Effects of Rhizobium Inoculation on N2 Fixation, Phytochemical Profiles and Rhizosphere Soil Microbes of Cancer Bush Lessertia frutescens (L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and Soils
2.2. Rhizobium Strain and Inoculum Preparation
2.3. Experimental Design
2.4. Plant Sampling and Preparation
2.5. Determination of Functional Diversity
2.6. Determination of Soil Microbial Enzymatic Activity
2.7. Determination of Total Phenolics and Flavonoids
2.8. Determination of Ferric Reducing Antioxidant Power (FRAP) Assay
2.9. Determination of Soluble Sugar Concentration
2.10. Estimation of Nitrogen Fixation
2.11. Measuring 15N/14N Ratios, %Ndfa, and N-fixed
2.11.1. Nitrogen Content
2.11.2. Percent N Derived from Atmospheric Fixation (%Ndfa)
2.12. Statistical Analysis
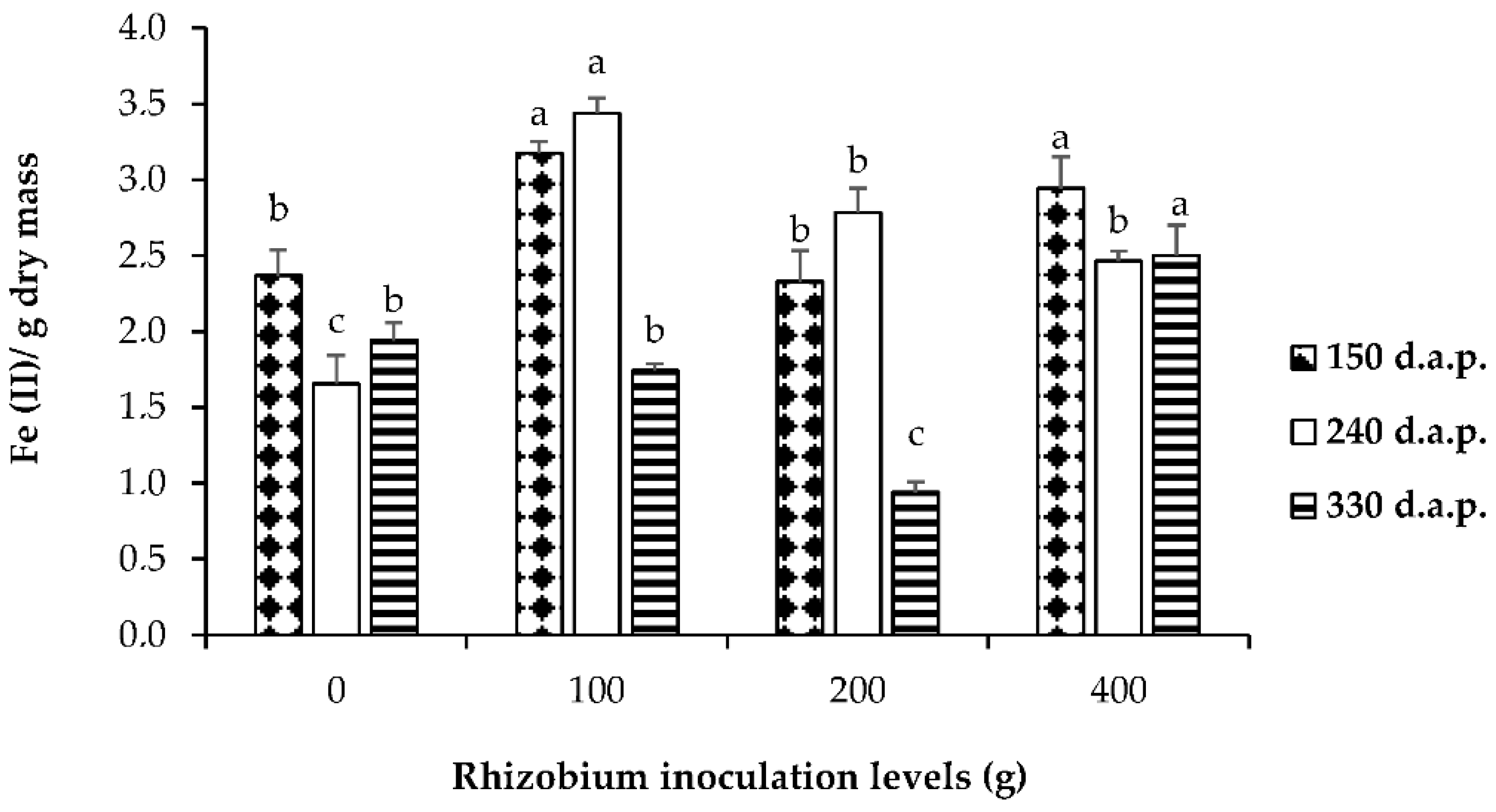
3. Results
3.1. Total Phenolics and Flavonoids
3.2. Ferric Reducing Antioxidant Power (FRAP) Assay
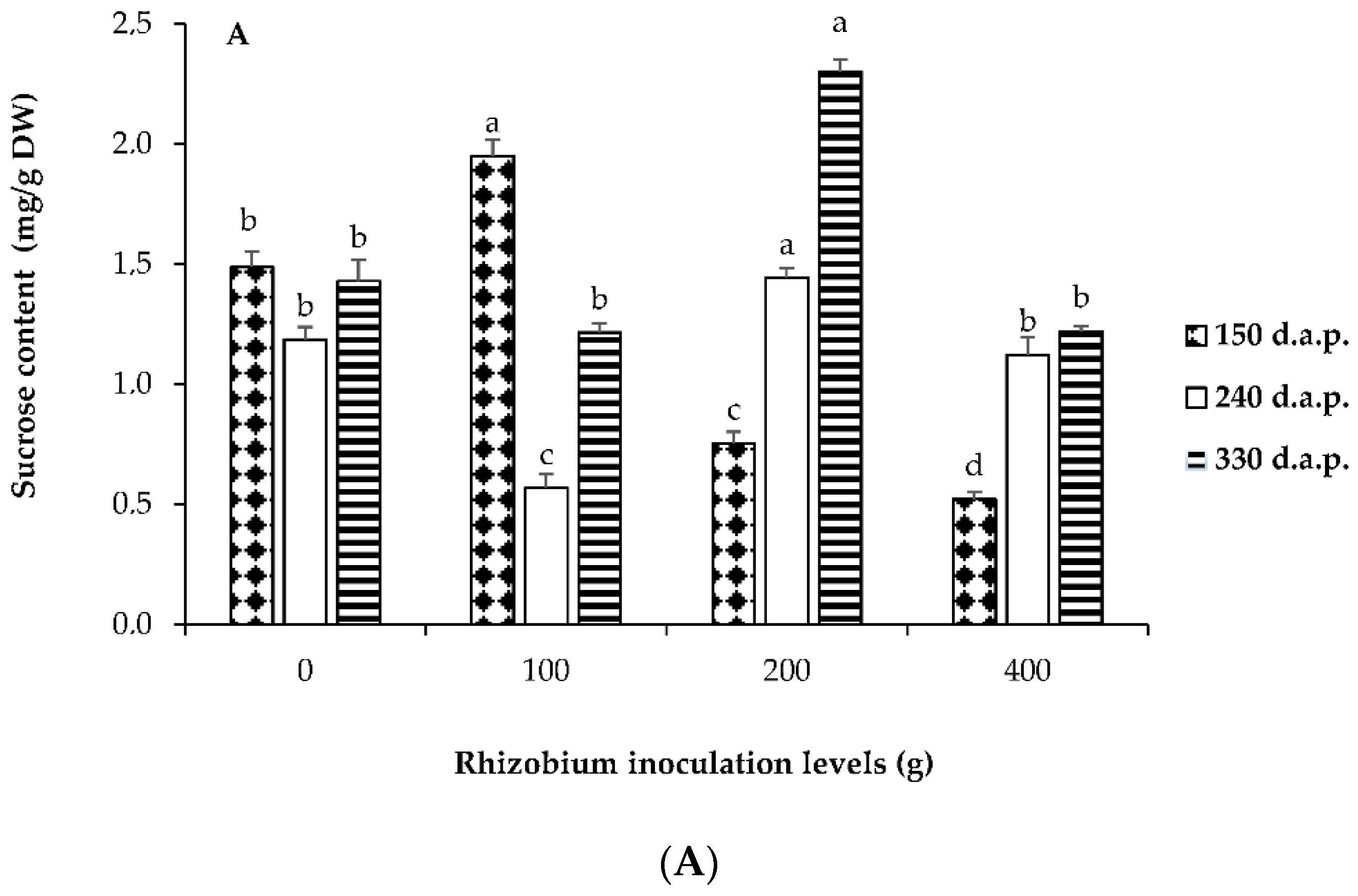
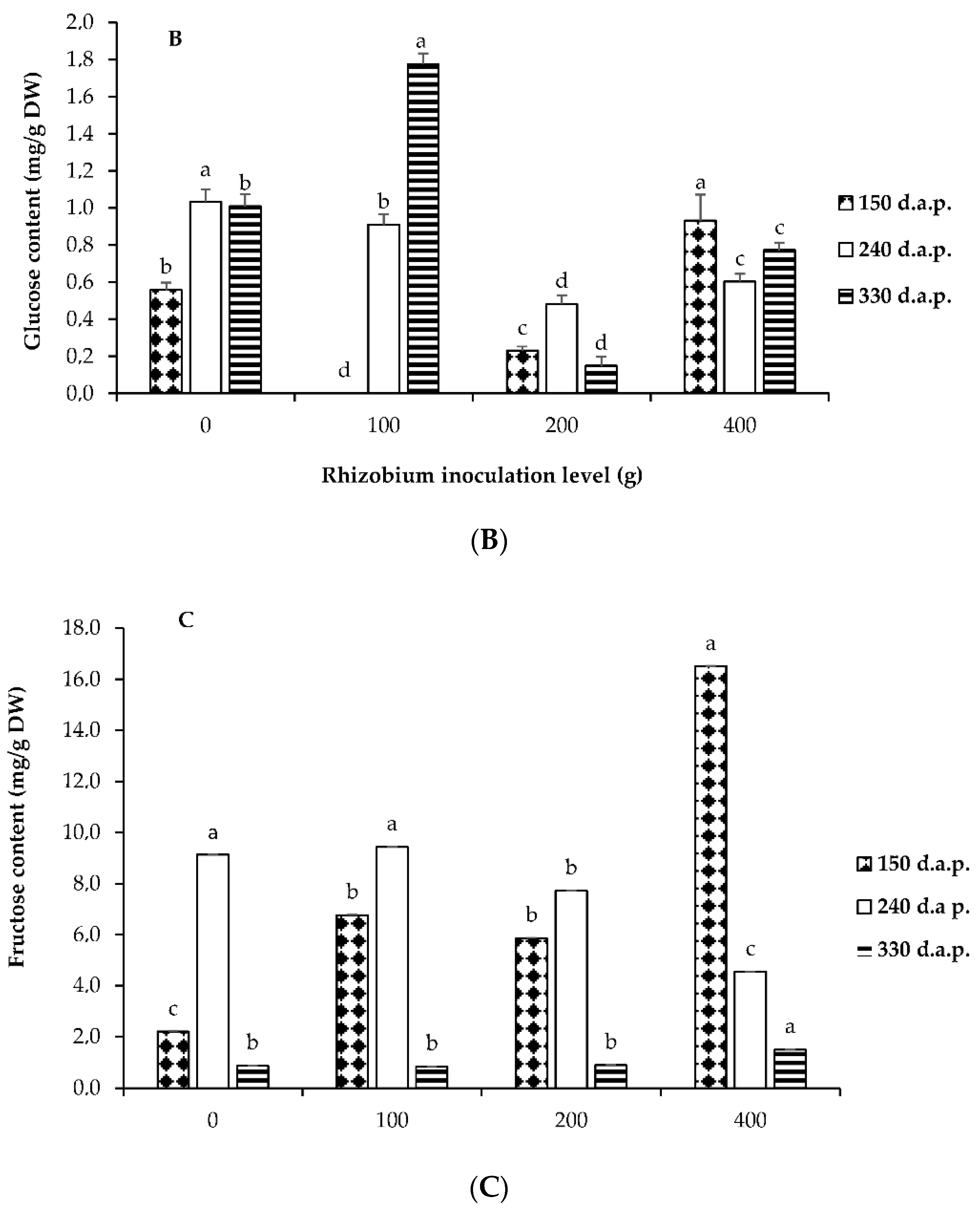
3.3. Soluble Sugars
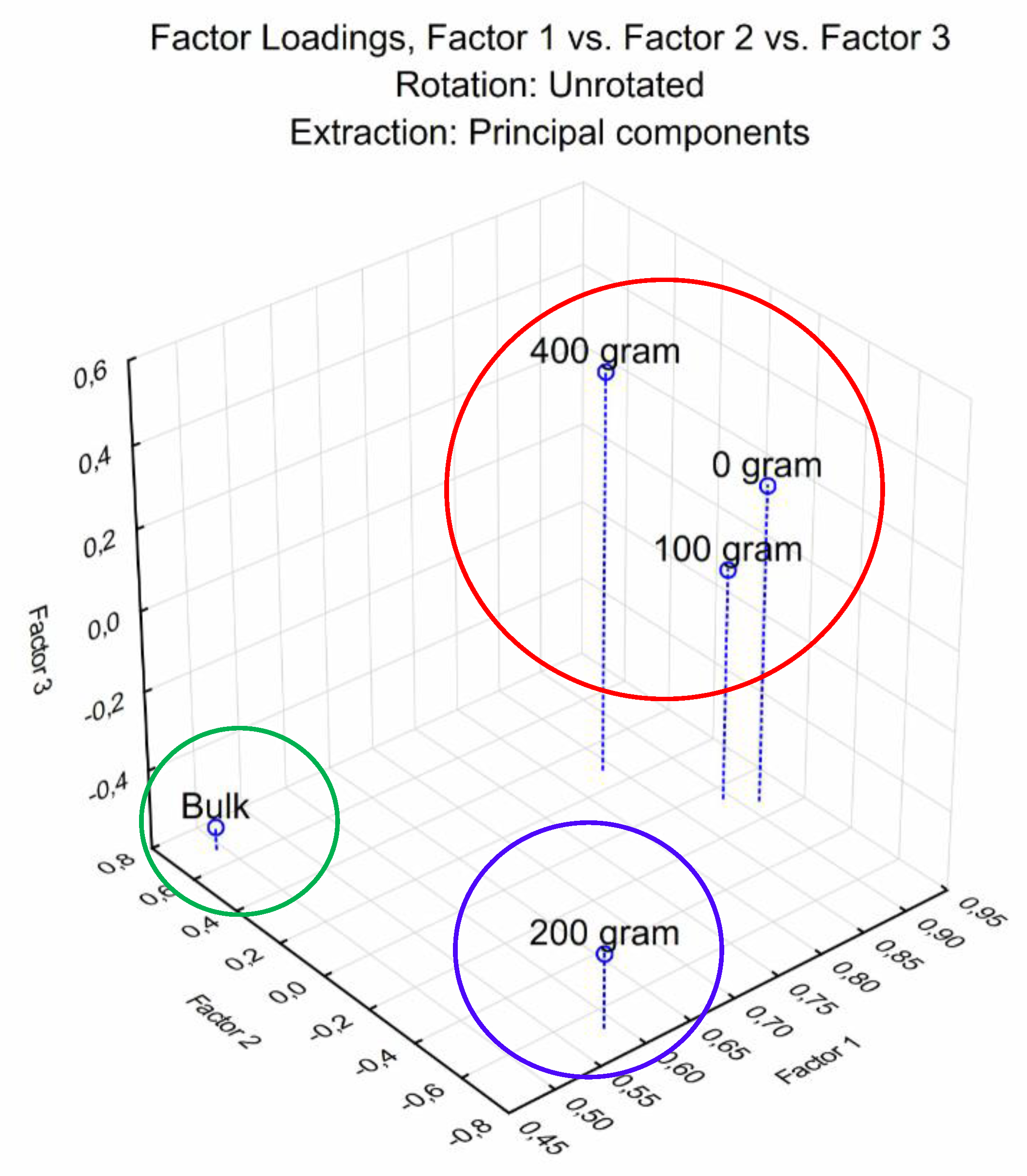
3.4. Carbon Substrate Utilization Profiles (CSUP)
3.5. Diversity Indices of Sampled Soil of L. frutescens and Bulk Soil
3.6. Soil Microbial Enzymatic Activity, viz. Microbial Activity
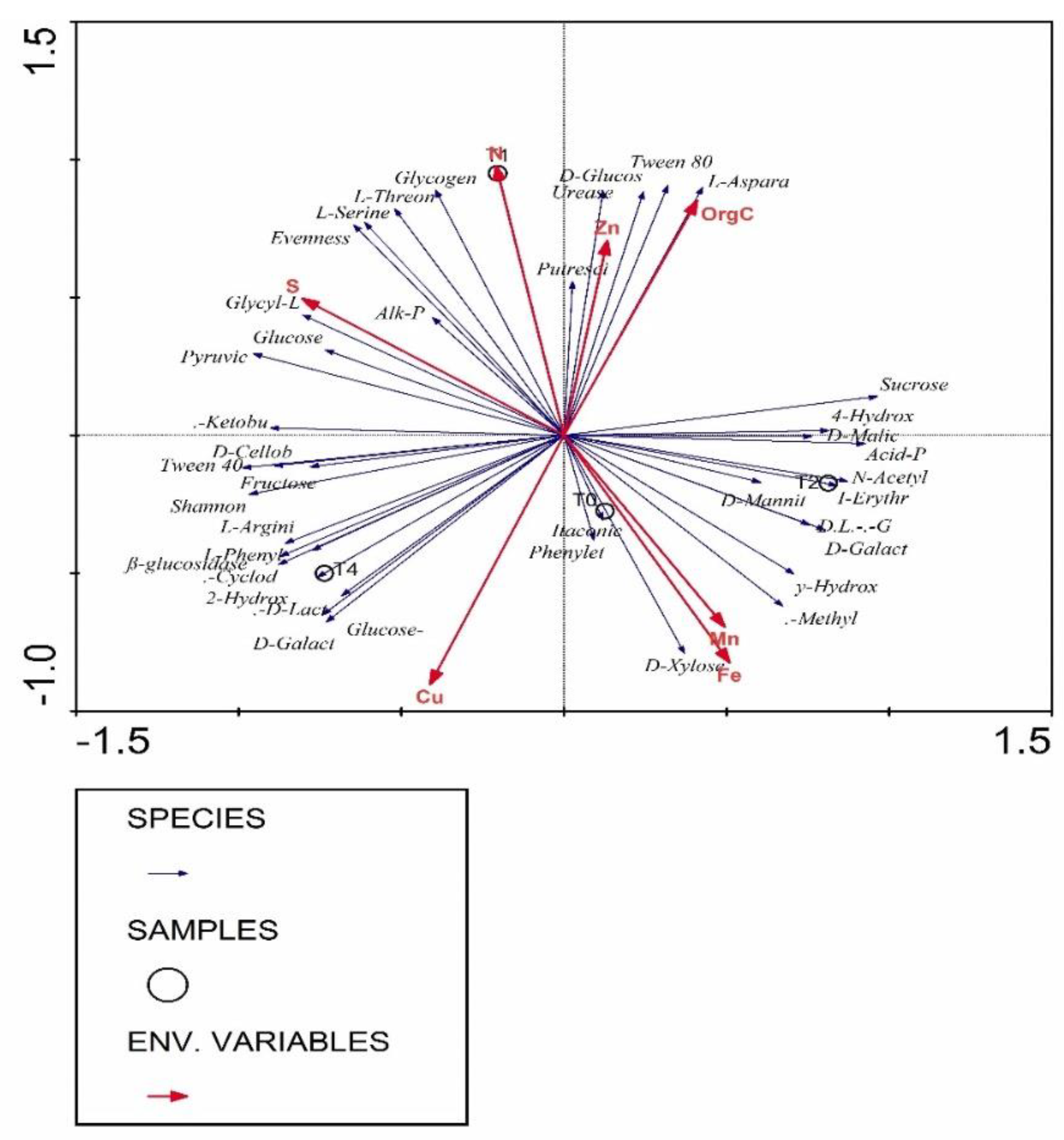
3.7. Correlation Analysis of Parameters Using Redundancy Analysis (RDA)
3.8. Comparing Plant Growth and Symbiotic N Fixation in Response to Rhizobium Inoculation
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Solaiman, Z.M.; Anawar, H.M. Rhizosphere microbes interactions in medicinal plants. In Plant-Growth Promoting Rhizobacteria (PGPR) and Medicinal Plants; Egamberdieva, D., Shrivastava, S., Varma, A., Eds.; Springer: Cham, Switzerland, 2015; pp. 19–41. [Google Scholar]
- Hayat, R.; Ali, S.; Amara, U.; Khalid, R.; Ahmed, I. Soil beneficial bacteria and their role in plant growth promotion: A review. Ann. Microbiol. 2010, 60, 1–20. [Google Scholar] [CrossRef]
- Thilakarathna, M.S.; Chapagain, T.; Ghimire, B.; Pudasaini, R.; Tamang, B.B.; Gurung, K.; Choi, K.; Rai, L.; Magar, S.; BK, B.; et al. Evaluating the effectiveness of rhizobium inoculants and micronutrients as technologies for Nepalese Common bean smallholder farmers in the Real-World Context of highly variable hillside environments and indigenous farming practices. Agriculture 2019, 9, 20. [Google Scholar] [CrossRef]
- Sarr, P.S.; Okon, J.W.; Begoude, D.A.; Araki, S.; Ambang, Z.; Shibata, M.; Funakawa, S. Symbiotic N2-fixation estimated by the 15N tracer technique and growth of Pueraria phaseoloides (Roxb.) Benth. Inoculated with Bradyrhizobium strain in field conditions. Scientifica 2016, 7026859. [Google Scholar]
- Arora, N.K.; Kang, S.C.; Maheshwari, D.K. Isolation of siderophore-producing strains of Rhizobium meliloti and their biocontrol potential against Macrophomina phaseolina that causes charcoal rot of groundnut. Curr. Sci. 2001, 81, 673–677. [Google Scholar]
- Rajeshkumar, S.; Nisha, M.C.; Selvaraj, T. Variability in growth, nutrition, and phytochemical constituents of Plectranthus amboinicus (Lour) Spreng. As influenced by indigenous arbuscular mycorrhizal fungi. Maejo Int. J. Sci. Technol. 2008, 2, 431–439. [Google Scholar]
- Geneva, M.P.; Stancheva, I.V.; Boychinova, M.B.; Mincheva, N.H.; Yonova, P.A. Effects of foliar fertilization and arbuscular mycorrhizal colonization on Salvia officinalis L. growth, antioxidant capacity, and essential oil composition. J. Sci. Food Agric. 2010, 90, 696–702. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, M.; Li, Y.; Wu, A.; Huang, J. Effects of arbuscular mycorrhizal fungi on growth and nitrogen uptake of Chrysanthemum morifolium under salt stress. PLoS ONE 2018, 13, e0196408. [Google Scholar]
- Gogoi, P.; Singh, R.K. Differential effect of some arbuscular mycorrhizal fungi on growth of Piper longum L. (Piperaceae). Indian J. Sci. Technol. 2011, 4, 119–125. [Google Scholar] [CrossRef]
- Tariq, M.; Hameed, S.; Yasmeen, T.; Ali, A. Non-rhizobial bacteria for improved nodulation and grain yield of Mung bean [Vigna radiata (L.) Wilczek]. Afr. J. Biotechnol. 2012, 11, 15012–15019. [Google Scholar]
- Street, R.A.; Prinsloo, G. Commercially important medicinal plants of South Africa: A Review. J. Chem. 2012, 2013, 205048. [Google Scholar] [CrossRef]
- Van Wyk, B.E.; Gericke, N. People’s Plants: A Guide to Useful Plants of Southern Africa; Briza Publications: Pretoria, South Africa, 2000. [Google Scholar]
- Makunga, P.; Colling, J.; Stander, M.A.; Nokwanda, P. Nitrogen supply, and abiotic stress influence canavanine synthesis and the productivity of in vitro regenerated Sutherlandia frutescens micro shoots. J. Plant Physiol. 2010, 167, 1521–1524. [Google Scholar]
- Gibson, D. Ambiguities in the making of an African medicine: Clinical trials of Sutherlandia frutescens (L.) R. Br (Lessertia frutescens). Afr. Sociol Rev. 2011, 15, 1–7. [Google Scholar]
- Moteetee, A.N.; Van Wyk, B.E. The concept of Musa-pelo and medicinal use of shrubby legumes (Fabaceae) in Lesotho. Bothalia 2007, 37, 75–77. [Google Scholar] [CrossRef]
- Van Wyk, B.E.; Albrecht, C. A Review of taxonomy, ethnobotany, chemistry, and pharmacology of Sutherlandia frutescens (Fabaceae). J. Ethnopharmacol. 2008, 119, 620–629. [Google Scholar] [CrossRef]
- Tobwala, S.; Fan, W.; Hines, C.J.; Folk, W.R.; Ercal, N. Antioxidant potential of Sutherlandia frutescens and its protective effects against oxidative stress in various cell cultures. BMC Complement. Altern. Med. 2014, 14, 1–11. [Google Scholar] [CrossRef]
- Silva, L.R.; Bento, C.; Gonçalves, A.C.; Flores-Félix, J.D.; Ramírez-Bahena, M.H.; Peix, A.; Velázquez, E. Legume bioactive compounds: Influence of rhizobial inoculation. AIMS Microbiol. 2017, 3, 267–278. [Google Scholar] [CrossRef]
- Silva, L.R.; Peix, Á.; Albuquerque, C. Bioactive compounds of legumes as health promoters. In Natural Bioactive Compounds from Fruits and Vegetables as Health Promoters. Part 2. Sharjah; Silva, L.R., Silva, B.M., Eds.; Bentham Science Publishers: Sharjah, UAE, 2016; pp. 3–27. [Google Scholar]
- Couto, C.; Silva, L.R.; Valentão, P.; Velázquez, E.; Peix, A.; Andrade, P.B. Effects induced by the nodulation with Bradyrhizobium japonicum on Glycine max (Soybean) metabolism and antioxidant potential. Food Chem. 2011, 127, 1487–1495. [Google Scholar] [CrossRef]
- Shaik, S.; Singh, N.; Nicholas, A. Comparison of the selected secondary metabolite content present in the Cancer-bush Lessertia (Sutherlandia) frutescens L. extracts. Afr. J. Tradit. Complement. 2011, 8, 429–434. [Google Scholar]
- Bhat, A.A.; Prabhu, K.S.; Kuttikrishnan, S.; Krishnankutty, R.; Ramzi, J.B.; Mohammad, M.; Uddin, S. Potential therapeutic targets of Guggulsterone in cancer. Nutr. Metab. 2017, 14, 23. [Google Scholar] [CrossRef]
- Mendes, R.; Garbeva, P.; Raaijmakers, J.M. The rhizosphere microbiome: Significance of plant beneficial plant pathogenic and human pathogenic microorganisms. FEMS Microbiol. Rev. 2013, 37, 634–663. [Google Scholar] [CrossRef]
- Doran, J.W.; Safley, M. Defining and assessing soil health and sustainable productivity. In Biological Indicators of Soil Health; Pankhurst, C., Doube, B.M., Gupta, V.V.S.R., Eds.; CAB International: Willingford, UK, 1997. [Google Scholar]
- Habig, J.; Swanepoel, C. Effects of conservation agriculture and fertilization on soil microbial diversity and activity. Environment 2015, 2, 358–384. [Google Scholar] [CrossRef]
- Doran, J.W.; Zeiss, M.R. Soil health and sustainability: Managing the biotic component of soil quality. Appl. Soil Ecol. 2000, 15, 3–11. [Google Scholar] [CrossRef]
- Jorquera, M.A.; Shaharoona, B.; Nadeem, S.M.; de la Luz Mora, M.; Crowley, D.E. Plant growth-promoting rhizobacteria associated with ancient clones of creosote bush (Larrea tridentata). Microb. Ecol. 2012, 64, 1008–1017. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, B.; Joe, M.M.; Jaleel, C.A. Response of some medicinal plants to vesicular-arbuscular inoculations. J. Sci. Res. 2009, 1, 381–386. [Google Scholar] [CrossRef]
- Li, H.B.; Wong, C.C.; Cheng, K.W.; Chen, F.F. Antioxidant properties in vitro and total phenolic contents in methanol extracts from medicinal plants. Food Sci. Technol. 2008, 41, 385–390. [Google Scholar] [CrossRef]
- Ibrahim, M.H.; Jaafar, H.Z.E.; Rahmat, A.; Rahman, Z.A. Involvement of nitrogen on flavonoids, glutathione, anthocyanin, ascorbic acid and antioxidant activities of Malaysian medicinal plant Labisia pumila Blume (Kacip). Int. J. Mol. Sci. 2012, 13, 393–408. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Unkovich, M.J.; Herridge, D.F.; Peoples, M.B.; Cadisch, G.; Boddey, R.M.; Giller, K.E. Measuring Plant-Associated Nitrogen Fixation in Agricultural Systems; ACIAR: Canberra, Australia, 2008.
- Shearer, G.; Kohl, D.H. N2-fixation in field settings, estimations based on natural 15N abundance. Aust. J. Plant Physiol. 1986, 13, 699–756. [Google Scholar]
- Mitchell, R.J.; Hester, A.J.; Campbell, C.D.; Chapman, S.J.; Cameron, C.M.; Hewison, R.J.; Potts, J.M. Is vegetation composition or soil chemistry the best predictor of the soil microbial community? Plant Soil 2010, 333, 417–430. [Google Scholar] [CrossRef]
- Mathiventhan, U.; Sivakanesan, R. Total phenolic content and total antioxidant activity of sixteen commonly consumed green leafy vegetables stored under different conditions. Eur. Int. J. Sci. Technol. 2013, 2, 123–132. [Google Scholar]
- Cappellari, L.; Santoro, M.V.; Nievas, F.; Giordano, W.E.; Banchio, F. Increase of secondary metabolite content in marigold by inoculation with plant growth-promoting rhizobacteria. Appl. Soil Ecol. 2013, 70, 16–22. [Google Scholar] [CrossRef]
- Salla, T.D.; Ramos da Silva, T.; Astarita, L.V.; Santarém, E.R. Streptomyces rhizobacteria modulate the secondary metabolism of Eucalyptus plants. Plant Physiol. Biochem. 2014, 85, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Larose, G.; Cheˆnevert, R.; Moutoglis, P.; Gagne´, S.; Piche´, Y.; Vierheilig, H. Flavonoid levels in roots of Medicago sativa are modulated by the developmental stage of the symbiosis and the root-colonizing arbuscular mycorrhizal fungus. J. Plant Physiol. 2002, 159, 1329–1339. [Google Scholar] [CrossRef]
- Pedone-Bonfim, M.V.L.; Lins, M.A.; Coelho, I.R.; Santana, A.S.; Silva, F.S.B.; Maia, L.C. Mycorrhizal technology and phosphorus in the production of primary and secondary metabolites in cebil (Anadenanthera colubrina (Vell.) Brenan) seedlings. J. Sci. Food Agric. 2013, 93, 1479–1484. [Google Scholar] [CrossRef] [PubMed]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: An overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef] [PubMed]
- Vurukonda, S.; Giovanardi, D.; Stefani, E. Plant growth-promoting and biocontrol activity of Streptomyces spp. as Endophytes. Int. J. Mol. Sci. 2018, 19, 952. [Google Scholar] [CrossRef]
- Ibrahim, M.H.; Jaafar, H.Z.; Karimi, E.; Ghasemzadeh, A. Impact of organic and inorganic fertilizer application on the phytochemical and antioxidant activity of Kacip Fatimah (Labisia pumila Benth). Molecules 2013, 18, 10973–10988. [Google Scholar] [CrossRef]
- Ceccarelli, N.; Curadi, M.; Martelloni, L.; Sbrana, C.; Picciarelli, P.; Giovannetti, M. Mycorrhizal colonization impacts on phenolic content and antioxidant properties of artichoke leaves and flower head two years after field transplant. Plant Soil 2010, 335, 311–323. [Google Scholar] [CrossRef]
- Ponce, M.A.; Scervino, J.M.; Erra, B.R.; Ocampo, J.A.; Godeas, A. Flavonoids from shoots and roots of Trifolium repens (white clover) grown in presence or absence of the arbuscular mycorrhizal fungus Glomus intraradices. Phytochemistry 2004, 65, 1925–1930. [Google Scholar] [CrossRef]
- Zubek, S.; Rola, K.; Szewczyk, A. Enhanced concentrations of elements and secondary metabolites in Viola tricolor L. induced by arbuscular mycorrhizal fungi. Plant Soil 2015, 390, 129. [Google Scholar] [CrossRef]
- Singh, R.; Soni, S.K.; Kalra, A. Synergy between Glomus fasciculatum and a beneficial pseudomonas in reducing root diseases and improving yield and forskolin content Coleus forskohlii Briq. Under organic field conditions. Mycorrhiza 2013, 23, 35–44. [Google Scholar] [CrossRef]
- Abdel-Lateif, K.; Bogusz, D.; Hocher, V. The role of flavonoids in the establishment of plant roots endosymbioses with arbuscular mycorrhiza fungi, rhizobia, and Frankia bacteria. Plant Signal. Behav. 2012, 7, 636–641. [Google Scholar] [CrossRef] [PubMed]
- Phillips, D.A.; Tsai, S.M. Flavonoids as plant signals to rhizosphere microbes. Mycorrhiza 1992, 1, 55–58. [Google Scholar] [CrossRef]
- Morandi, D. Occurrence of phytoalexins and phenolic compounds in endomycorrhizal interactions, and their potential role in biological control. Plant Soil 1996, 185, 241–251. [Google Scholar] [CrossRef]
- Veeru, P.; Kishor, M.P.; Meenakshi, M. Screening of medicinal plant extracts for antioxidant activity. J. Med. Plant Res. 2009, 3, 608–612. [Google Scholar]
- Fernandes, A.C.; Cromarty, A.D.; Albrecht, C.; Rensburg, C.E.J. The antioxidant potential of Sutherlandia frutescens. J. Ethnopharmacol. 2004, 95, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Wingler, A.; Masclaux-Daubresse, C.; Fischer, A.M. Sugars, senescence, and aging in plants and heterotrophic organisms. J. Exp. Bot. 2009, 60, 1063–1066. [Google Scholar] [CrossRef]
- Koch, K. Sucrose metabolism: Regulatory mechanisms and pivotal roles in sugar sensing and plant development. Curr. Opin. Plant Biol. 2004, 7, 235–246. [Google Scholar] [CrossRef]
- Ferguson, B.J.; Lin, M.H.; Gresshoff, P.M. Regulation of legume nodulation by acidic growth conditions. Plant Signa.l Behav. 2013, 8. [Google Scholar] [CrossRef]
- Canarini, A.; Kaiser, C.; Merchant, A.; Richter, A.; Wanek, W. Root exudation of primary metabolites: Mechanisms and their roles in plant responses to environmental stimuli. Front Plant Sci. 2019, 10, 157. [Google Scholar] [CrossRef]
- Girvan, M.S.; Bullimore, J.; Pretty, J.N.; Osborn, A.M.; Ball, A.S. Soil type is the primary determinant of the composition of the total and active bacterial communities in arable soils. Appl. Environ. Microbiol. 2003, 69, 1800–1809. [Google Scholar] [CrossRef]
- Gomez, E.; Ferreras, L.; Toresani, S. Soil bacterial functional diversity as influenced by organic amendment application. Bioresour. Technol. 2006, 97, 1484–1489. [Google Scholar] [CrossRef]
- Hofman, J.; Švihálek, J.; Holoubek, I. Evaluation of functional diversity of soil microbial communities—A case study. Plant Soil Environ. 2004, 50, 141–148. [Google Scholar] [CrossRef]
- Brockett, B.F.T.; Prescott, C.E.; Grayston, S.J. Soil moisture is the major factor influencing microbial community structure and enzyme activities across seven biogeoclimatic zones in Western Canada. Soil Biol. Biochem. 2012, 44, 9–20. [Google Scholar] [CrossRef]
- Pitumpe Arachchige, P.S.; Rosso, L.H.M.; Hansel, F.D.; Ramundo, B.; Torres, A.R.; Ciampitti, I.A.; Jagadish, S.V.K. Temporal biological nitrogen fixation pattern in soybean inoculated with Bradyrhizobium. Agrosyst. Geosci. Environ. 2020, 3, e20079. [Google Scholar] [CrossRef]
- Gelfand, I.; Robertson, G.P. A reassessment of the contribution of soybean biological nitrogen fixation to reactive N in the environment. Biogeochemistry 2015, 123, 175–184. [Google Scholar] [CrossRef]

| Depth (cm) | P | N | K | Mg | Fe | Ca | pH |
|---|---|---|---|---|---|---|---|
| mg/kg | H2O | ||||||
| 0–30 | 54.43 | 0.04 | 287.67 | 275.33 | 12.36 | 919.33 | 7.06 |
| 30–60 | 50.10 | 0.03 | 265.33 | 329.33 | 9.85 | 1005.00 | 7.30 |
| Total Phenolic Content (mg GAE/g) | Total Flavonoid Content (mg QE/g) | |||||
|---|---|---|---|---|---|---|
| Harvesting Period (d.a.p.) | Harvesting Period (d.a.p.) | |||||
| Inoculation Level (g) | 150 | 240 | 330 | 150 | 240 | 330 |
| 0 | 31.08 ± 0.54 b | 64.69 ± 1.09 ab | 46.02 ± 3.42 b | 0.46 ± 0.15 ab | 1.36 ± 0.34 ab | 1.62 ± 0.42 a |
| 100 | 39.02 ± 2.20 a | 62.89 ± 2.41 ab | 60.17 ± 1.89 a | 0.64 ± 0.03 a | 1.74 ± 0.34 a | 1.17 ± 0.09 b |
| 200 | 36.83 ± 0.92 a | 88.77 ± 5.40 a | 64.64 ± 0.28 a | 0.48 ± 0.06 ab | 1.45 ± 0.03 ab | 1.66 ± 0.06 a |
| 400 | 37.26 ± 8.45 a | 48.33 ± 1.12 c | 58.07 ± 1.53 a | 0.19 ± 0.04 c | 1.42 ± 0.16 ab | 1.60 ± 0.18 a |
| F-statistics | 0.6 ns | 30.0 *** | 14.3 *** | 4.6 ** | 0.70 ns | 0.36 ns |
| Treatments | CSU |
|---|---|
| T0 | 0.653 a |
| T1 | 0.497 b |
| T2 | 0.239 c |
| T4 | 0.597 ab |
| BULK | 0.294 c |
| Treatments | Shannon-Weaver | Evenness |
|---|---|---|
| T0 | 2.22 a | 0.73 ab |
| T1 | 2.18 a | 0.79 b |
| T2 | 2.06 a | 0.67 a |
| T4 | 2.32 a | 0.76 ab |
| BULK | 1.88 a | 0.66 ab |
| Treatment | ß-Glucosidase Activity (p-Nitrophenol µg/g/h) | Alkaline Phosphatase Activity (p-Nitrophenol µg/g/h) | Acid Phosphatase Activity (p-Nitrophenol µg/g/h) | Urease Activity (NH4-N µg/g/h) | Overall Enzyme Activity (µg/g/h) |
|---|---|---|---|---|---|
| T0 | 1104.585 a | 1646.923 a | 1502.064 a | 73.157 a | 1081.682 |
| T1 | 1084.815 a | 1634.502 a | 1503.120 a | 78.427 a | 1075.216 |
| T2 | 1069.766 a | 1497.299 a | 1599.061 a | 76.059 a | 1060.546 |
| T4 | 1108.302 a | 1559.537 a | 1476.979 a | 74.168 a | 1054.747 |
| Bulk | 1055.473 a | 1712.644 a | 1641.505 a | 88.063 a | 1124.421 |
| Treatment | Dry Matter | N Content | N | 15N | Ndfa | N Fixed | Soil N Uptake |
|---|---|---|---|---|---|---|---|
| g | g | % | ‰ | % | (kg/ha) | (kg/ha) | |
| 150 d.a.p. | |||||||
| T0 | 59.54 ± 2.75 c | 1.29 ± 0.10 b | 2.15 ± 0.08 b | 3.81 ± 0.18 a | 10.23 ± 2.71 c | 2.81 ± 0.79 b | 23.43 ± 1.54 b |
| T1 | 68.02 ± 8.11 b | 1.45 ± 0.23 b | 2.12 ± 0.14 b | 3.42 ± 0.31 ab | 16.27 ± 4.87 b | 4.92 ± 1.60 ab | 24.76 ± 4.16 b |
| T2 | 60.89 ± 5.81 b | 1.28 ± 0.08 b | 2.14 ± 0.08 ab | 2.25 ± 0.24 b | 34.26 ± 3.73 a | 8.95 ± 1.03 a | 17.24 ± 1.50 c |
| T4 | 89.83 ± 8.07 a | 2.12 ± 0.29 a | 2.36 ± 0.26 a | 3.71 ± 0.30 a | 11.72 ± 4.70 c | 5.13 ± 2.51 ab | 38.21 ± 5.73 a |
| 240 d.a.p. | |||||||
| T0 | 53.75 ± 7.49 b | 11.12 ± 4.62 b | 2.15 ± 0.08 ab | 3.81 ± 0.18 a | 10.28 ± 2.71 c | 21.24 ± 5.08 d | 25.78 ± 4.87 ab |
| T1 | 75.75 ± 3.32 a | 15.47 ± 2.37 a | 2.12 ± 0.14 ab | 3.42 ± 0.31 ab | 16.27 ± 4.87 b | 56.66 ± 25.79 ab | 29.08 ± 2.43 a |
| T2 | 74.25 ± 9.83 a | 15.98 ± 1.93 a | 2.14 ± 0.08 ab | 2.25 ± 0.24 b | 34.26 ± 3.73 a | 110.86 ± 17.18 a | 25.31 ± 2.12 ab |
| T4 | 77.50 ± 9.56 a | 17.41 ± 1.40 a | 2.36 ± 0.26 a | 3.71 ± 0.30 a | 11.72 ± 4.71 c | 43.45 ± 19.67 c | 31.81 ± 2.67 a |
| 330 d.a.p. | |||||||
| T0 | 54.22 ± 2.11 b | 12.81 ± 1.36 b | 2.17 ± 0.08 a | 3.10 ± 0.11 a | 11.22 ± 2.10 ab | 27.50 ± 8.64 ab | 23.88 ± 2.94 a |
| T1 | 42.08 ± 1.11 c | 10.62 ± 1.80 b | 2.21 ± 0.07 a | 3.00 ± 0.25 a | 15.38 ± 1.11 a | 37.89 ± 7.42 a | 17.89 ± 9.37 b |
| T2 | 40.89 ± 2.51 c | 18.26 ± 2.17 a | 2.14 ± 0.08 a | 2.00 ± 0.11 b | 14.27 ± 2.11 a | 13.22 ± 7.65 b | 24.49 ± 7.72 a |
| T4 | 86.00 ± 11.07 a | 10.37 ± 1.49 b | 2.07 ± 0.16 a | 3.26 ± 0.11 a | 8.23 ± 0.11 b | 16.11 ± 6.04 b | 19.51 ± 11.27 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makgato, M.J.; Araya, H.T.; du Plooy, C.P.; Mokgehle, S.N.; Mudau, F.N. Effects of Rhizobium Inoculation on N2 Fixation, Phytochemical Profiles and Rhizosphere Soil Microbes of Cancer Bush Lessertia frutescens (L.). Agronomy 2020, 10, 1675. https://doi.org/10.3390/agronomy10111675
Makgato MJ, Araya HT, du Plooy CP, Mokgehle SN, Mudau FN. Effects of Rhizobium Inoculation on N2 Fixation, Phytochemical Profiles and Rhizosphere Soil Microbes of Cancer Bush Lessertia frutescens (L.). Agronomy. 2020; 10(11):1675. https://doi.org/10.3390/agronomy10111675
Chicago/Turabian StyleMakgato, Manaka J., Hintsa T. Araya, Christian P. du Plooy, Salmina N. Mokgehle, and Fhatuwani N. Mudau. 2020. "Effects of Rhizobium Inoculation on N2 Fixation, Phytochemical Profiles and Rhizosphere Soil Microbes of Cancer Bush Lessertia frutescens (L.)" Agronomy 10, no. 11: 1675. https://doi.org/10.3390/agronomy10111675
APA StyleMakgato, M. J., Araya, H. T., du Plooy, C. P., Mokgehle, S. N., & Mudau, F. N. (2020). Effects of Rhizobium Inoculation on N2 Fixation, Phytochemical Profiles and Rhizosphere Soil Microbes of Cancer Bush Lessertia frutescens (L.). Agronomy, 10(11), 1675. https://doi.org/10.3390/agronomy10111675





