Effects of Drip Irrigation Emitter Density with Various Irrigation Levels on Physiological Parameters, Root, Yield, and Quality of Cherry Tomato
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site and Materials
2.2. Experimental Design
2.3. Sampling and Measurements
2.4. Statistical Analyses
3. Results
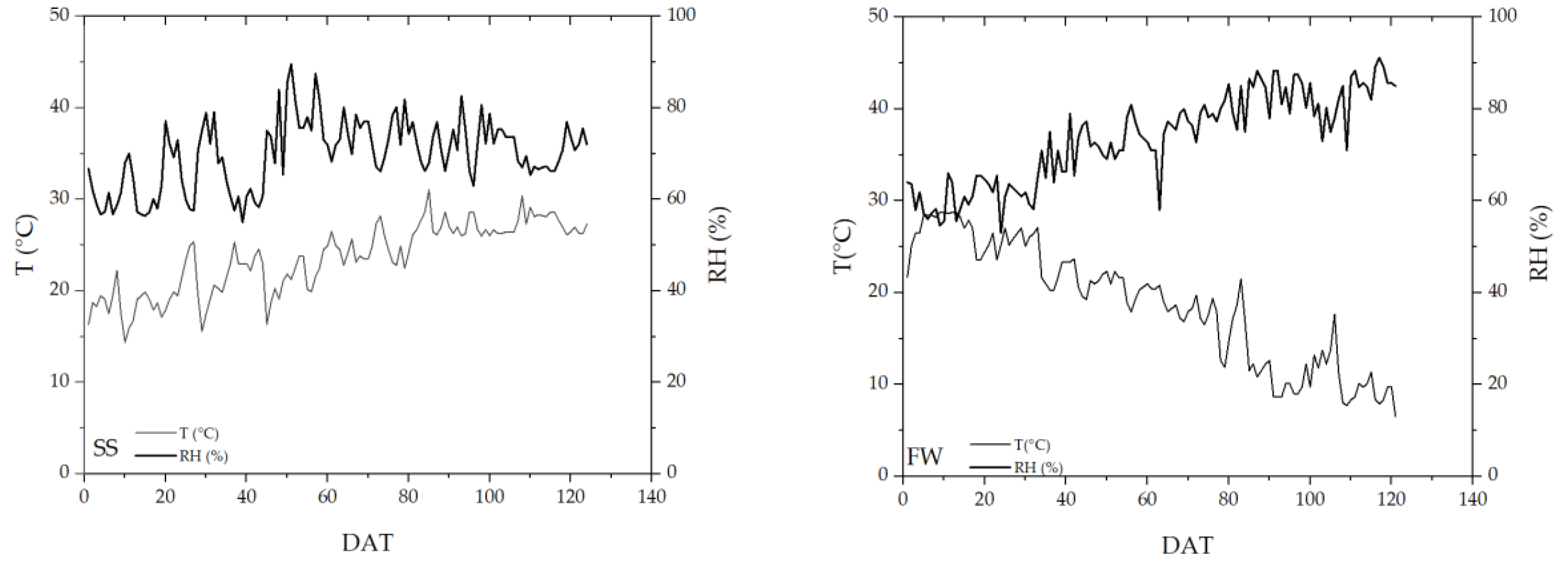
3.1. Greenhouse Climate
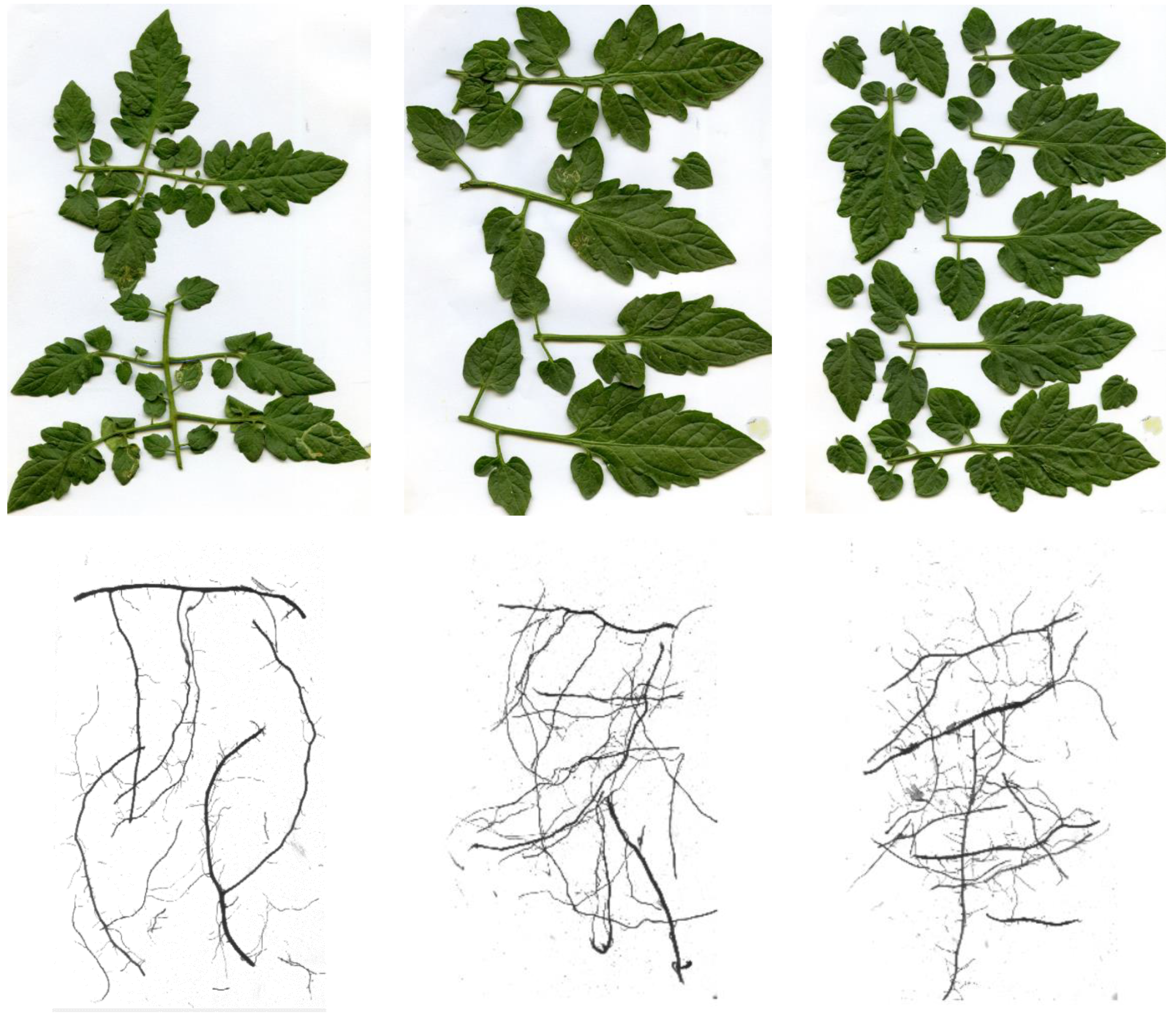
3.2. Root Morphology
3.3. Leaf Morphology and Yield
3.4. Plant Physiological Parameters
3.5. Fruit Morphology and Fruit Quality
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Padayachee, A.; Day, L.; Howell, K.; Gidley, M. Complexity and health functionality of plant cell wall fibers from fruits and vegetables. Crit. Rev. Food Sci. Nutr. 2017, 57, 59–81. [Google Scholar] [CrossRef]
- Sturm, R.; An, R. Obesity and economic environments. CA A Cancer J. Clin. 2014, 64, 337–350. [Google Scholar] [CrossRef] [PubMed]
- Ilahy, R.; Piro, G.; Tlili, I.; Riahi, A.; Sihem, R.; Ouerghi, I.; Hdider, C.; Lenucci, M.S. Fractionate analysis of the phytochemical composition and antioxidant activities in advanced breeding lines of high-lycopene tomatoes. Food Funct. 2016, 7, 574–583. [Google Scholar] [CrossRef]
- Heber, D. Colorful Cancer Prevention: A-Carotene, Lycopene, and Lung Cancer. Am. J. Clin. Nutr. 2000, 72, 901–902. [Google Scholar] [CrossRef]
- Toor, R.K.; Savage, G.P. Antioxidant activity in different fractions of tomatoes. Food Res. Int. 2005, 38, 487–494. [Google Scholar] [CrossRef]
- Zhang, Y.-J.; Gan, R.-Y.; Li, S.; Zhou, Y.; Li, A.-N.; Xu, D.-P.; Li, H.-B. Antioxidant phytochemicals for the prevention and treatment of chronic diseases. Molecules 2015, 20, 21138–21156. [Google Scholar] [CrossRef] [PubMed]
- Gruda, N. Impact of environmental factors on product quality of greenhouse vegetables for fresh consumption. Crit. Rev. Plant. Sci. 2005, 24, 227–247. [Google Scholar] [CrossRef]
- Flores, F.B.; Sanchez-Bel, P.; Estan, M.T.; Martinez-Rodriguez, M.M.; Moyano, E.; Morales, B.; Campos, J.F.; Garcia-Abellán, J.O.; Egea, M.I.; Fernández-Garcia, N. The effectiveness of grafting to improve tomato fruit quality. Sci. Hortic. 2010, 125, 211–217. [Google Scholar] [CrossRef]
- Giuliani, M.M.; Nardella, E.; Gatta, G.; De Caro, A.; Quitadamo, M. Processing tomato cultivated under water deficit conditions: The effect of azoxystrobin. III Int. Symp. Tomato Dis. 2010, 914, 287–294. [Google Scholar] [CrossRef]
- Merah, O. Potential importance of water status traits for durum wheat improvement under mediterranean conditions. J. Agric. Sci. 2001, 137, 139–145. [Google Scholar] [CrossRef]
- Kato, Y.; Kamoshita, A.; Yamagishi, J. Preflowering abortion reduces spikelet number in upland rice (oryza sativa l.) under water stress. Crop. Sci. 2008, 48, 2389–2395. [Google Scholar] [CrossRef]
- Naseri, A.; Rezania, A.R.; Albaji, M. Investigation of soil quality for different irrigation systems in lali plain, iran. J. Food Agric. Environ. 2009, 7, 955–960. [Google Scholar]
- Beltrano, J.; Ronco, M.; Montaldi, E. Drought stress syndrome in wheat is provoked by ethylene evolution imbalance and reversed by rewatering, aminoethoxyvinylglycine, or sodium benzoate. J. Plant. Growth Regul. 1999, 18, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Shabbir, A.; Arshad, M.; Bakhsh, A.; Usman, M.; Shakoor, A.; Ahmad, I.; Ahmad, A. Apparent and real water productivity for cotton-wheat zone of punjab, pakistan. Pak. J. Agri. Sci. 2012, 49, 357–363. [Google Scholar]
- Zhai, Y.; Shao, X.; Xing, W.; Wang, Y.; Hung, T.; Xu, H. Effects of drip irrigation regimes on tomato fruit yield and water use efficiency. J. Food Agric. Environ. 2010, 8, 709–713. [Google Scholar]
- Gerçek, S.; Demirkaya, M.; Işik, D. Water pillow irrigation versus drip irrigation with regard to growth and yield of tomato grown under greenhouse conditions in a semi-arid region. Agric. Water Manag. 2017, 180, 172–177. [Google Scholar] [CrossRef]
- Fereres, E.; Soriano, M.A. Deficit irrigation for reducing agricultural water use. J. Exp. Bot. 2006, 58, 147–159. [Google Scholar] [CrossRef]
- Nagaz, K.; Masmoudi, M.; Ben Mechlia, N. Yield response of drip irrigated onion under full and deficit irrigation with saline water in arid regions of tunisia. ISRN Agron. 2012, 212, 562315. [Google Scholar] [CrossRef]
- Sezen, S.M.; Yazar, A.; Daşgan, Y.; Yucel, S.; Akyıldız, A.; Tekin, S.; Akhoundnejad, Y. Evaluation of crop water stress index (cwsi) for red pepper with drip and furrow irrigation under varying irrigation regimes. Agric. Water Manag. 2014, 143, 59–70. [Google Scholar] [CrossRef]
- Çolak, Y.B.; Yazar, A.; Sesveren, S.; Colak, I. Evaluation of yield and leaf water potantial (lwp) for eggplant under varying irrigation regimes using surface and subsurface drip systems. Sci. Hortic. 2017, 219, 10–21. [Google Scholar] [CrossRef]
- Lu, J.; Shao, G.; Cui, J.; Wang, X.; Keabetswe, L. Yield, fruit quality and water use efficiency of tomato for processing under regulated deficit irrigation: A meta-analysis. Agric. Water Manag. 2019, 222, 301–312. [Google Scholar] [CrossRef]
- Rasool, G.; Guo, X.; Wang, Z.; Chen, S.; Ullah, I. The interactive responses of fertigation levels under buried straw layer on growth, physiological traits and fruit yield in tomato plant. J. Plant. Interact. 2019, 14, 552–563. [Google Scholar] [CrossRef]
- Besharat, S.; Nazemi, A.H.; Sadraddini, A.A. Parametric modeling of root length density and root water uptake in unsaturated soil. Turk. J. Agric. For. 2010, 34, 439–449. [Google Scholar]
- Li, Q.; Dong, B.; Qiao, Y.; Liu, M.; Zhang, J. Root growth, available soil water, and water-use efficiency of winter wheat under different irrigation regimes applied at different growth stages in north china. Agric. Water Manag. 2010, 97, 1676–1682. [Google Scholar] [CrossRef]
- Xia, J.; Liu, M.-Y.; Jia, S.-F. Water security problem in north china: Research and perspective. Pedospher 2005, 15, 563–575. [Google Scholar]
- Fageria, N. Influence of dry matter and length of roots on growth of five field crops at varying soil zinc and copper levels. J. Plant. Nutr. 2005, 27, 1517–1523. [Google Scholar] [CrossRef]
- Li, X.; Šimůnek, J.; Shi, H.; Yan, J.; Peng, Z.; Gong, X. Spatial distribution of soil water, soil temperature, and plant roots in a drip-irrigated intercropping field with plastic mulch. Eur. J. Agron. 2017, 83, 47–56. [Google Scholar] [CrossRef]
- Wang, C.; Liu, W.; Li, Q.; Ma, D.; Lu, H.; Feng, W.; Xie, Y.; Zhu, Y.; Guo, T. Effects of different irrigation and nitrogen regimes on root growth and its correlation with above-ground plant parts in high-yielding wheat under field conditions. Field Crop. Res. 2014, 165, 138–149. [Google Scholar] [CrossRef]
- Bar-Yosef, B. Advances in fertigation. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 1999; Volume 65, pp. 1–77. [Google Scholar]
- Palomo, M.; Moreno, F.; Fernández, J.; Dıaz-Espejo, A.; Girón, I. Determining water consumption in olive orchards using the water balance approach. Agric. Water Manag. 2002, 55, 15–35. [Google Scholar] [CrossRef]
- Chu, G.; Chen, T.; Wang, Z.; Yang, J.; Zhang, J. Reprint of “morphological and physiological traits of roots and their relationships with water productivity in water-saving and drought-resistant rice”. Field Crop. Res. 2014, 165, 36–48. [Google Scholar] [CrossRef]
- Ondrasek, G.; Romic, D.; Romic, M.; Tomic, F.; Mustac, I. Salt distribution in peat substrate grown with melon (cucumis melo l.). Int. Symp. Grow. Media 2005, 779, 307–312. [Google Scholar] [CrossRef]
- Valdés, R.; Miralles, J.; Ochoa, J.; Bañón, S.; Sánchez-Blanco, M.J. The number of emitters alters salt distribution and root growth in potted gerbera. HortScience 2014, 49, 160–165. [Google Scholar] [CrossRef]
- Qiu, R.; Du, T.; Kang, S. Root length density distribution and associated soil water dynamics for tomato plants under furrow irrigation in a solar greenhouse. J. Arid Land 2017, 9, 637–650. [Google Scholar] [CrossRef]
- Wang, X.; Yun, J.; Shi, P.; Li, Z.; Li, P.; Xing, Y. Root growth, fruit yield and water use efficiency of greenhouse grown tomato under different irrigation regimes and nitrogen levels. J. Plant. Growth Regul. 2019, 38, 400–415. [Google Scholar] [CrossRef]
- Zotarelli, L.; Scholberg, J.M.; Dukes, M.D.; Muñoz-Carpena, R.; Icerman, J. Tomato yield, biomass accumulation, root distribution and irrigation water use efficiency on a sandy soil, as affected by nitrogen rate and irrigation scheduling. Agric. Water Manag. 2009, 96, 23–34. [Google Scholar] [CrossRef]
- Wang, X.; Xing, Y. Evaluation of the effects of irrigation and fertilization on tomato fruit yield and quality: A principal component analysis. Sci. Rep. 2017, 7, 350. [Google Scholar] [CrossRef]
- Huang, S.; Yan, H.; Zhang, C.; Wang, G.; Acquah, S.J.; Yu, J.; Li, L.; Ma, J.; Darko, R.O. Modeling evapotranspiration for cucumber plants based on the shuttleworth-wallace model in a venlo-type greenhouse. Agric. Water Manag. 2020, 228, 105861. [Google Scholar] [CrossRef]
- Hao, L.; Duan, A.-W.; Li, F.-S.; Sun, J.-S.; Wang, Y.-C.; Sun, C.-T. Drip irrigation scheduling for tomato grown in solar greenhouse based on pan evaporation in north china plain. J. Integr. Agric. 2013, 12, 520–531. [Google Scholar]
- Kurunç, A.; Ünlükara, A. Growth, yield, and water use of okra (abelmoschus esculentus) and eggplant (solanum melongena) as influenced by rooting volume. N. Z. J. Crop. Hortic. Sci. 2009, 37, 201–210. [Google Scholar] [CrossRef]
- Wang, W.-H.; Chen, J.; Liu, T.-W.; Chen, J.; Han, A.-D.; Simon, M.; Dong, X.-J.; He, J.-X.; Zheng, H.-L. Regulation of the calcium-sensing receptor in both stomatal movement and photosynthetic electron transport is crucial for water use efficiency and drought tolerance in arabidopsis. J. Exp. Bot. 2014, 65, 223–234. [Google Scholar] [CrossRef]
- Oikeh, S.; Kling, J.; Horst, W.; Chude, V.; Carsky, R. Growth and distribution of maize roots under nitrogen fertilization in plinthite soil. Field Crop. Res. 1999, 62, 1–13. [Google Scholar] [CrossRef]
- Ryser, P.; Lambers, H. Root and leaf attributes accounting for the performance of fast-and slow-growing grasses at different nutrient supply. Plant and Soil 1995, 170, 251–265. [Google Scholar] [CrossRef]
- Donald, C.; Hamblin, J. The biological yield and harvest index of cereals as agronomic and plant breeding criteria. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 1976; Volume 28, pp. 361–405. [Google Scholar]
- Gur, A.; Osorio, S.; Fridman, E.; Fernie, A.R.; Zamir, D. Hi2-1, a qtl which improves harvest index, earliness and alters metabolite accumulation of processing tomatoes. Theor. Appl. Genet. 2010, 121, 1587–1599. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yan, S.; Fan, J.; Zhang, F.; Xiang, Y.; Zheng, J.; Guo, J. Responses of growth, fruit yield, quality and water productivity of greenhouse tomato to deficit drip irrigation. Sci. Hortic. 2021, 275, 109710. [Google Scholar] [CrossRef]
- Rosales, M.A. Producción y Calidad Nutricional en Frutos de Tomate Cherry Cultivados en dos Invernaderos Mediterráneos Experimentales: Respuestas Metabólicas y Fisiológicas. Ph.D. Thesis, Universidad de Granada, Granada, Spain, 2008. [Google Scholar]
- Forde, B.; Lorenzo, H. The nutritional control of root development. Plant. Soil 2001, 232, 51–68. [Google Scholar] [CrossRef]
- Liu, C.; Jin, S.; Zhou, L.; Jia, Y.; Li, F.; Xiong, Y.; Li, X. Effects of plastic film mulch and tillage on maize productivity and soil parameters. Eur. J. Agron. 2009, 31, 241–249. [Google Scholar] [CrossRef]
- Ren, X.; Jia, Z.; Chen, X. Rainfall concentration for increasing corn production under semiarid climate. Agric. Water Manag. 2008, 95, 1293–1302. [Google Scholar] [CrossRef]
- Valdés, R.; Ochoa, J.; Sánchez-Blanco, M.; Franco, J.; Bañón, S. Irrigation volume and the number of emitters per pot affect root growth and saline ion contents in weeping fig. Agric. Agric. Sci. Procedia 2015, 4, 356–364. [Google Scholar] [CrossRef]
- Guo, X.; Kang, S.; Suo, L. Effects of regulated deficit irrigation on root growth in maize. Irrig. Drain. 2001, 20, 25–27. [Google Scholar]
- Jha, S.K.; Gao, Y.; Liu, H.; Huang, Z.; Wang, G.; Liang, Y.; Duan, A. Root development and water uptake in winter wheat under different irrigation methods and scheduling for north china. Agric. Water Manag. 2017, 182, 139–150. [Google Scholar] [CrossRef]
- Garnier, E.; Salager, J.-L.; Laurent, G.; Sonié, L. Relationships between photosynthesis, nitrogen and leaf structure in 14 grass species and their dependence on the basis of expression. New Phytol. 1999, 143, 119–129. [Google Scholar] [CrossRef]
- Wahl, S.; Ryser, P. Root tissue structure is linked to ecological strategies of grasses. New Phytol. 2000, 148, 459–471. [Google Scholar] [CrossRef]
- Hummel, I.; Vile, D.; Violle, C.; Devaux, J.; Ricci, B.; Blanchard, A.; Garnier, É.; Roumet, C. Relating root structure and anatomy to whole-plant functioning in 14 herbaceous mediterranean species. New Phytol. 2007, 173, 313–321. [Google Scholar] [CrossRef]
- Sorgonà, A.; Abenavoli, M.R.; Cacco, G.; Gelsomino, A. Growth of tomato and zucchini seedlings in orange waste compost media: Ph and implication of dosage. Compos. Sci. Util. 2011, 19, 189–196. [Google Scholar] [CrossRef]
- Wang, X.; Wang, W.; Huang, J.; Peng, S.; Xiong, D. Diffusional conductance to co2 is the key limitation to photosynthesis in salt-stressed leaves of rice (oryza sativa). Physiol. Plant. 2018, 163, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Takai, T.; Kondo, M.; Yano, M.; Yamamoto, T. A quantitative trait locus for chlorophyll content and its association with leaf photosynthesis in rice. Rice 2010, 3, 172–180. [Google Scholar] [CrossRef]
- Lawlor, D.W.; Cornic, G. Photosynthetic carbon assimilation and associated metabolism in relation to water deficits in higher plants. Plant Cell Environ. 2002, 25, 275–294. [Google Scholar] [CrossRef] [PubMed]
- Hanping, M.; Ullah, I.; Jiheng, N.; Javed, Q.; Azeem, A. Estimating tomato water consumption by sap flow measurement in response to water stress under greenhouse conditions. J. Plant. Interact. 2017, 12, 402–413. [Google Scholar] [CrossRef]
- Agbna, G.H.; Dongli, S.; Zhipeng, L.; Elshaikh, N.A.; Guangcheng, S.; Timm, L.C. Effects of deficit irrigation and biochar addition on the growth, yield, and quality of tomato. Sci. Hortic. 2017, 222, 90–101. [Google Scholar] [CrossRef]
- Kuşçu, H.; Turhan, A.; Demir, A.O. The response of processing tomato to deficit irrigation at various phenological stages in a sub-humid environment. Agric. Water Manag. 2014, 133, 92–103. [Google Scholar] [CrossRef]
- Zhang, H.; Xiong, Y.; Huang, G.; Xu, X.; Huang, Q. Effects of water stress on processing tomatoes yield, quality and water use efficiency with plastic mulched drip irrigation in sandy soil of the hetao irrigation district. Agric. Water Manag. 2017, 179, 205–214. [Google Scholar] [CrossRef]
- Hooshmand, M.; Albaji, M.; Ansari, N.A.Z. The effect of deficit irrigation on yield and yield components of greenhouse tomato (solanum lycopersicum) in hydroponic culture in ahvaz region, iran. Sci. Hortic. 2019, 254, 84–90. [Google Scholar] [CrossRef]
- Kulkarni, M.; Phalke, S. Evaluating variability of root size system and its constitutive traits in hot pepper (capsicum annum l.) under water stress. Sci. Hortic. 2009, 120, 159–166. [Google Scholar] [CrossRef]
- Mohawesh, O. Field evaluation of deficit irrigation effects on tomato growth performance, water-use efficiency and control of parasitic nematode infection. South. Afr. J. Plant. Soil 2016, 33, 125–132. [Google Scholar] [CrossRef]
- Aguirre, L.; Johnson, D.A. Root morphological development in relation to shoot growth in seedlings of four range grasses. Rangel. Ecol. Manag. J. Range Manag. Arch. 1991, 44, 341–346. [Google Scholar] [CrossRef]
- Flenet, F.; Bouniols, A.; Saraiva, C. Sunflower response to a range of soil water contents. Eur. J. Agron. 1996, 5, 161–167. [Google Scholar] [CrossRef]
- Moccia, S.; Chiesa, A.; Oberti, A.; Tittonell, P. Yield and quality of sequentially grown cherry tomato and lettuce under long-term conventional, low-input and organic soil management systems. Eur. J. Hortic. Sci. 2006, 71, 183–191. [Google Scholar]
- Chen, G.; Cao, H.; Liang, J.; Ma, W.; Guo, L.; Zhang, S.; Jiang, R.; Zhang, H.; Goulding, K.W.; Zhang, F. Factors affecting nitrogen use efficiency and grain yield of summer maize on smallholder farms in the north china plain. Sustainability 2018, 10, 363. [Google Scholar] [CrossRef]
- Giuliani, M.; Nardella, E.; Gagliardi, A.; Gatta, G. Deficit irrigation and partial root-zone drying techniques in processing tomato cultivated under mediterranean climate conditions. Sustainability 2017, 9, 2197. [Google Scholar] [CrossRef]
- Sharayeie, P.; Sobhani, A.; Rahimian, M. Effect of different levels of irrigation water and potassium fertilizer on water use efficiency and quality of tomato fruit of petvarli ch. J. Agric. Eng. Res. 2006, 27, 75–86. [Google Scholar]
- Wang, C.; Gu, F.; Chen, J.; Yang, H.; Jiang, J.; Du, T.; Zhang, J. Assessing the response of yield and comprehensive fruit quality of tomato grown in greenhouse to deficit irrigation and nitrogen application strategies. Agric. Water Manag. 2015, 161, 9–19. [Google Scholar] [CrossRef]
- Du, Y.-D.; Cao, H.-X.; Liu, S.-Q.; Gi, X.-B.; Cao, Y.-X. Response of yield, quality, water and nitrogen use efficiency of tomato to different levels of water and nitrogen under drip irrigation in northwestern china. J. Integr. Agric. 2017, 16, 1153–1161. [Google Scholar] [CrossRef]
- Machado, R.M.; Maria do Rosàrio, G.O. Tomato root distribution, yield and fruit quality under different subsurface drip irrigation regimes and depths. Irrig. Sci. 2005, 24, 15–24. [Google Scholar] [CrossRef]
- Zomorodi, S.; Emami, A. Study the effects of deficit irrigation on the yield, quality and storability of tomato. J. Agric. Eng. Res. 2006, 27, 19–30. [Google Scholar]
| Soil Property | Particle Size Distribution (%) | Bulk Density (g cm−3) | Field Capacity (cm3 cm−3) | Organic Matter (g kg−1) | ||
|---|---|---|---|---|---|---|
| Sand | Silt | Clay | ||||
| Range | 34% | 23% | 43% | 1.31 | 0.36 | 31.23 |
| Treatments | Emitter Density (Number of Emitters Per Plant), N | Irrigation Level (% of Crop Evapotranspiration), W |
|---|---|---|
| N1W1 (control) | 1 | 100 |
| N2W1 | 2 | 100 |
| N3W1 | 3 | 100 |
| N4W1 | 4 | 100 |
| N1W2 | 1 | 75 |
| N2W2 | 2 | 75 |
| N3W2 | 3 | 75 |
| N4W2 | 4 | 75 |
| Factors | Root Length | Root Average Diameter | Root Dry Mass | Root Surface Area | Root Volume | Specific Root Length | Root Fineness | Root Tissue Density |
|---|---|---|---|---|---|---|---|---|
| (m) | (mm) | (g) | (m2) | (cm3) | (mg−1) | (cm cm−3) | (g cm−3) | |
| Emitter density (N) | ||||||||
| N1 | 482.49 d | 0.351 a | 4.78 c | 0.536 c | 47.64 c | 100.58 d | 1048.6 a | 0.105 a |
| N2 | 558.41 c | 0.358 a | 5.17 c | 0.633 b | 57.40 b | 107.91 c | 1008.0 a | 0.094 b |
| N3 | 617.14 b | 0.362 a | 5.33 b | 0.708 a | 64.71 ab | 115.54 b | 991.3 a | 0.086 bc |
| N4 | 667.83 a | 0.362 a | 5.39 a | 0.766 a | 70.31 a | 123.47 a | 987.7 a | 0.081 c |
| Water Level (W) | ||||||||
| W1 | 617.13 a | 0.361 a | 5.27 a | 0.707 a | 64.58 a | 116.51 a | 992.9 a | 0.086 b |
| W2 | 545.81 b | 0.356 a | 5.07 a | 0.615 b | 55.54 b | 107.24 b | 1024.9 a | 0.096 a |
| Season (S) | ||||||||
| SS | 638.41 a | 0.368 a | 5.53 a | 0.743 a | 69.05 a | 115.11 a | 955.6 b | 0.086 b |
| FW | 524.52 b | 0.349 b | 4.81 b | 0.579 b | 50.98 b | 108.64 b | 1062.2 a | 0.097 a |
| ANOVA | ||||||||
| N | *** | ns | *** | *** | *** | *** | ns | *** |
| W | *** | ns | * | *** | ** | *** | ns | ** |
| S | *** | ** | *** | *** | *** | *** | ** | *** |
| N * W | ns | ns | ns | ns | ns | ns | ns | ns |
| N * S | ns | ns | ns | ns | * | ns | ns | ns |
| W*S | ns | ns | ns | ns | ns | ns | ns | ns |
| N * W * S | ns | ns | ns | ns | ns | ns | ns | ns |
| Factors | Leaf Area | Leaf Area Index | Specific Leaf Area | Leaf Area Ratio | Yield | Harvest Index | WUE | WUEB |
|---|---|---|---|---|---|---|---|---|
| (cm2) | (-) | (cm2 g−1) | (cm2 g−1) | (kg plant−1) | (-) | (kg m−3) | (kg m−3) | |
| Emitter density (N) | ||||||||
| N1 | 3672.7 b | 2.92 b | 151.52 c | 79.01 c | 1.86 b | 0.661 | 41.04 b | 62.23 b |
| N2 | 4541.9 a | 3.62 a | 176.15 b | 92.16 b | 2.15 a | 0.675 | 47.20 a | 70.11 a |
| N3 | 4844.0 a | 3.86 a | 184.20 ab | 96.47 ab | 2.20 a | 0.676 | 48.46 a | 71.87 a |
| N4 | 5104.7 a | 4.06 a | 192.94 a | 100.75 a | 2.24 a | 0.676 | 49.17 a | 72.89 a |
| Water Level (W) | ||||||||
| W1 | 4837.7 a | 3.85 a | 182.89 a | 96.03 a | 2.16 a | 0.679 | 40.91 b | 60.33 b |
| W2 | 4243.9 b | 3.38 b | 169.52 b | 88.17 b | 2.06 b | 0.665 | 52.02 a | 78.22 a |
| Season (S) | ||||||||
| SS | 5034.7 a | 4.01 a | 187.08 a | 98.10 a | 2.35 a | 0.682 | 43.33 b | 63.56 b |
| FW | 4047.0 b | 3.22 b | 165.33 b | 86.10 b | 1.88 b | 0.662 | 49.60 a | 74.99 a |
| ANOVA | ||||||||
| N | *** | *** | *** | *** | *** | ns | *** | *** |
| W | ** | ** | ** | ** | ** | ns | *** | *** |
| S | *** | *** | *** | *** | *** | ns | *** | *** |
| N * W | ns | ns | ns | ns | ns | ns | ns | ns |
| N * S | ns | ns | ns | ns | ns | ns | ns | ns |
| W * S | ns | ns | ns | ns | ns | ns | ns | ns |
| N * W*S | ns | ns | ns | ns | ns | ns | ns | ns |
| Factors | Photosynthetic rate (Pn) | Transpiration Rate (Tr) | Stomatal Conductance (gs) | Leaf Intercellular CO2 Concentration (Ci) | Instantaneous Water Use Efficiency (WUEleaf = Pn/Tr) |
|---|---|---|---|---|---|
| (μmol (CO2) m2 s−1) | (mmol m2 s−1) | (mol (H2O) m−2 s−1) | (μmol mol−1) | (μmol mol−1) | |
| Emitter density (N) | |||||
| N1 | 13.35 c | 11.38 b | 0.42 c | 270.27 c | 1.17 b |
| N2 | 15.07 b | 12.11 a | 0.49 b | 299.34 b | 1.25 a |
| N3 | 15.47 ab | 12.34 a | 0.52 a | 310.49 ab | 1.25 a |
| N4 | 15.81 a | 12.44 a | 0.53 a | 320.63 a | 1.27 a |
| Water Level (W) | |||||
| W1 | 15.44 a | 12.93 a | 0.51 a | 318.44 a | 1.19 b |
| W2 | 14.41 b | 11.20 b | 0.47 b | 281.93 b | 1.28 a |
| Season (S) | |||||
| SS | 16.61 a | 12.76 a | 0.51 a | 311.19 a | 1.30 a |
| FW | 13.24 b | 11.37 b | 0.47 b | 289.17 b | 1.17 b |
| ANOVA | |||||
| N | *** | ** | *** | *** | ** |
| W | *** | *** | *** | *** | *** |
| S | *** | *** | *** | *** | *** |
| N * W | ns | ns | ns | ns | ns |
| N * S | ns | ns | ns | ns | ns |
| W * S | ns | ns | ns | ns | ns |
| N * W * S | ns | ns | ns | ns | ns |
| Factors | Unit Fresh Weight | Number of Fruits Per Plant | Fruit Diameter | Fruit Height | Total Soluble Solids | pH |
|---|---|---|---|---|---|---|
| (g) | (-) | (mm) | (mm) | (⁰Brix) | (-) | |
| Emitter density (N) | ||||||
| N1 | 29.72 b | 62.44 b | 28.31 b | 30.56 | 4.22 a | 4.13 |
| N2 | 30.99 ab | 69.14 a | 29.29 ab | 31.00 | 4.10 ab | 4.14 |
| N3 | 31.62 a | 69.50 a | 29.96 a | 31.72 | 4.05 ab | 4.14 |
| N4 | 31.92 a | 69.88 a | 30.31 a | 31.91 | 4.02 b | 4.15 |
| Water Level (W) | ||||||
| W1 | 31.45 a | 68.51 a | 29.90 a | 31.66 | 3.84 b | 4.16 |
| W2 | 30.67 a | 66.97 a | 29.03 a | 30.94 | 4.35 a | 4.11 |
| Season (S) | ||||||
| SS | 32.23 a | 72.79 a | 30.08 a | 31.45 | 4.08 a | 4.08 |
| FW | 29.90 b | 62.69 b | 28.86 b | 31.15 | 4.11 a | 4.19 |
| ANOVA | ||||||
| N | * | *** | * | ns | ns | ns |
| W | ns | * | ns | ns | *** | ns |
| S | *** | *** | ** | ns | ns | ns |
| N * W | ns | ns | ns | ns | ns | ns |
| N * S | ns | ns | ns | ns | ns | ns |
| W * S | ns | ns | ns | ns | ns | ns |
| N * W * S | ns | ns | ns | ns | ns | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shabbir, A.; Mao, H.; Ullah, I.; Buttar, N.A.; Ajmal, M.; Lakhiar, I.A. Effects of Drip Irrigation Emitter Density with Various Irrigation Levels on Physiological Parameters, Root, Yield, and Quality of Cherry Tomato. Agronomy 2020, 10, 1685. https://doi.org/10.3390/agronomy10111685
Shabbir A, Mao H, Ullah I, Buttar NA, Ajmal M, Lakhiar IA. Effects of Drip Irrigation Emitter Density with Various Irrigation Levels on Physiological Parameters, Root, Yield, and Quality of Cherry Tomato. Agronomy. 2020; 10(11):1685. https://doi.org/10.3390/agronomy10111685
Chicago/Turabian StyleShabbir, Abdul, Hanping Mao, Ikram Ullah, Noman Ali Buttar, Muhammad Ajmal, and Imran Ali Lakhiar. 2020. "Effects of Drip Irrigation Emitter Density with Various Irrigation Levels on Physiological Parameters, Root, Yield, and Quality of Cherry Tomato" Agronomy 10, no. 11: 1685. https://doi.org/10.3390/agronomy10111685
APA StyleShabbir, A., Mao, H., Ullah, I., Buttar, N. A., Ajmal, M., & Lakhiar, I. A. (2020). Effects of Drip Irrigation Emitter Density with Various Irrigation Levels on Physiological Parameters, Root, Yield, and Quality of Cherry Tomato. Agronomy, 10(11), 1685. https://doi.org/10.3390/agronomy10111685






