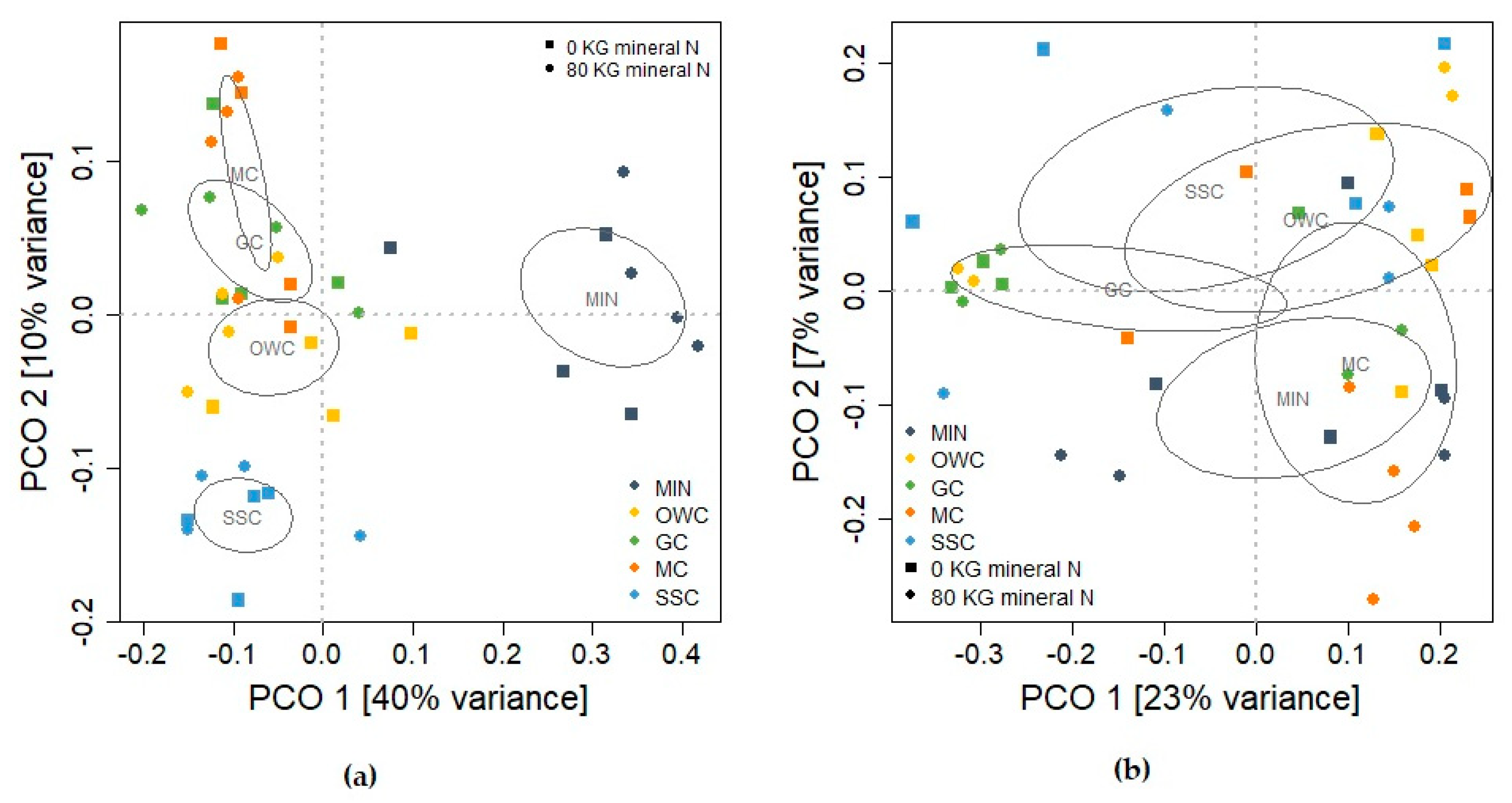
3.4. Soil Microbial Community
4. Discussion
4.1. Crop Yield and Physicochemical Soil Properties
4.2. Soil Microbial Biomass and Enzyme Activities
4.3. Soil Microbial Community
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Spiegel, H.; Baumgarten, A.; Dersch, G.; Pfundtner, E.; Sandén, T. Impact of mineral P fertilization on trace elements in cropland soils. In Sustainable Agriculture Reviews 29; Lal, R., Francaviglia, R., Eds.; Springer: Cham, Switzerland, 2019; pp. 93–110. [Google Scholar]
- Lehtinen, T.; Dersch, G.; Söllinger, J.; Baumgarten, A.; Schlatter, N.; Aichberger, K.; Spiegel, H. Long-term amendment of four different composttypes on a loamy silt Cambisol: Impact on soil organic matter, nutrients and yields. Arch. Agron. Soil Sci. 2017, 63, 663–673. [Google Scholar] [CrossRef]
- Amundson, R.; Berhe, A.A.; Hopmans, J.W.; Olson, C.; Sztein, A.E.; Sparks, D.L. Soil and human security in the 21st century. Science 2015, 348, 1261071. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Blanco, J.; Lazcano, C.; Christensen, T.H.; Muñoz, P.; Rieradevall, J.; Møller, J.; Antón, A.; Boldrin, A. Compost benefits for agriculture evaluated by life cycle assessment. Review. Agron. Sustain. Dev. 2013, 33, 721–732. [Google Scholar] [CrossRef]
- Aguilera, J.; Motavalli, P.; Gonzales, M.A.; Valdivia, C. Initial and residual effects of organic and inorganic amendments on soil properties in a potato-based cropping system in the Bolivian Andean Highlands. Am. J. Exp. Agric. 2012, 2, 641–666. [Google Scholar]
- Shafi, M.; Bakht, J.; Jan, M.; Shah, Z. Soil C and N dynamics and maize (Zea may L.) yield as affected by cropping systems and residue management in North-western Pakistan. Soil Tillage Res. 2007, 94, 520–529. [Google Scholar] [CrossRef]
- Tiemann, L.K.; Grandy, A.S.; Atkinson, E.E.; Marin-Spiotta, E.; McDaniel, M.D. Crop rotational diversity enhances belowground communities and functions in an agroecosystem. Ecol. Lett. 2015, 18, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Soman, C.; Li, D.; Wander, M.M.; Kent, A.D. Long-term fertilizer and crop-rotation treatments differentially affect soil bacterial community structure. Plant Soil 2017, 413, 145–159. [Google Scholar] [CrossRef]
- Rebollido, R.; Martínez, J.; Aguilera, Y.; Melchor, K.; Koerner, I.; Stegmann, R. Microbial populations during composting process of organic fraction of municipal solid waste. Appl. Ecol. Environ. Res. 2008, 6, 61–67. [Google Scholar] [CrossRef]
- Ryckeboer, J.; Mergaert, J.; Coosemans, J.; Deprins, K.; Swings, J. Microbiological aspects of biowaste during composting in a monitored compost bin. J. Appl. Microbiol. 2002, 94, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Mao, H.; Li, X. Functional characteristics and influence factors of microbial community in sewage sludge composting with inorganic bulking agent. Bioresour. Technol. 2018, 249, 527–535. [Google Scholar] [CrossRef]
- Meada, K.; Hanajima, D.; Toyoda, S.; Yoshida, N.; Morioka, R.; Osada, T. Microbiology of nitrogen cycle in animal manure compost. Microb. Biotechnol. 2011, 4, 700–709. [Google Scholar] [CrossRef]
- Chelius, M.K.; Triplett, E.W. The Diversity of archaea and bacteria in association with the roots of Zea Mays L. Microb. Ecol. 2001, 41, 252–263. [Google Scholar] [CrossRef] [PubMed]
- Dangi, S.R.; Bañuelos, G.; Buyer, J.S.; Hanson, B.; Gerik, J. Microbial community biomass and structure in saline and non-saline soils associated with salt- and boron-tolerant poplar clones grown for the phytoremediation of selenium. Int. J. Phytoremediation 2018, 20, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.K.; Ramesh, A.; Sharma, M.P.; Joshi, O.P.; Govaerts, B.; Steenwerth, K.L. Microbial Community structure and diversity as indicators for evaluating soil quality. In Biodiversity, Biofuels, Agroforestry and Conservation Agriculture; Lichtfouse, E., Ed.; Springer: Dordrecht, The Netherlands, 2010; pp. 317–358. [Google Scholar]
- Cui, H.; Yu, S.; Jing, Z.; Haiyan, C.; Long, C.; Dong-Mei, Z. Effect of different grain sizes of hydroxyapatite on soil heavy metal bioavailability and microbial community composition. Agric. Ecosyst. Environ. 2018, 267, 165–173. [Google Scholar] [CrossRef]
- Chang, E.; Chung, R.; Tsai, Y. Effect of different application rates of organic fertilizer on soil enzyme activity and microbial population. Soil Sci. Plant Nutr. 2010, 53, 132–140. [Google Scholar] [CrossRef]
- Ros, M.; Pascual, J.A.; Garcia, C.; Hernandez, M.T.; Insam, H. Hydrolase activities, microbial biomass and bacterial community in a soil after long-term amendment with different composts. Soil Biol. Biochem. 2006, 38, 3443–3452. [Google Scholar] [CrossRef]
- Ros, M.; Klammer, S.; Knapp, B.; Aichberger, K.; Insam, H. Long-term effects of compost amendment of soil on functional and structural diversity and microbial activity. Soil Use Manag. 2006, 22, 209–218. [Google Scholar] [CrossRef]
- Spiegel, H.; Mosleitner, T.; Sandén, T.; Zaller, J.G. Effects of two decades of organic and mineral fertilization of arable crops on earthworms and standardized litter decomposition. J. Land Manag. Food Environ. 2018, 69, 17–28. [Google Scholar] [CrossRef]
- ÖNORM L1083. Chemical Analysis of Soils—Determination of Acidity. Available online: https://shop.austrian-standards.at/Preview.action;jsessionid=D692D196DF05E2BB172509BDE6B78C4F?preview=&dokkey=380294&selectedLocale=de (accessed on 15 October 2020).
- ÖNORM L1080. Chemical Analyses of Soils—Determination of Organic Carbon by Dry Combustion with and without Consideration of Carbonates. Available online: https://shop.austrian-standards.at/action/de/public/details/470251/OENORM_L_1080_2013_03_15;jsessionid=00270D8EC9C601EDEF91D5F92544A9C0 (accessed on 15 October 2020).
- ÖNORM EN16168. Sludge, Treated Biowaste and Soil—Determination of Total Nitrogen Using Dry Combustion Method. Available online: https://shop.austrian-standards.at/action/de/public/details/446011/OENORM_EN_16168_2012_10_01;jsessionid=2F15397C05DF6D81471D3E416159C852 (accessed on 15 October 2020).
- Tatzber, M.; Schlatter, N.; Baumgartner, A.; Dersch, G.; Körner, R.; Lehtinen, T.; Unger, G.; Mifek, E.; Spiegel, H. KMnO4 determination of active carbon for laboratory routines: Three long-term field experiments in Austria. Soil Res. 2015, 53, 190–204. [Google Scholar] [CrossRef]
- DIN EN 13346. Characterization of Sludges—Determination of Trace Elements and Phosphorus—Aqua Regia Extraction Methods. Available online: https://www.beuth.de/de/norm/din-en-13346/36017256 (accessed on 15 October 2020).
- Öhlinger, R. Dry Matter and Water Content. In Methods in Soil Biology; Schinner, F., Öhlinger, R., Kandeler, E., Margesin, R., Eds.; Springer: Heidelberg, Germany, 1996; p. 385. [Google Scholar]
- Öhlinger, R. Maximum Water-Holding Capacity. In Methods in Soil Biology; Schinner, F., Öhlinger, R., Kandeler, E., Margesin, R., Eds.; Springer: Heidelberg, Germany, 1996; pp. 385–386. [Google Scholar]
- Kandeler, E. Ammonium. In Methods in Soil Biology; Schinner, F., Öhlinger, R., Kandeler, E., Margesin, R., Eds.; Springer: Heidelberg, Germany, 1996; pp. 406–408. [Google Scholar]
- Kandeler, E. N-Mineralization under waterlogged conditions. In Methods in Soil Biology; Schinner, F., Öhlinger, R., Kandeler, E., Margesin, R., Eds.; Springer: Heidelberg, Germany, 1996; pp. 141–143. [Google Scholar]
- ÖNORM L1093. Chemical Analyses of Soils—Determination of Calciumchloride-Extractable Magnesium. Available online: https://shop.austrian-standards.at/action/de/public/details/377609/OENORM_L_1093_2010_12_01 (accessed on 15 October 2020).
- ÖNORM L1086-1. Chemical Analyses of Soils—Extraction of the Effective Exchangeable Cations Ca++, K+, Mg++, Na+ and Al+++, Fe+++, Mn++ and H+ by Bariumchloride Solution and Determination of the Exchange Capacity. Available online: https://shop.austrian-standards.at/action/de/public/details/518044/OENORM_L_1086-1_2014_03_15;jsessionid=BEF9832C9871F0717DAD63E77B17ABD5 (accessed on 15 October 2020).
- Tabatabai, M.A.; Bremner, J.M. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Eivazi, F.; Tabatabai, M.A. Glucosidases and galactosidases in soils. Soil Biol. Biochem. 1988, 20, 601–606. [Google Scholar] [CrossRef]
- Hupfauf, S.; Etemadi, M.; Insam, H.; Podmirseg, S.M. CoMA—Comparative Microbiome Analysis. 2017. Available online: https://www.uibk.ac.at/microbiology/services/coma.html (accessed on 27 January 2020).
- R Development Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. 2013. ISBN 3-900051-07-0. Available online: http://www.R-project.org (accessed on 27 January 2020).
- Willis, A.; Martin, B.D.; Trinh, P.; Barger, K.; Bunge, J. Breakaway: Species Richness Estimation and Modeling. R Package Version 4.7.1. 2020. Available online: https://adw96.github.io/breakaway/ (accessed on 20 August 2020).
- Gloor, G.B.; Macklaim, J.M.; Fernandes, A.D. Displaying variation in large datasets: A visual summary of effect sizes. J. Comput. Graph. Stat. 2016, 25, 971–979. [Google Scholar] [CrossRef]
- Swinton, J. Vennerable: Venn and Euler Area-Proportional Diagrams. R Package Version 3.0/r82. 2013. Available online: https://R-Forge.R-project.org/projects/vennerable/ (accessed on 30 April 2020).
- Van den Boogaart, K.G.; Tolosana-Delgado, R.; Bren, M. Compositions: Compositional Data Analysis. R Package Version 1.40-5. 2020. Available online: https://CRAN.R-project.org/package=compositions (accessed on 20 August 2020).
- Ros, M.; Hernandez, M.T.; Garcia, C. Soil microbial activity after restoration of a semiarid soil by organic amendments. Soil Biol. Biochem. 2003, 35, 463–469. [Google Scholar] [CrossRef]
- Diacono, M.; Montemurro, F. Long-term effects of organic amendments on soil fertility. A review. Agron. Sustain. Dev. 2010, 30, 401–422. [Google Scholar] [CrossRef]
- Smith, J.L.; Doran, J.W. Measurement and Use of pH and Electric Conductivity for Soil Quality Analysis. In Methods for Assessing Soil Quality (SSSA Special Publication 49); Doran, J.W., Jones, A.J., Eds.; Soil Science Society of America: Madison, WI, USA, 1996; pp. 169–185. [Google Scholar]
- Gesamte Rechtsvorschrift für Oö. Bodengrenzwerte-Verordnung 2006, Fassung vom 18.05.2020. Available online: https://www.ris.bka.gv.at/GeltendeFassung.wxe?Abfrage=LROO&Gesetzesnummer=20000409 (accessed on 18 May 2020).
- Geisseler, D.; Scow, K.M. Long-term effects of mineral fertilizers on soil microorganisms—A review. Soil Biol. Biochem. 2014, 75, 54–63. [Google Scholar] [CrossRef]
- Ladd, J.N. Origin and range of enzyme in soil. In Soil Enzyme; Burns, R.G., Ed.; Academic Press: New York, NY, USA, 1978; pp. 51–96. [Google Scholar]
- Caldwell, B.A. Enzyme activities as a component of soil biodiversity: A review. Pedobiologia 2005, 49, 637–644. [Google Scholar] [CrossRef]
- Demetz, M.; Insam, H. Phosphorus availability in a forest soil determined with a respiratory assay compared to chemical methods. Geoderma 1999, 89, 259–271. [Google Scholar] [CrossRef]
- Tao, J.; Liu, X.; Liang, Y.; Niu, J.; Xiao, Y.; Gu, Y.; Ma, L.; Meng, D.; Zhang, Y.; Huang, W.; et al. Maize growth responses to soil microbes and soil properties after fertilization with different green manures. Appl. Microbiol. Biotechnol. 2017, 101, 1289–1299. [Google Scholar] [CrossRef]
- Glassmann, S.I.; Weihe, C.; Li, J.; Albright, M.B.N.; Looby, C.I.; Martiny, A.C.; Treseder, K.K.; Allison, S.D.; Martiny, J.B.H. Decompositions responses to climate depend on microbial community composition. Proc. Natl. Acad. Sci. USA 2018, 115, 11994–11999. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.M.; Benedetti, A.; Insam, H.; Nuti, M.P.; Smalla, K.; Torsvik, V.; Nannipieri, P. Microbial diversity in soil: Ecological theories, the contribution of molecular techniques and the impact of transgenic plants and transgenic microorganisms. Biol. Fertil. Soils 2004, 40, 363–385. [Google Scholar] [CrossRef]
- Perin, L.; Martínez-Aguilar, L.; Castro-González, R.; Estrada-de los Santos, P.; Cabellos-Avelar, T.; Guedes, H.V.; Reis, V.M.; Caballero-Mellado, J. Diazotrophic Burkholderia species associated with field-grown maize and sugarcane. Plant Microbiol. 2006, 72, 3103–3110. [Google Scholar] [CrossRef] [PubMed]
- Steenhoudt, O.; Vanderleyden, J. Azospirillum, a free-living nitrogen-fixing bacterium closely associated with grasses: Genetic, biochemical and ecological aspects. Fems Microbiol. Rev. 2000, 24, 487–506. [Google Scholar] [CrossRef] [PubMed]



| Compost | OWC | GC | MC | SSC |
|---|---|---|---|---|
| pH | 7.85 | 7.26 | 8.15 | 7.1 |
| Ntot (g kg−1) | 10.5 | 6.6 | 5.9 | 14.0 |
| Corg (g kg−1) | 225 | 116 | 183 | 231 |
| C/N | 21.4 | 17.6 | 31.0 | 16.5 |
| P (g kg−1) | 2.0 | 1.6 | 1.9 | 9.7 |
| K (g kg−1) | 4.8 | 5.5 | 7.8 | 9.1 |
| Cu (mg kg−1) | 101 | 33 | 24 | 67 |
| Zn (mg kg−1) | 233 | 118 | 155 | 260 |
| Ni (mg kg−1) | 21.5 | 28.6 | 23.6 | 59.0 |
| Cr (mg kg−1) | 39.5 | 50.5 | 53.1 | 27.0 |
| Pb (mg kg−1) | 29.6 | 23.1 | <LOD | 23.0 |
| Cd (mg kg−1) | 0.50 | 0.20 | 0.30 | 0.47 |
| Hg (mg kg−1) | 0.10 | 0.09 | 0.04 | 0.20 |
| Treatment | EC (µS cm−1) | pH | Corg (g kg−1) | SOM (g kg−1) | DM (%) | AC (%) |
|---|---|---|---|---|---|---|
| 0 kg N | 36.0 ± 7.68 ab | 7.09 ± 0.15 ab | 12.6 ± 0.12 a | 40.8 ± 3.49 ab | 84.7 ± 1.02 | 4.5 ± 0.22 ab |
| 80 kg N | 24.0 ± 2.41 a | 7.00 ± 0.16 a | 12.6 ± 0.07 a | 37.6 ± 0.92 a | 85.5 ± 0.14 | 4.33 ± 0.22 ab |
| OWC | 30.4 ± 1.75 a | 7.25 ± 0.07 bc | 15.5 ± 0.79 ab | 44.7 ± 2.90 bc | 83.6 ± 1.09 | 4.36 ± 0.22 ab |
| GC | 34.0 ± 3.28 ab | 7.20 ± 0.08 bc | 15.4 ± 1.47 ab | 47.5 ± 3.09 bc | 84.1 ± 0.45 | 5.22 ± 1.43 b |
| MC | 30.7 ± 4.87 a | 7.14 ± 0.11 abc | 14.3 ± 1.46 ab | 50.3 ± 2.08 bc | 84.6 ± 0.41 | 4.28 ± 0.15 ab |
| SSC | 39.6 ± 3.94 ab | 7.38 ± 0.02 c | 16.8 ± 1.54 b | 44.5 ± 2.63 c | 83.6 ± 0.27 | 4.14 ± 0.14 ab |
| OWC + N | 34.2 ± 3.95 a | 7.26 ± 0.09 bc | 15.2 ± 0.27 ab | 48.0 ± 0.94 bc | 84.0 ± 0.71 | 4.05 ± 0.11 ab |
| GC + N | 34.0 ± 6.84 a | 7.23 ± 0.02 abc | 15.9 ± 0.93 b | 52.2 ± 1.43 bc | 84.0 ± 0.42 | 4.01 ± 0.11 ab |
| MC + N | 35.8 ± 8.80 a | 7.16 ± 0.11 abc | 14.7 ± 1.08 ab | 44.0 ± 2.43 bc | 84.2 ± 0.50 | 3.98 ± 0.23 ab |
| SSC + N | 49.4 ± 10.5 b | 7.40 ± 0.03 c | 16.2 ± 0.69 b | 44.9 ± 0.64 bc | 84.4 ± 0.64 | 3.82 ± 0.06 a |
| N(tot) (g kg−1) | Ammonification Rate (µg N g−1 DM) | NH4 (µg N g DM−1) | Mg (mg kg−1) | K (mg kg−1) | P (mg kg−1) | |
| 0 kg N | 1.40 ± 0.03 ab | 1.88 ± 0.13 ab | 4.47 ± 0.18 | 116 ± 6.1 b | 121 ± 20.3 ab | 89.0 ±19.3 a |
| 80 kg N | 1.38 ± 0.12 a | 1.76 ± 0.42 ab | 4.31 ± 0.22 | 119 ± 7.8 b | 191 ± 7.8 abc | 97.1 ± 44.9 a |
| OWC | 1.67 ± 0.07 bc | 2.64 ± 0.41 ab | 4.70 ± 0.38 | 104 ± 14.5 ab | 143 ± 29.9 abc | 140 ± 34.3 a |
| GC | 1.65 ± 0.14 bc | 2.66 ± 0.68 ab | 5.11 ± 0.73 | 135 ± 11.1 b | 168 ± 25.3 abc | 123 ± 13.6 a |
| MC | 1.54 ± 0.12 abc | 2.62 ± 0.34 ab | 3.52 ± 1.57 | 126 ± 14.6 b | 203 ± 27.5 c | 139 ± 33.9 a |
| SSC | 1.74 ± 0.13 c | 2.81 ± 0.31 b | 4.71 ± 0.88 | 73 ± 6.2 a | 134 ± 16.3 abc | 312 ± 56.5 b |
| OWC + N | 1.63 ± 0.03 abc | 2.51 ± 0.49 ab | 3.88 ± 0.80 | 104 ± 22.8 ab | 121 ± 15.4 ab | 149 ± 48.2 a |
| GC + N | 1.74 ± 0.07 c | 2.45 ± 0.37 ab | 3.54 ± 0.10 | 134 ± 11.5 b | 161 ± 29.3 abc | 121 ± 5.0 a |
| MC + N | 1.60 ± 0.09 abc | 2.55 ± 0.40 ab | 3.70 ± 1.44 | 129 ± 19.0 b | 199 ± 35.9 abc | 141 ± 46.7 a |
| SSC + N | 1.75 ± 0.06 c | 1.54 ± 0.23 a | 3.82 ± 0.65 | 74 ± 13.2 a | 133 ± 28.3 abc | 297 ± 35.9 b |
| Caexcha (cmolc kg−1) | Kexcha (cmolc kg−1) | Mgexcha (cmolc kg−1) | Naexcha (cmolc kg−1) | WHC (%) | Co (mg kg−1) | |
| 0 kg N | 14.2 ± 1.13 ab | 0.42 ± 0.07 | 1.43 ± 0.07 b | 0.03 ± 0.00 a | 28.8 ± 7.52 | 5.76 ± 0.23 a |
| 80 kg N | 13.0 ± 0.71 a | 0.42 ± 0.10 | 1.40 ± 0.13 b | 0.03 ± 0.00 a | 31.7 ± 1.06 | 5.95 ab |
| OWC | 16.0 ± 1.04 b | 0.49 ± 0.09 | 1.29 ± 0.15 ab | 0.03 ± 0.00 a | 34.6 ± 2.21 | 7.50 ± 1.66 ab |
| GC | 15.0 ± 0.64 ab | 0.60 ± 0.09 | 1.59 ± 0.13 b | 0.03 ± 0.00 a | 32.9 ± 1.16 | 7.65 ± 0.41 ab |
| MC | 14.6 ± 1.26 ab | 0.68 ± 0.06 | 1.50 ± 0.17 b | 0.04 ± 0.00 a | 32.9 ± 1.46 | 8.58 ± 0.83 b |
| SSC | 18.8 ± 0.81 cd | 0.45 ± 0.06 | 0.91 ± 0.09 a | 0.06 ± 0.01 b | 33.5 ± 1.80 | 5.54 ± 1.49 a |
| OWC + N | 16.3 ± 1.09 bcd | 0.42 ± 0.05 | 1.25 ± 0.26 ab | 0.03 ± 0.00 a | 34.0 ± 2.05 | 6.66 ± 0.72 ab |
| GC + N | 15.8 ± 0.31 abd | 0.53 ± 0.09 | 1.61 ± 0.14 b | 0.04 ± 0.01 a | 33.9 ± 2.52 | 6.76 ± 0.66 ab |
| MC + N | 14.6 ± 1.52 ab | 0.64 ± 0.11 | 1.49 ± 0.20 b | 0.04 ± 0.01 a | 34.7 ± 1.84 | 6.71 ± 1.00 ab |
| SSC + N | 18.4 ± 0.74 cd | 0.43 ± 0.09 | 0.95 ± 0.15 a | 0.07 ± 0.01 b | 31.0 ± 2.37 | 6.51 ± 0.81 ab |
| Cr (mg kg−1) | Cu (mg kg−1) | Ni (mg kg−1) | Zn (mg kg−1) | Cd (mg kg−1) | Pb (mg kg−1) | |
| 0 kg N | 18.5 ± 1.07 | 16.91 ± 1.36 | 20.91 ± 0.96 | 52.6 ± 2.89 | 3.70 ± 0.20 | 15.45 ± 0.87 |
| 80 kg N | 18.05 ± NA | 15.65 ± NA | 20.02 ± NA | 50.05 ± NA | 3.75 ± NA | 15.35 ± NA |
| OWC | 19.6 ± 1.25 | 18.6 ± 1.14 | 22.0 ± 1.38 | 61.4 ± 6.85 | 4.01 ± 0.25 | 17.4 ± 0.47 |
| GC | 19.0 ± 1.57 | 18.2 ± 1.65 | 20.7 ± 2.59 | 60.5 ± 6.65 | 3.81 ± 0.16 | 16.8 ± 0.77 |
| MC | 19.7 ± 1.22 | 19.9 ± 3.06 | 23.3 ± 5.44 | 61.9 ± 2.19 | 5.25 ± 2.52 | 16.9 ± 0.54 |
| SSC | 16.5 ± 5.10 | 17.7 ± 5.99 | 18.6 ± 6.00 | 57.5 ± 18.06 | 3.39 ± 1.04 | 14.0 ± 4.52 |
| OWC + N | 20.5 ± 1.10 | 18.5 ± 0.81 | 21.7 ± 0.83 | 58.3 ± 1.92 | 4.05 ± 0.11 | 17.5 ± 0.35 |
| GC + N | 19.3 ± 1.27 | 18.2 ± 0.95 | 21.3 ± 1.26 | 56.9 ± 3.56 | 3.91 ± 0.27 | 17.5 ± 1.11 |
| MC + N | 18.9 ± 1.35 | 18.3 ± 1.01 | 20.8 ± 0.88 | 54.0 ± 1.37 | 3.85 ± 0.13 | 15.8 ± 0.50 |
| SSC + N | 19.9 ± 0.89 | 21.0 ± 0.72 | 21.6 ± 0.66 | 63.78 ± 1.95 | 3.90 ± 0.17 | 16.0 ± 0.45 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurzemann, F.R.; Plieger, U.; Probst, M.; Spiegel, H.; Sandén, T.; Ros, M.; Insam, H. Long-Term Fertilization Affects Soil Microbiota, Improves Yield and Benefits Soil. Agronomy 2020, 10, 1664. https://doi.org/10.3390/agronomy10111664
Kurzemann FR, Plieger U, Probst M, Spiegel H, Sandén T, Ros M, Insam H. Long-Term Fertilization Affects Soil Microbiota, Improves Yield and Benefits Soil. Agronomy. 2020; 10(11):1664. https://doi.org/10.3390/agronomy10111664
Chicago/Turabian StyleKurzemann, Felix R., Ulrich Plieger, Maraike Probst, Heide Spiegel, Taru Sandén, Margarita Ros, and Heribert Insam. 2020. "Long-Term Fertilization Affects Soil Microbiota, Improves Yield and Benefits Soil" Agronomy 10, no. 11: 1664. https://doi.org/10.3390/agronomy10111664
APA StyleKurzemann, F. R., Plieger, U., Probst, M., Spiegel, H., Sandén, T., Ros, M., & Insam, H. (2020). Long-Term Fertilization Affects Soil Microbiota, Improves Yield and Benefits Soil. Agronomy, 10(11), 1664. https://doi.org/10.3390/agronomy10111664








