Phenotypic Analysis of Germination Time of Individual Seeds Affected by Microenvironment and Management Factors for Cohort Research in Plant Factory
Abstract
1. Introduction
2. Materials and Methods
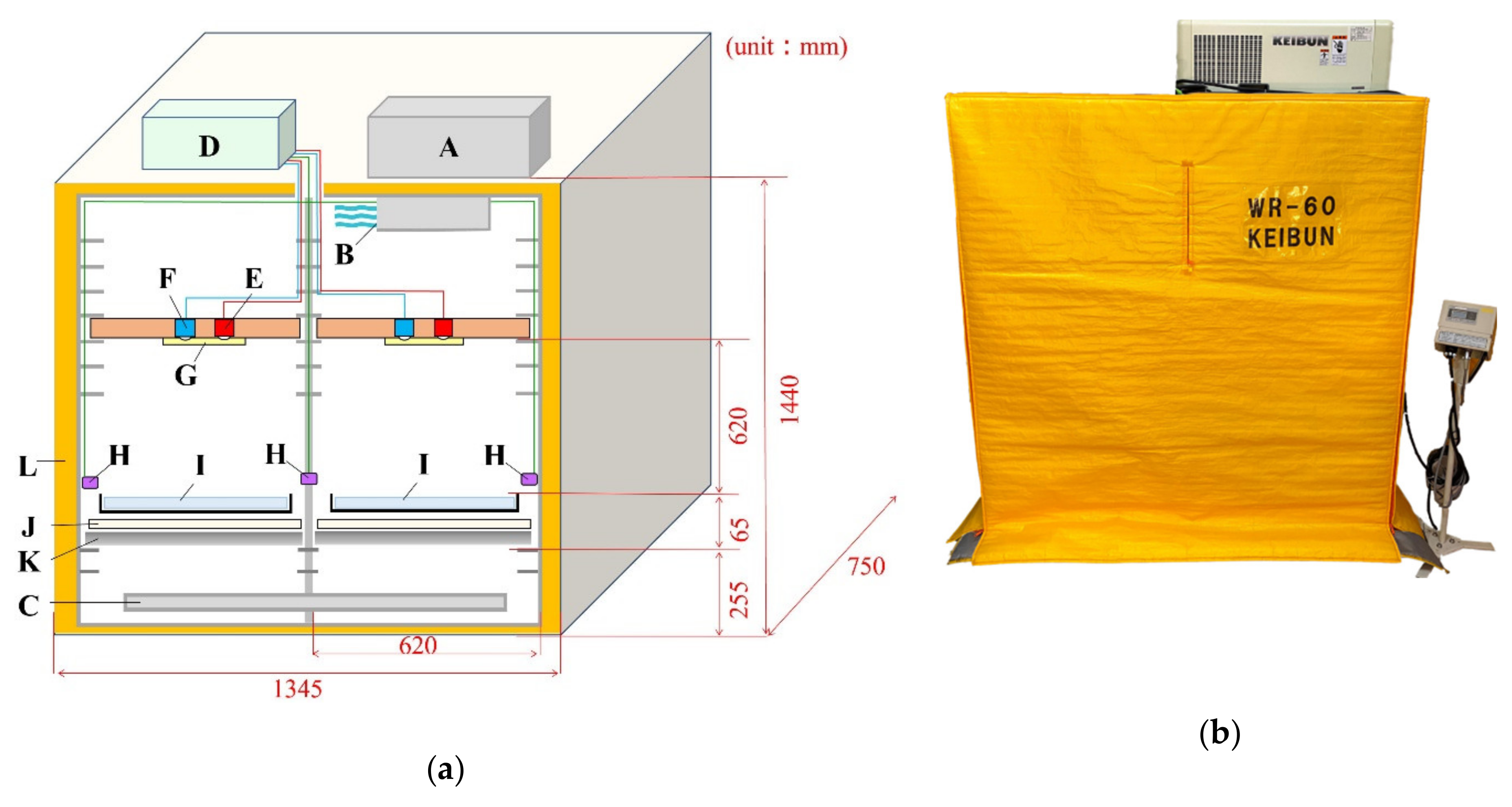
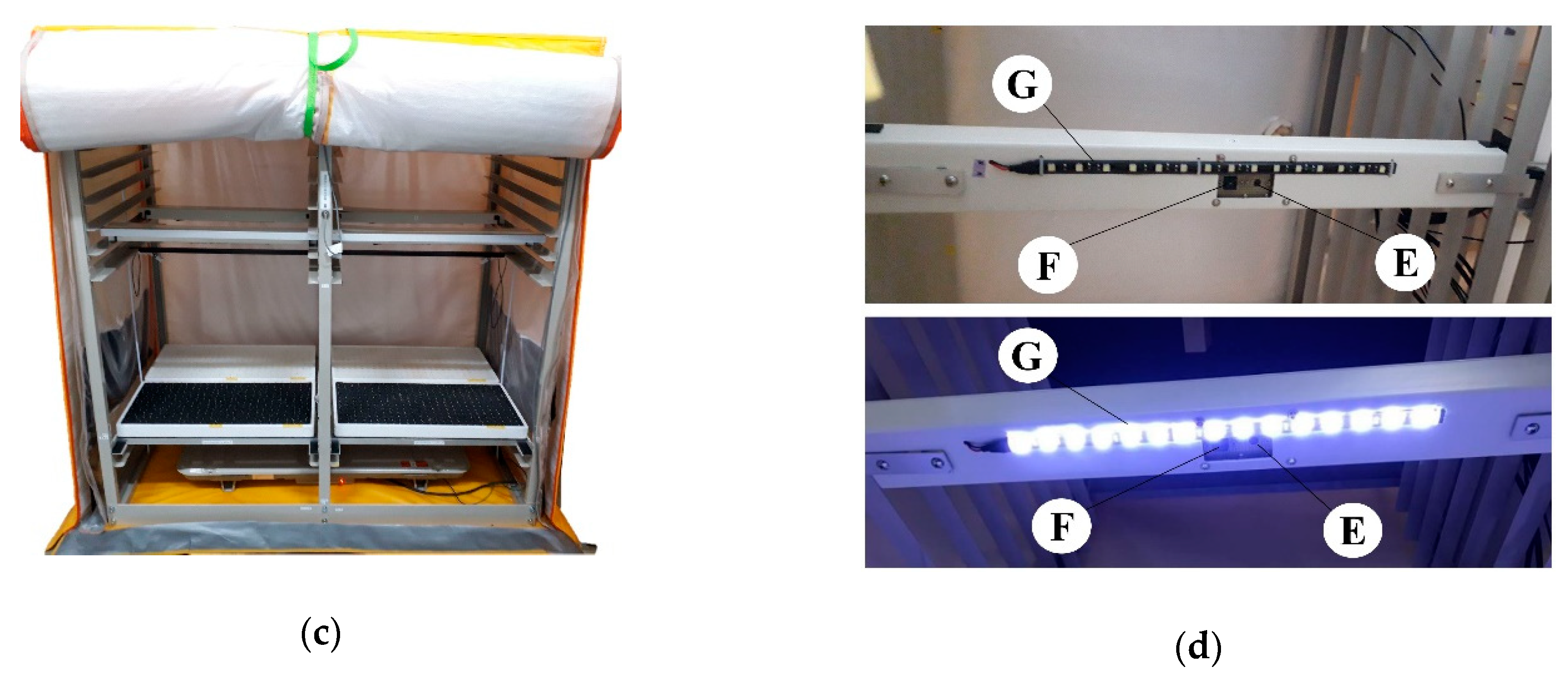
2.1. Description of the System
2.2. Phenotyping and Microenvironmental Sensing Units
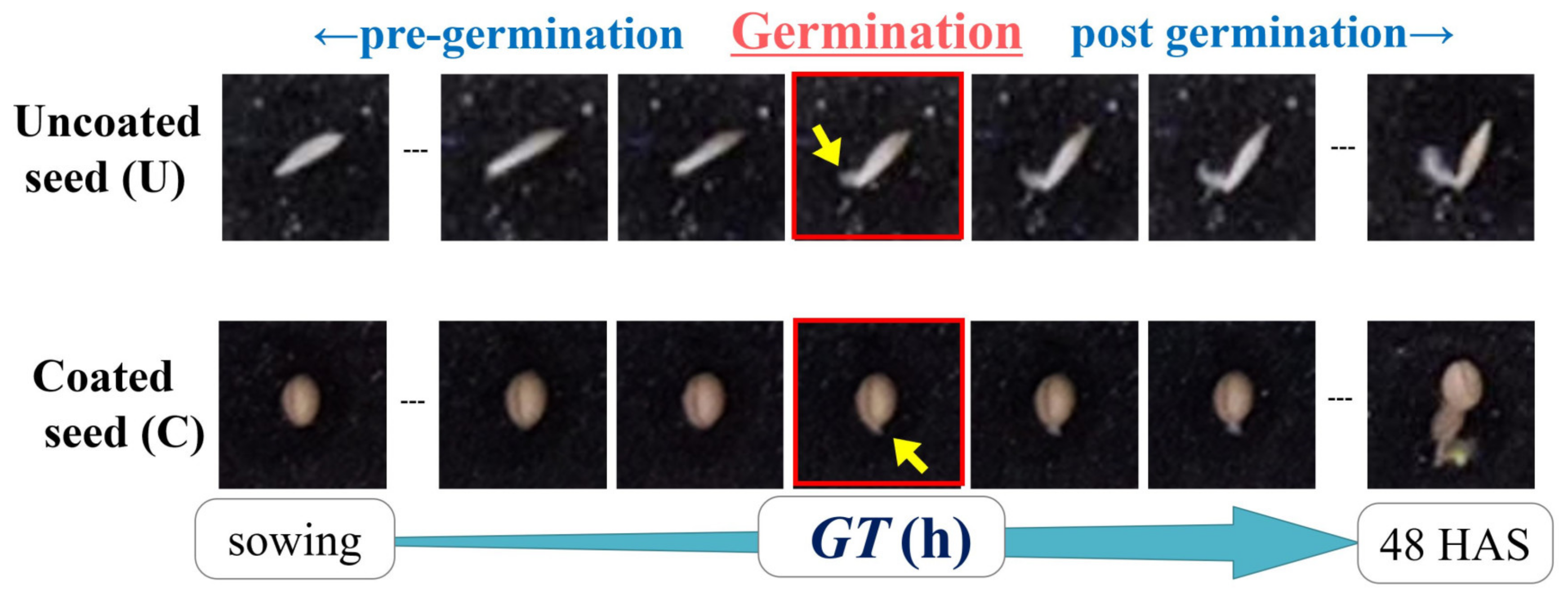
2.3. Germination Time (GT) Acquired from RGB Image
2.4. Experimental Design
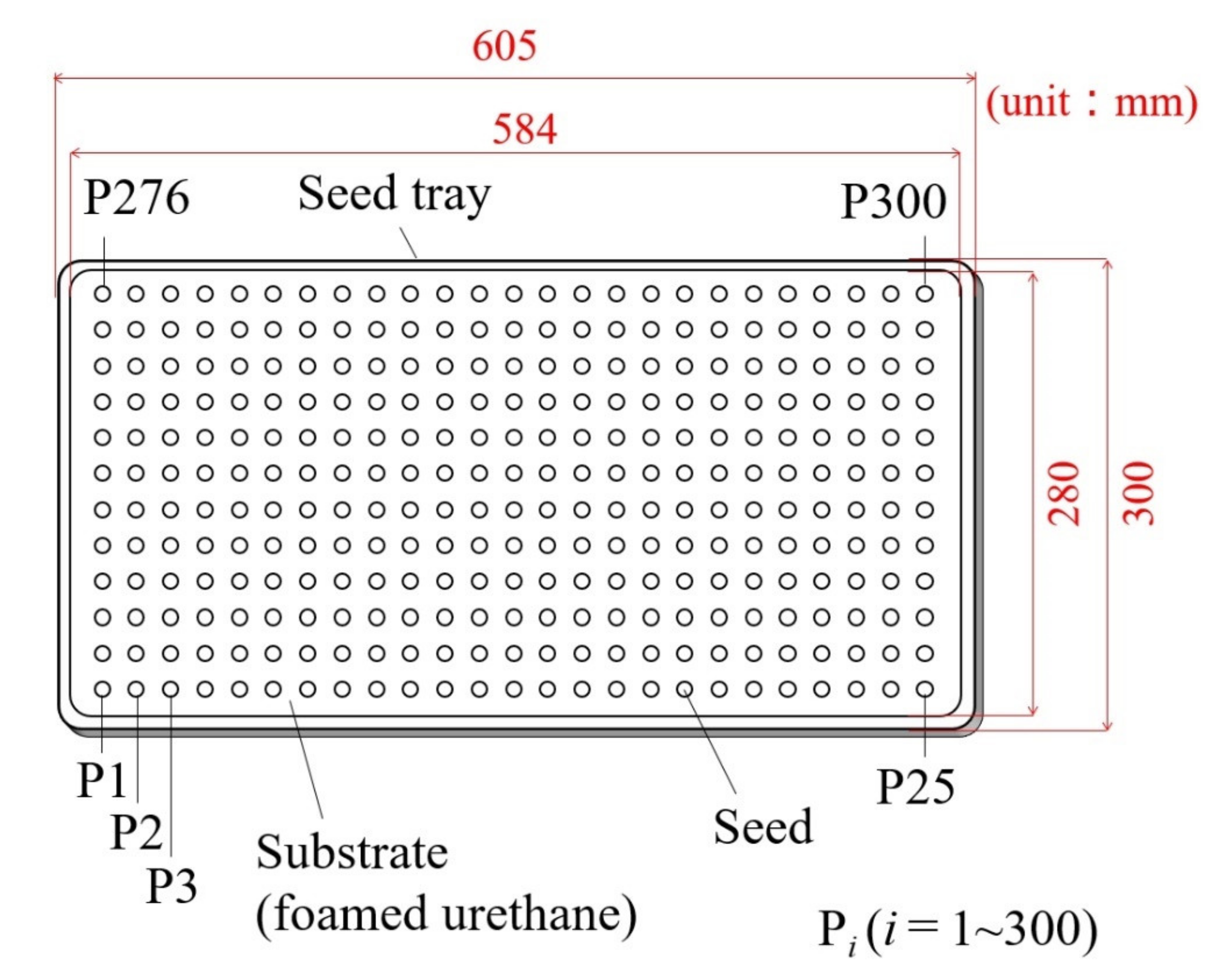
2.4.1. Data Acquisition
2.4.2. Variables and Symbols
2.4.3. Plant Material and Culture Conditions
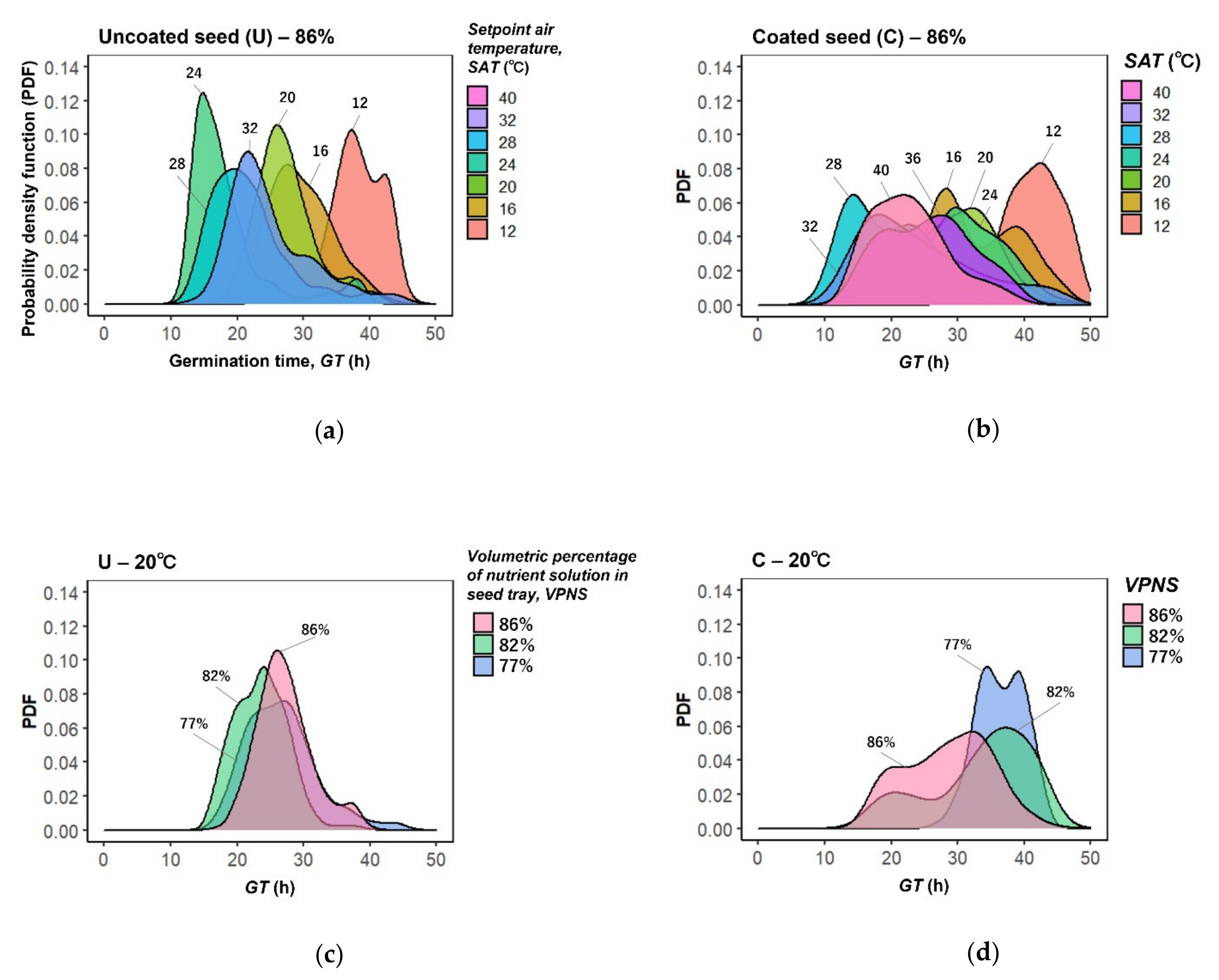
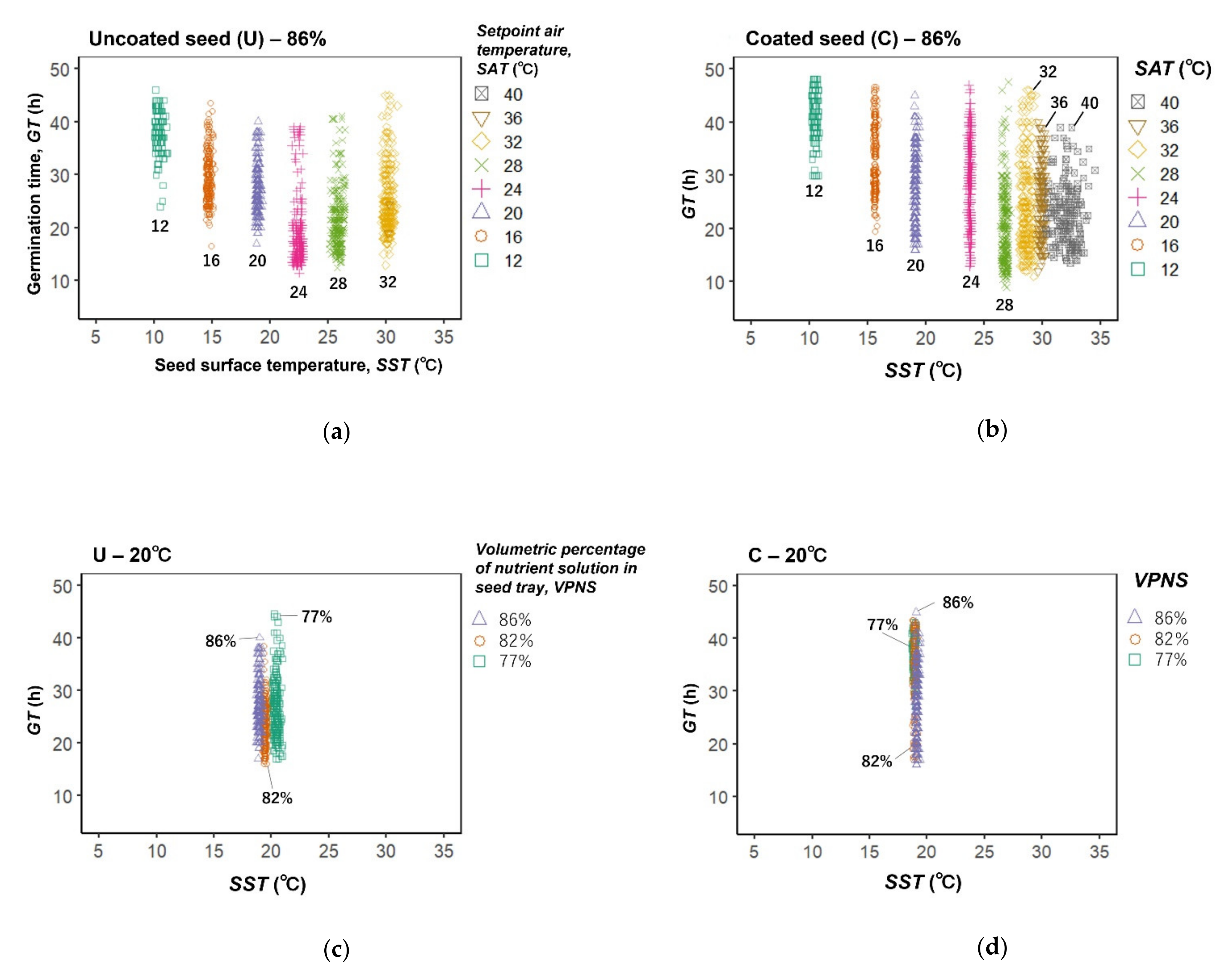
3. Results
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kumar, J.; Pratap, A.; Kumar, S. Phenomics in Crop Plants: Trends, Options and Limitations; Springer: New Delhi, India, 2015; p. 296. [Google Scholar]
- Kozai, T.; Lu, N.; Hasegawa, R.; Nunomura, O.; Nozaki, T.; Amagai, Y. Plant Cohort Research and Its Application. In Smart Plant Factory: The Next Generation Indoor Vertical Farms; Kozai, T., Ed.; Springer: Berlin, Germany, 2018; pp. 413–432. [Google Scholar]
- Fiorani, F.; Schurr, U. Future Scenarios for Plant Phenotyping. Annu. Rev. Plant Biol. 2013, 64, 267–291. [Google Scholar] [CrossRef] [PubMed]
- Golbach, F.; Kootstra, G.; Damjanovic, S.; Otten, G.; Van de Zedde, R. Validation of plant part measurements using a 3D reconstruction method suitable for high-throughput seedling phenotyping. Mach. Vis. Appl. 2016, 27, 663–680. [Google Scholar] [CrossRef]
- Kuijken, R.C.; van Eeuwijk, F.A.; Marcelis, L.F.; Bouwmeester, H.J. Root phenotyping: From component trait in the lab to breeding. J Exp Bot. 2015, 66, 5389–5401. [Google Scholar] [CrossRef] [PubMed]
- Guo, W. Automated Characterization of Plant Growth and Flowering Dynamics Using RGB images. In Smart Plant Factory: The Next Generation Indoor Vertical Farms; Kozai, T., Ed.; Springer: Berlin, Germany, 2018; pp. 385–393. [Google Scholar]
- Ninomiya, S.; Baret, F.; Cheng, Z.-M. Plant phenomics: Emerging transdisciplinary science. Plant Phenomics 2019, 2019, 2765120. [Google Scholar] [CrossRef]
- Goto, E. Plant production in a closed plant factory with artificial lighting. Acta Hortic. 2012, 956, 37–49. [Google Scholar] [CrossRef]
- Kozai, T. Resource use efficiency of closed plant production system with artificial light: Concept, estimation and application to plant factory. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2013, 89, 447–461. [Google Scholar] [CrossRef] [PubMed]
- Leong, R.; Urano, D. Molecular Breeding for Plant Factory: Strategies and technology. In Smart Plant Factory: The Next Generation Indoor Vertical Farms; Kozai, T., Ed.; Springer: Berlin, Germany, 2018; pp. 301–323. [Google Scholar]
- Kozai, T.; Amagai, Y.; Hayashi, E. Towards sustainable plant factories with artificial lighting (PFALs): From greenhouses to vertical farms. In Achieving Sustainable Greenhouse Cultivation; Marcelis, L., Heuvelink, E., Eds.; Burleigh Dodds Science Publishing: Cambridge, UK, 2019; pp. 177–204. [Google Scholar]
- Kozai, T.; Hayashi, E.; Amagai, Y. Plant factories with artificial lighting (PFALs) toward sustainable plant production. Acta Hortic. 2020, 1273, 251–260. [Google Scholar] [CrossRef]
- Ohyama, K.; Yamaguchi, J.; Enjoji, A. Resource Utilization Efficiencies in a Closed System with Artificial Lighting during Continuous Lettuce Production. Agronomy 2020, 10, 723. [Google Scholar] [CrossRef]
- SharathKumar, M.; Heuvelink, E.; Marcelis, L.F.M. Vertical Farming: Moving from Genetic to Environmental Modification. Trends Plant Sci. 2020, 25, 724–727. [Google Scholar] [CrossRef] [PubMed]
- Nagano, S.; Moriyuki, S.; Wakamori, K.; Mineno, H.; Fukuda, H. Leaf-Movement-Based Growth Prediction Model Using Optical Flow Analysis and Machine Learning in Plant Factory. Front. Plant Sci. 2019, 10, 227. [Google Scholar] [CrossRef] [PubMed]
- Soltani, E.; Ghaderi-Far, F.; Baskin, C.C.; Baskin, J.M. Problems with using mean germination time to calculate rate of seed germination. Aust. J. Bot. 2015, 63. [Google Scholar] [CrossRef]
- Bewley, J.D.; Bradford, K.J.; Hilhorst, H.W.M.; Nonogaki, H. Seeds: Physiology of Development, Germination and Dormancy, 3rd ed.; Springer: New York, NY, USA, 2013; p. 392. [Google Scholar]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography, and Evolution of Dormancy and Germination, 2nd ed.; Elsevier/Academic Press: San Diego, CA, USA, 2014; p. 1586. [Google Scholar]
- Gustin, J.L.; Settles, A.M. Seed Phenomics. In Phenomics: How Next-Generation Phenotyping is Revolutionizing Plant Breeding; Fritsche-Neto., R., Borém, A., Eds.; Springer: Cham, Switzerland, 2015; pp. 67–82. [Google Scholar]
- Ducournau, S.; Feutry, A.; Plainchault, P.; Revollon, P.; Vigouroux, B.; Wagner, M.H. Using computer vision to monitor germination time course of sunflower (Helianthus annuus L.) seeds. Seed Sci. Technol. 2005, 33, 329–340. [Google Scholar] [CrossRef]
- Shinohara, T. Seed Vigour as a New Seed Quality lndex and lts Utility. Jpn. Soc. Agric. Technol. Manag. 2009, 16, 1–9. (In Japanese) [Google Scholar]
- Joosen, R.V.; Kodde, J.; Willems, L.A.; Ligterink, W.; van der Plas, L.H.; Hilhorst, H.W. GERMINATOR: A software package for high-throughput scoring and curve fitting of Arabidopsis seed germination. Plant J. 2010, 62, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Ligterink, W.; Hilhorst, H.W.M. High-Throughput Scoring of Seed Germination. In Plant Hormones; Kleine-Vehn, J., Sauer, M., Eds.; Humana Press: New York, NY, USA, 2017; Volume 1497, pp. 57–72. [Google Scholar] [CrossRef]
- Hayashi, E.; Kozai, T. Phenotyping and AI-based Environmental Control and Breeding for PFAL. In Smart Plant Factory: The Next Generation Indoor Vertical Farms; Kozai, T., Ed.; Springer: Berlin, Germany, 2018; pp. 405–411. [Google Scholar]
- Hasegawa, R. Data warehouse for plant phenotyping in plant factories. In Proceedings of the Meeting of Japanese Society of Agricultural, Biological and Environmental Engineers and Scientists (JSABEES), Tokyo, Japan, 18–21 September 2018; pp. 224–225. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing:Vienna, Austria, 2019. Available online: https://www.r-project.org/ (accessed on 8 August 2020).
- International Seed Testing Association (ISTA). International Rules for Seed Testing. International Seed Testing Association: Zurich, Switzerland, 2019. Available online: https://www.seedtest.org/en/international-rules-for-seed-testing-_content---1--1083 (accessed on 17 April 2020).
- Maruo, T.; Ito, T.; Ishii, S. Studies on the feasible management of nutrient solution in hydroponically growth lettuce (Lactuca sativa L.). Tech. Bull. Fac. Hort. Chiba Univ 1992, 46, 235–240. [Google Scholar]


| Symbol | Variable name/Description | Unit |
|---|---|---|
| AT | Air temperature inside the germination container box (GCB) | °C |
| Average AT at 48 h after sowing (HAS) | ||
| C | Coated seed | - |
| GP | Germination percentage per 300 seeds 48 HAS | % |
| GR | Hourly germination rate of individual seed (inverse of GT) | h−1 |
| Average GR per 300 seeds (GP divided by ) | % h−1 | |
| GT | Germination time course (from seed sowing to outbreak of the radicle from seed coat) of individual seed | h |
| Average GT per 300 seeds | ||
| SST | Seed surface temperature of individual seed | °C |
| Average SST per 300 seeds 48 HAS | ||
| SAT | Setpoint air temperature of GCB | °C |
| U | Uncoated seed | - |
| VPNS | Volumetric percentage of nutrient solution in seed tray | % |
| Treatments | Measured | Calculated | |
|---|---|---|---|
| (1) | SAT: 12 °C, 16 °C, 20 °C, 24 °C, 28 °C, 32 °C, 36 °C, 40 °C | GT, SST, AT, GP | GR, CVt (St/) CVt (St/) |
| VPNS: 86% | |||
| Seed type: U and C | |||
| (2) | SAT: 20 °C | ||
| VPNS: 77%, 82%, 86% | |||
| Seed type: U and C | |||
| Seed Type | VPNS (%) | SAT (°C) | Measured Value | Calculated Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 (°C) | (h) | (°C) | GP (%) | Identified Percent 2 (%) | [GP/] (% h−1) | |||||
| U | 86 | 40 | 39.4 | 25.0 | 33.3 | 0 | - | - | 0.04 | 0.00 |
| 36 | 35.9 | 21.8 | 30.3 | 1 | 67 | 0.2 | 0.02 | 0.05 | ||
| 32 | 31.9 | 24.9 | 30.2 | 81 | 98 | 0.2 | 0.01 | 3.25 | ||
| 28 | 27.9 | 21.7 | 25.9 | 100 | 83 | 0.3 | 0.01 | 4.60 | ||
| 24 | 24.0 | 18.6 | 22.5 | 100 | 77 | 0.3 | 0.01 | 5.38 | ||
| 20 | 20.4 | 27.2 | 18.9 | 100 | 90 | 0.2 | 0.01 | 3.68 | ||
| 16 | 16.4 | 29.7 | 14.7 | 100 | 79 | 0.2 | 0.01 | 3.36 | ||
| 12 | 12.9 | 38.2 | 10.4 | 100 | 46 | 0.1 | 0.02 | 2.62 | ||
| 82 | 20 | 19.9 | 23.6 | 19.4 | 100 | 86 | 0.2 | 0.00 | 4.23 | |
| 77 | 20 | 20.0 | 26.7 | 20.4 | 100 | 89 | 0.2 | 0.01 | 3.74 | |
| C | 86 | 40 | 38.3 | 22.8 | 32.2 | 91 | 62 | 0.3 | 0.03 | 3.98 |
| 36 | 35.2 | 25.6 | 30.0 | 86 | 83 | 0.3 | 0.01 | 3.36 | ||
| 32 | 31.9 | 24.3 | 28.7 | 79 | 97 | 0.4 | 0.01 | 3.24 | ||
| 28 | 27.9 | 20.2 | 26.8 | 99 | 95 | 0.4 | 0.01 | 4.93 | ||
| 24 | 25.8 | 29.2 | 23.8 | 100 | 99 | 0.2 | 0.00 | 3.42 | ||
| 20 | 20.4 | 28.5 | 19.1 | 100 | 86 | 0.2 | 0.00 | 3.51 | ||
| 16 | 16.4 | 33.0 | 15.6 | 100 | 81 | 0.2 | 0.01 | 3.02 | ||
| 12 | 12.3 | 41.1 | 10.4 | 100 | 47 | 0.1 | 0.02 | 2.43 | ||
| 82 | 20 | 19.9 | 33.4 | 18.9 | 100 | 42 | 0.2 | 0.01 | 3.00 | |
| 77 | 20 | 19.9 | 36.5 | 18.7 | 48 | 83 | 0.1 | 0.01 | 1.32 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayashi, E.; Amagai, Y.; Maruo, T.; Kozai, T. Phenotypic Analysis of Germination Time of Individual Seeds Affected by Microenvironment and Management Factors for Cohort Research in Plant Factory. Agronomy 2020, 10, 1680. https://doi.org/10.3390/agronomy10111680
Hayashi E, Amagai Y, Maruo T, Kozai T. Phenotypic Analysis of Germination Time of Individual Seeds Affected by Microenvironment and Management Factors for Cohort Research in Plant Factory. Agronomy. 2020; 10(11):1680. https://doi.org/10.3390/agronomy10111680
Chicago/Turabian StyleHayashi, Eri, Yumiko Amagai, Toru Maruo, and Toyoki Kozai. 2020. "Phenotypic Analysis of Germination Time of Individual Seeds Affected by Microenvironment and Management Factors for Cohort Research in Plant Factory" Agronomy 10, no. 11: 1680. https://doi.org/10.3390/agronomy10111680
APA StyleHayashi, E., Amagai, Y., Maruo, T., & Kozai, T. (2020). Phenotypic Analysis of Germination Time of Individual Seeds Affected by Microenvironment and Management Factors for Cohort Research in Plant Factory. Agronomy, 10(11), 1680. https://doi.org/10.3390/agronomy10111680





