Biofuel Production with Castor Bean: A Win–Win Strategy for Marginal Land
Abstract
:1. Introduction
2. Botanical Aspects and Ecological Characteristics
2.1. Botanical Aspects
2.2. Ecological Niche
3. Tolerance to Abiotic Stress
3.1. Drought Resistance
3.2. Salt Resistance
4. Agronomic Features
4.1. Growth Requirements
4.2. Planting Density
4.3. Irrigation
4.4. Fertilization
5. Castor Bean Products
Castor Bean Biodiesel
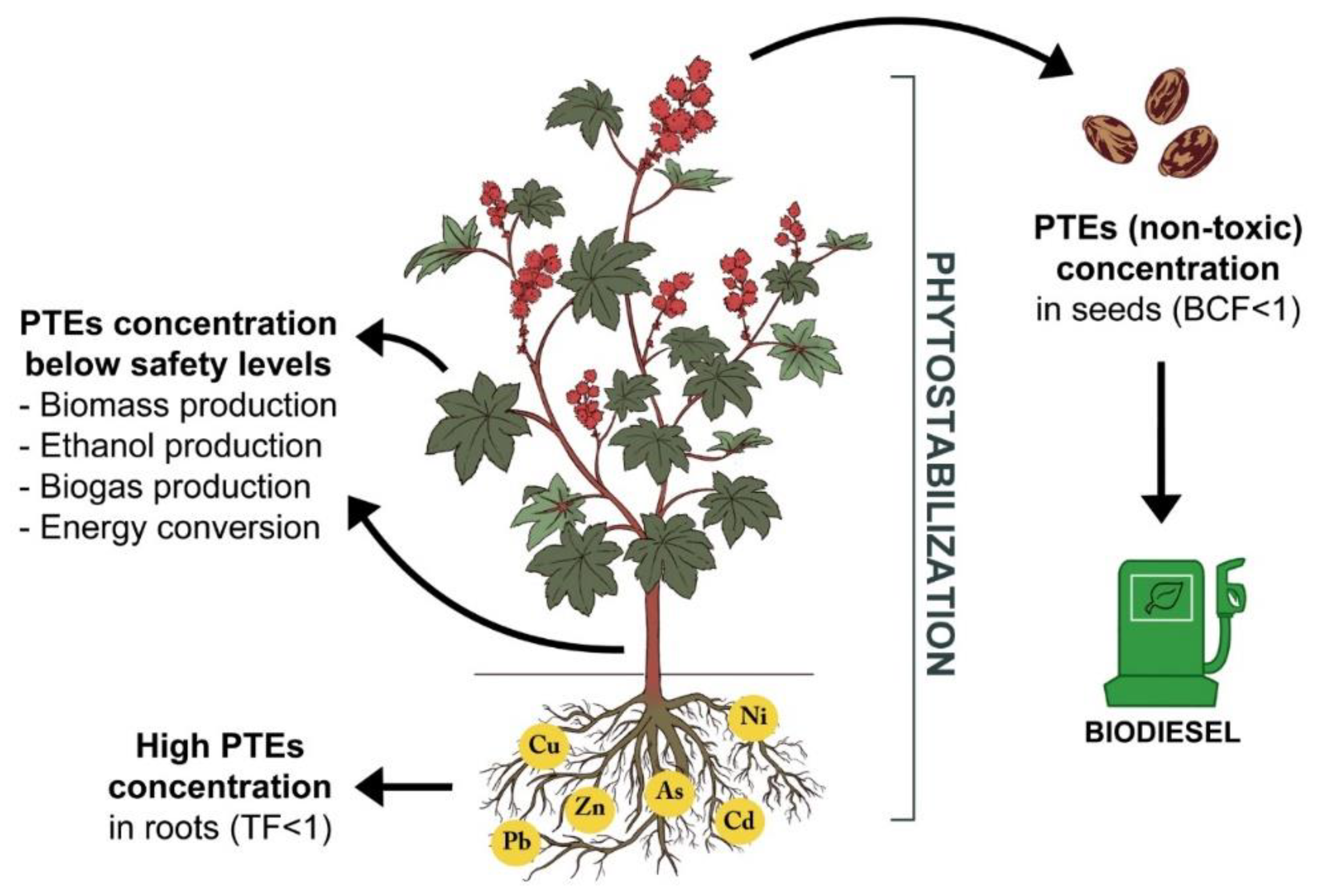
6. Phytoremediation Potential
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fagnano, M. Definition of a site as contaminated: Problems related to agricultural soils. Ital. J. Agron. 2018, 13 (Suppl. 1), 1–5. [Google Scholar]
- Pošćić, F.; Fellet, G.; Fagnano, M.; Fiorentino, N.; Marchiol, L. Linking phytotechnologies to bioeconomy; varietal screening of high biomass and energy crops for phytoremediation of Cr and Cu contaminated soils. Ital. J. Agron. 2019, 14, 43–49. [Google Scholar] [CrossRef] [Green Version]
- Chatzakis, M.K.; Tzanakakis, V.A.; Mara, D.D.; Angelakis, A.N. Irrigation of Castor Bean (Ricinus communis L.) and Sunflower (Helianthus annus L.) Plant Species with Municipal Wastewater Effluent: Impacts on Soil Properties and Seed Yield. Water 2011, 3, 1112–1127. [Google Scholar] [CrossRef] [Green Version]
- Bentivoglio, D.; Rasetti, M. Biofuel sustainability: review of implications for land use and food price. Ital. Rev. Agric. Econ. 2015, 70, 7–31. [Google Scholar] [CrossRef]
- Lora, E.E.S.; Palacio, J.C.E.; Rocha, M.H.; Renó, M.L.G.; Venturini, O.J.; del Olmo, O.A. Issues to consider, existing tools and constraints in biofuels sustainability assessments. Energy 2011, 36, 2097–2110. [Google Scholar] [CrossRef]
- Malins, C.; Searle, S.; Baral, A. A Guide for the Perplexed to the Indirect Effects of Biofuels Production. Available online: http://www.theicct.org/guide-perplexed-indirect-effects-biofuels-production (accessed on 29 July 2020).
- Pérez, A.P.; Eugenio, N.R. Status of Local Soil Contamination in Europe: Revision of the Indicator “Progress in the Management Contaminated Sites in Europe”; Office of the European Union: Luxembourg, 2018. [Google Scholar] [CrossRef]
- Bauddh, K.; Singh, B.; Korstad, J. Phytoremediation Potential of Bioenergy Plants; Springer: Berlin/Heidelberg, Germany, 2017; pp. 1–472. [Google Scholar]
- Kiran, B.R.; Prasad, M.N.V. Ricinus communis L. (Castor bean), a potential multi-purpose environmental crop for improved and integrated phytoremediation. EuroBiotech J. 2017, 1, 101–116. [Google Scholar] [CrossRef] [Green Version]
- Fiorentino, N.; Ventorino, V.; Rocco, C.; Cenvinzo, V.; Agrelli, D.; Gioia, L.; Di Mola, I.; Adamo, P.; Pepe, O.; Fagnano, M. Giant reed growth and effects on soil biological fertility in assisted phytoremediation of an industrial polluted soil. Sci. Total Environ. 2017, 575, 1375–1383. [Google Scholar] [CrossRef]
- Fagnano, M.; Fiorentino, N.F. Guest the Ecoremed protocol for an integrated agronomic approach to characterization and remediation of contaminated soils. Ital. J. Agron. 2018, 13, 1–68. [Google Scholar] [CrossRef] [Green Version]
- Giudicianni, P.; Pindozzi, S.; Grottola, C.M.; Stanzione, F.; Faugno, S.; Fagnano, M.; Fiorentino, N.; Ragucci, R. Pyrolysis for exploitation of biomasses selected for soil phytoremediation: Characterization of gaseous and solid products. Waste Manag. 2017, 61, 288–299. [Google Scholar] [CrossRef]
- Grottola, C.M.; Giudicianni, P.; Pindozzi, S.; Stanzione, F.; Faugno, S.; Fagnano, M.; Fiorentino, N.; Ragucci, R. Steam assisted slow pyrolysis of contaminated biomasses: Effect of plant parts and process temperature on heavy metals fate. Waste Manag. 2019, 85, 232–241. [Google Scholar] [CrossRef]
- Liu, C.F.; Li, Y.H.; Shi, G.R. Utilize Heavy Metal-Contaminated Farmland to Develop Bioenergy. Adv. Mater. Res. 2011, 414, 254–261. [Google Scholar] [CrossRef]
- Dastyar, W.; Raheem, A.; He, J.; Zhao, M. Biofuel Production Using Thermochemical Conversion of Heavy Metal-Contaminated Biomass (HMCB) Harvested from Phytoextraction Process. Chem. Eng. J. 2019, 358, 759–785. [Google Scholar] [CrossRef]
- Babita, M.; Maheswari, M.; Rao, L.; Shanker, A.K.; Rao, D.G. Osmotic adjustment, drought tolerance and yield in castor (Ricinus communis L.) hybrids. Environ. Exp. Bot. 2010, 69, 243–249. [Google Scholar] [CrossRef]
- Bauddh, K.; Singh, R.P. Growth, tolerance efficiency and phytoremediation potential of Ricinus communis (L.) and Brassica juncea (L.) in salinity and drought affected cadmium contaminated soil. Ecotoxicol. Environ. Saf. 2012, 85, 13–22. [Google Scholar] [CrossRef]
- Sausen, T.L.; Rosa, L.M.G. Growth and carbon assimilation limitations in Ricinus communis (Euphorbiaceae) under soil water stress conditions. Acta Bot. Bras. 2010, 24, 648–654. [Google Scholar] [CrossRef]
- Jeschke, W.D.; Wolf, O. Effect of NaCI Salinity on Growth, Development, Ion Distribution, and Ion Translocation in Castor Bean (Ricinus communis L.). J. Plant. Physiol. 1988, 132, 45–53. [Google Scholar] [CrossRef]
- Pinheiro, H.A.; Silva, J.V.; Endres, L.; Ferreira, V.M.; Camara, C.D.A.; Cabral, F.F.; Oliveira, J.F.; De Carvalho, L.W.T.; Dos Santos, J.M.; Filho, B.G.D.S. Leaf gas exchange, chloroplastic pigments and dry matter accumulation in castor bean (Ricinus communis L) seedlings subjected to salt stress conditions. Ind. Crop. Prod. 2008, 27, 385–392. [Google Scholar] [CrossRef]
- Anjani, K. Castor genetic resources: A primary gene pool for exploitation. Ind. Crop. Prod. 2012, 35, 1–14. [Google Scholar] [CrossRef]
- McKeon, T.A. Castor (Ricinus communis L.). In Industrial Oil Crops; Elsevier: Amsterdam, The Netherlands, 2016; pp. 75–112. [Google Scholar]
- Falasca, S.L.; Ulberich, A.C.; Ulberich, E. Developing an agro-climatic zoning model to determine potential production areas for castor bean (Ricinus communis L.). Ind. Crop. Prod. 2012, 40, 185–191. [Google Scholar] [CrossRef]
- Anjani, K. A re-evaluation of castor (Ricinus communis L.) as a crop plant. CAB Rev. 2014, 9, 1–21. [Google Scholar] [CrossRef]
- Salihu, B.Z.; Gana, A.K.; Apuyor, B.O. Castor Oil Plant (Ricinus communis L.): Botany, Ecology and Uses. Int. J. Sci. Res. 2014, 3, 1333–1341. [Google Scholar]
- Milani, M.; Nbreg, M.B.; Nóbrega, M.M. Castor Breeding. In Plant Breeding from Laboratories to Fields; IntechOpen: London, UK, 2013; p. 9. [Google Scholar]
- Vallejos, M.; Rondanini, D.; Wassner, D.F. Water relationships of castor bean (Ricinus communis L.) seeds related to final seed dry weight and physiological maturity. Eur. J. Agron. 2011, 35, 93–101. [Google Scholar] [CrossRef]
- Koutroubas, S.; Papakosta, D.; Doitsinis, A. Adaptation and yielding ability of castor plant (Ricinus communis L.) genotypes in a Mediterranean climate. Eur. J. Agron. 1999, 11, 227–237. [Google Scholar] [CrossRef]
- Velasco, L.; Fernández-Cuesta, Á.; Pascual-Villalobos, M.J.; Fernández-Martínez, J.M. Variability of seed quality traits in wild and semi-wild accessions of castor collected in Spain. Ind. Crop. Prod. 2015, 65, 203–209. [Google Scholar] [CrossRef]
- Wang, M.L.; Morris, J.B.; Pinnow, D.L.; Davis, J.; Raymer, P.; Pederson, G.A. A survey of the castor oil content, seed weight and seed-coat colour on the United States Department of Agriculture germplasm collection. Plant. Genet. Resour. 2010, 8, 229–231. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.L.; Morris, J.B.; Tonnis, B.; Pinnow, D.; Davis, J.; Raymer, P.; Pederson, G.A. Screening of the Entire USDA Castor Germplasm Collection for Oil Content and Fatty Acid Composition for Optimum Biodiesel Production. J. Agric. Food Chem. 2011, 59, 9250–9256. [Google Scholar] [CrossRef]
- Gómez, J.J.M.; Saadaoui, E.; Cervantes, E. Seed Shape of Castor Bean (Ricinus communis L.) Grown in Different Regions of Tunisia. J. Agric. Ecol. Res. Int. 2016, 8, 1–11. [Google Scholar] [CrossRef]
- Anastasi, U.; Sortino, O.; Cosentino, S.L.; Patanè, C. Seed yield and oil quality of perennial castor bean in a Mediterranean environment. Int. J. Plant Prod. 2014, 9, 99–116. [Google Scholar]
- Melo, E.E.C.; Guilherme, L.R.G.; Nascimento, C.W.A.D.; Penha, H.G.V. Availability and Accumulation of Arsenic in Oilseeds Grown in Contaminated Soils. Water Air Soil Pollut. 2011, 223, 233–240. [Google Scholar] [CrossRef]
- Palanivel, T.M.; Pracejus, B.; Victor, R. Phytoremediation potential of castor (Ricinus communis L.) in the soils of the abandoned copper mine in Northern Oman: Implications for arid regions. Environ. Sci. Pollut. Res. 2020, 27, 17359–17369. [Google Scholar] [CrossRef]
- Yang, J.; Yang, J.; Huang, J. Role of co-planting and chitosan in phytoextraction of As and heavy metals by Pteris vittata and castor bean–A field case. Ecol. Eng. 2017, 109, 35–40. [Google Scholar] [CrossRef]
- Da Silva, W.R.; da Silva, F.B.V.; Araújo, D.N.C.W.; Nascimento, C.W.A.D. Assessing human health risks and strategies for phytoremediation in soils contaminated with As, Cd, Pb, and Zn by slag disposal. Ecotoxicol. Environ. Saf. 2017, 144, 522–530. [Google Scholar] [CrossRef]
- De Abreu, C.A.; Coscione, A.R.; Pires, A.M.; Paz-Ferreiro, J. Phytoremediation of a soil contaminated by heavy metals and boron using castor oil plants and organic matter amendments. J. Geochem. Explor. 2012, 123, 3–7. [Google Scholar] [CrossRef]
- Coscione, A.R.; Berton, R.S. Barium extraction potential by mustard, sunflower and castor bean. Sci. Agric. 2009, 66, 59–63. [Google Scholar] [CrossRef] [Green Version]
- Shi, G.; Xia, S.; Ye, J.; Huang, Y.; Liu, C.; Zhang, Z. PEG-simulated drought stress decreases cadmium accumulation in castor bean by altering root morphology. Environ. Exp. Bot. 2015, 111, 127–134. [Google Scholar] [CrossRef]
- Ye, W.-L.; Guo, G.; Wu, F.; Fan, T.; Lu, H.; Chen, H.; Li, X.; Ma, Y. Absorption, translocation, and detoxification of Cd in two different castor bean (Ricinus communis L.) cultivars. Environ. Sci. Pollut. Res. 2018, 25, 28899–28906. [Google Scholar] [CrossRef]
- Zhang, H.; Guo, Q.; Yang, J.; Chen, T.; Zhu, G.; Peters, M.; Wei, R.; Tian, L.; Wang, C.; Tan, D.; et al. Cadmium accumulation and tolerance of two castor cultivars in relation to antioxidant systems. J. Environ. Sci. 2014, 26, 2048–2055. [Google Scholar] [CrossRef]
- Bauddh, K.; Singh, K.; Singh, R.P. Ricinus communis L. A Value Added Crop for Remediation of Cadmium Contaminated Soil. Bull. Environ. Contam. Toxicol. 2015, 96, 265–269. [Google Scholar] [CrossRef]
- Bauddh, K.; Singh, R.P. Cadmium Tolerance and Its Phytoremediation by Two Oil Yielding Plants Ricinus Communis (L.) and Brassica Juncea (L.) From The Contaminated Soil. Int. J. Phytoremediation 2012, 14, 772–785. [Google Scholar] [CrossRef]
- Bauddh, K.; Singh, R.P. Effects of organic and inorganic amendments on bio-accumulation and partitioning of Cd in Brassica juncea and Ricinus communis. Ecol. Eng. 2015, 74, 93–100. [Google Scholar] [CrossRef]
- González-Chávez, M.C.A.; Olivares, A.R.; Carrillo-González, R.; Leal, E.R. Crude oil and bioproducts of castor bean (Ricinus communis L.) plants established naturally on metal mine tailings. Int. J. Environ. Sci. Technol. 2014, 12, 2263–2272. [Google Scholar] [CrossRef]
- Olivares, A.R.; Carrillo-González, R.; González-Chávez, M.D.C.A.; Hernández, R.M.S. Potential of castor bean (Ricinus communis L.) for phytoremediation of mine tailings and oil production. J. Environ. Manag. 2013, 114, 316–323. [Google Scholar] [CrossRef]
- Pandey, V.C. Suitability of Ricinus communis L.cultivation for phytoremediation of fly ash disposal sites. Ecol. Eng. 2013, 57, 336–341. [Google Scholar] [CrossRef]
- Huang, H.; Yu, N.; Wang, L.; Gupta, D.; He, Z.; Wang, K.; Zhu, Z.; Yan, X.; Li, T.; Yang, X.-E. The phytoremediation potential of bioenergy crop Ricinus communis for DDTs and cadmium co-contaminated soil. Bioresour. Technol. 2011, 102, 11034–11038. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wen, C.; Liang, X.; He, C. Determination of the phytoremediation efficiency of Ricinus communis L. and methane uptake from cadmium and nickel-contaminated soil using spent mushroom substrate. Environ. Sci. Pollut. Res. 2018, 25, 32603–32616. [Google Scholar] [CrossRef]
- Costa, E.T.D.S.; Guilherme, L.R.G.; de Melo, E.E.C.; Ribeiro, B.T.; Inácio, E.D.S.B.; Severiano, E.D.C.; Faquin, V.; Hale, B.A. Assessing the Tolerance of Castor Bean to Cd and Pb for Phytoremediation Purposes. Biol. Trace Elem. Res. 2011, 145, 93–100. [Google Scholar] [CrossRef]
- Xiong, P.-P.; He, C.-Q.; Oh, K.; Chen, X.; Liang, X.; Liu, X.; Cheng, X.; Wu, C.-L.; Shi, Z.-C. Medicago sativa L. enhances the phytoextraction of cadmium and zinc by Ricinus communis L. on contaminated land in situ. Ecol. Eng. 2018, 116, 61–66. [Google Scholar] [CrossRef]
- Ren, C.; You, J.; Qi, Y.; Huang, G.; Hu, H. Effects of sulfur on toxicity and bioavailability of Cu for castor (Ricinus communis L.) in Cu-contaminated soil. Environ. Sci. Pollut. Res. 2017, 24, 27476–27483. [Google Scholar] [CrossRef]
- Zhou, X.; Huang, G.; Liang, D.; Liu, Y.; Yao, S.; Ali, U.; Hu, H. Influence of nitrogen forms and application rates on the phytoextraction of copper by castor bean (Ricinus communis L.). Environ. Sci. Pollut. Res. 2019, 27, 647–656. [Google Scholar] [CrossRef]
- Andreazza, R.; Bortolon, L.; Pieniz, S.; Camargo, F. Use of High-Yielding Bioenergy Plant Castor Bean (Ricinus communis L.) as a Potential Phytoremediator for Copper-Contaminated Soils. Pedosphere 2013, 23, 651–661. [Google Scholar] [CrossRef]
- Rehn, L.S.; Rodrigues, A.A.; Vasconcelos-Filho, S.C.; Rodrigues, D.A.; Moura, L.M.D.F.; Costa, A.C.; Carlos, L.; Sales, J.D.F.; Zuchi, J.; Angelini, L.P.; et al. Ricinus communis as a phytoremediator of soil mineral oil: Morphoanatomical and physiological traits. Ecotoxicology 2019, 29, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Rissato, S.R.; Galhiane, M.S.; Fernandes, J.R.; Gerenutti, M.; Gomes, H.M.; Ribeiro, R.; de Almeida, M.V. Evaluation of Ricinus communis L. for the Phytoremediation of Polluted Soil with Organochlorine Pesticides. BioMed Res. Int. 2015, 2015, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Pal, R.; Banerjee, A.; Kundu, R. Responses of Castor Bean (Ricinus communis L.) to Lead Stress. Proc. Natl. Acad. Sci. India Sect. B Boil. Sci. 2013, 83, 643–650. [Google Scholar] [CrossRef]
- González–Chávez, M.; Carrillo-González, R.; Cuellar-Sánchez, A.; Delgado-Alvarado, A.; Suárez-Espinosa, J.; Ríos-Leal, E.; Solís-Domínguez, F.A.; Maldonado-Mendoza, I.E. Phytoremediation assisted by mycorrhizal fungi of a Mexican defunct lead-acid battery recycling site. Sci. Total Environ. 2019, 650, 3134–3144. [Google Scholar] [CrossRef] [PubMed]
- Romeiro, S.; Lagôa, A.M.; Furlani, P.R.; de Abreu, C.A.; de Abreu, M.F.; Erismann, N.M. Lead uptake and tolerance of Ricinus communis L. Braz. J. Plant. Physiol. 2006, 18, 483–489. [Google Scholar] [CrossRef] [Green Version]
- Kiran, B.R.; Prasad, M. Defense manifestations of enzymatic and non-enzymatic antioxidants in Ricinus communis L. exposed to lead in hydroponics. EuroBiotech J. 2019, 3, 117–127. [Google Scholar] [CrossRef]
- Mallhi, Z.I.; Rizwan, M.; Mansha, A.; Ali, Q.; Asim, S.; Ali, S.; Hussain, A.; Alrokayan, S.H.; Khan, H.A.; Alam, P.; et al. Citric Acid Enhances Plant Growth, Photosynthesis, and Phytoextraction of Lead by Alleviating the Oxidative Stress in Castor Beans. Plants 2019, 8, 525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maggio, A.; de Pascale, S.; Ruggiero, C.; Barbieri, G. Physiological response of field-grown cabbage to salinity and drought stress. Eur. J. Agron. 2005, 23, 57–67. [Google Scholar] [CrossRef]
- Parvathaneni, L.; Prayaga, L.; Karusala, A. Selection of castor germplasm with important traits for drought tolerance under field conditions. Indian J. Plant. Physiol. 2017, 22, 295–303. [Google Scholar] [CrossRef]
- Silva, M.M.; Ferreira, L.T.; de Vasconcelos, F.M.T.; Willadino, L.; Camara, T.R.; de Oliveira, A.F.M. Water Stress-Induced Responses in the Growth, Cuticular Wax Composition, Chloroplast Pigments and Soluble Protein Content, and Redox Metabolism of Two Genotypes of Ricinus communis L. J. Plant. Growth Regul. 2020, 1–11. [Google Scholar] [CrossRef]
- Schurr, U.; Heckenberger, U.; Herdel, K.; Walter, A.; Feil, R. Leaf development in Ricinus communis during drought stress: Dynamics of growth processes, of cellular structure and of sink-source transition. J. Exp. Bot. 2000, 51, 1515–1529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tadayyon, A.; Nikneshan, P.; Pessarakli, M. Effects of drought stress on concentration of macro- and micro-nutrients in Castor (Ricinus communis L.) plant. J. Plant. Nutr. 2018, 41, 304–310. [Google Scholar] [CrossRef]
- Anjani, K.; Raoof, M.A.; Desai, A.G. Evaluation of world castor (Ricinus communis L.) germplasm for resistance to Fusarium wilt (Fusarium oxysporum f. sp. ricini). Eur. J. Plant. Pathol. 2014, 139, 567–578. [Google Scholar] [CrossRef]
- Sun, Y.; Niu, G.; Osuna, P.; Ganjegunte, G.; Auld, D.; Zhao, L.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Seedling emergence, growth, and leaf mineral nutrition of Ricinus communis L. cultivars irrigated with saline solution. Ind. Crop. Prod. 2013, 49, 75–80. [Google Scholar] [CrossRef]
- Wang, Y.; Jie, W.; Peng, X.; Hua, X.; Yan, X.; Zhou, Z.; Lin, J. Physiological Adaptive Strategies of Oil Seed Crop Ricinus communis Early Seedlings (Cotyledon vs. True Leaf) Under Salt and Alkali Stresses: From the Growth, Photosynthesis and Chlorophyll Fluorescence. Front. Plant. Sci. 2019, 9, 1–13. [Google Scholar] [CrossRef]
- Li, G.; Wan, S.; Zhou, J.; Yang, Z.; Qin, P. Leaf chlorophyll fluorescence, hyperspectral reflectance, pigments content, malondialdehyde and proline accumulation responses of castor bean (Ricinus communis L.) seedlings to salt stress levels. Ind. Crop. Prod. 2010, 31, 13–19. [Google Scholar] [CrossRef]
- Neto, M.C.L.; Lobo, A.K.M.; Martins, M.O.; Fontenele, A.V.; Silveira, J.A.G. Dissipation of excess photosynthetic energy contributes to salinity tolerance: A comparative study of salt-tolerant Ricinus communis and salt-sensitive Jatropha curcas. J. Plant. Physiol. 2014, 171, 23–30. [Google Scholar] [CrossRef]
- Zhou, G.; Ma, B.L.; Li, J.; Feng, C.; Lu, J.; Qin, P.-Y. Determining Salinity Threshold Level for Castor Bean Emergence and Stand Establishment. Crop Sci. 2010, 50, 2030–2036. [Google Scholar] [CrossRef]
- Li, G.; Zhang, H.; Wu, X.; Shi, C.; Huang, X.; Qin, P.-Y. Canopy reflectance in two castor bean varieties (Ricinus communis L.) for growth assessment and yield prediction on coastal saline land of Yancheng District, China. Ind. Crop Prod. 2011, 33, 395–402. [Google Scholar] [CrossRef]
- Nobre, R.G.; de Lima, G.S.; Medeiros, E.P.; de Lima, G.S.; Alves, A.N.; Gheyi, H.R. Teor de óleo e produtividade da mamoneira de acordo com a adubação nitrogenada e irrigação com água salina. Pesqui. Agropecuária Bras. 2012, 47, 991–999. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.-H.; Zhang, H.-S.; Li, G.; Liu, X.-C.; Qin, P.-Y. Ameliorative effect of castor bean (Ricinus communis L.) planting on physico-chemical and biological properties of seashore saline soil. Ecol. Eng. 2012, 38, 97–100. [Google Scholar] [CrossRef]
- Nobre, R.G.; De Lima, G.S.; Gheyi, H.R.; Lourenço, G.D.S.; Soares, L.A.D.A. Emergência, crescimento e produção da mamoneira sob estresse salino e adubação nitrogenada. Rev. Ciência Agronômica 2013, 44, 76–85. [Google Scholar] [CrossRef]
- Zhang, H.-S.; Li, G.; Qin, F.F.; Zhou, M.X.; Qin, P.-Y.; Pan, S. Castor bean growth and rhizosphere soil property response to different proportions of arbuscular mycorrhizal and phosphate-solubilizing fungi. Ecol. Res. 2013, 29, 181–190. [Google Scholar] [CrossRef]
- Zhang, T.; Hu, Y.; Zhang, K.; Tian, C.; Guo, J. Arbuscular mycorrhizal fungi improve plant growth of Ricinus communis by altering photosynthetic properties and increasing pigments under drought and salt stress. Ind. Crop. Prod. 2018, 117, 13–19. [Google Scholar] [CrossRef]
- Severino, L.S.; Auld, D.L.; Baldanzi, M.; Cândido, M.J.D.; Chen, G.; Crosby, W.L.; Tan, D.; He, X.; Lakshmamma, P.; Lavanya, C.; et al. A Review on the Challenges for Increased Production of Castor. Agron. J. 2012, 104, 853–880. [Google Scholar] [CrossRef] [Green Version]
- Alemaw, G.; Moges, A.; Aberra, D. Effect of plant and rowspacing on the yield and oil contents of castor (Ricinus communis L.) in the Central Rift Valley, Ethiopia. Ethiop. J. Agric. Sci. 2013, 24, 155–162. [Google Scholar]
- Alexopoulou, E.; Papatheohari, Y.; Zanetti, F.; Tsiotas, K.; Papamichael, I.; Christou, M.; Namatov, I.; Monti, A. Comparative studies on several castor (Ricinus communis L.) hybrids: Growth, yields, seed oil and biomass characterization. Ind. Crop. Prod. 2015, 75, 8–13. [Google Scholar] [CrossRef]
- Tamariz, M.N.B.; Chapingo, U.A.; Calzada, R.T.; Sánchez-Cohen, I.; Flores-Hernández, A. Castor seed yield at suboptimal soil moisture: Is it high enough? Int. J. Agric. Nat. Resour. 2019, 46, 253–265. [Google Scholar] [CrossRef]
- Cabrales, R.R.A.; Marrugo, N.J.L.; Plaza, T.G.A. Evaluation of seed yield and oil contents in four materials of Ricinus communis L. Agron. Colomb. 2011, 29, 43–48. [Google Scholar]
- Campbell, D.N.; Rowland, D.L.; Schnell, R.W.; Ferrell, J.A.; Wilkie, A.C. Developing a castor (Ricinus communis L.) production system in Florida, U.S.: Evaluating crop phenology and response to management. Ind. Crop. Prod. 2014, 53, 217–227. [Google Scholar] [CrossRef]
- Laureti, D.; Marras, G. Irrigation of castor (Ricinus communis L.) in Italy. Eur. J. Agron. 1995, 4, 229–235. [Google Scholar] [CrossRef]
- Severino, L.S.; Auld, D.L. A framework for the study of the growth and development of castor plant. Ind. Crop. Prod. 2013, 46, 25–38. [Google Scholar] [CrossRef]
- Severino, L.S.; Ferreira, G.B.; Moraes, C.R.D.A.; Gondim, T.M.D.S.; Cardoso, G.D.; Viriato, J.R.; Beltrão, N.E.D.M. Produtividade e crescimento da mamoneira em resposta à adubação orgânica e mineral. Pesqui. Agropecuária Bras. 2006, 41, 879–882. [Google Scholar] [CrossRef]
- Yousaf, M.M.; Hussain, M.; Shah, M.J.; Ahmed, B.; Zeshan, M.; Raza, M.M.; Ali, K. Yield Response of Castor (Ricinus communis L.) to NPK Fertilizers under Arid Climatic Conditions. Pak. J. Agric. Res. 2018, 31, 180–185. [Google Scholar] [CrossRef]
- Severino, L.S.; Maira, M.; de Almeida Moares, C.R.; de Souza Gondim, T.M.; Cardoso, G.D. Avaliação da produtividade e teor de óleo de dez genótipos de mamoneira cultivados em altitude inferior a 300 metros. Rev. Ciência Agronômica 2006, 37, 188–194. [Google Scholar]
- Severino, L.S.; Auld, D.L. Study on the effect of air temperature on seed development and determination of the base temperature for seed growth in castor (Ricinus communis L.). Aust. J. Crop Sci. 2014, 8, 290–295. [Google Scholar]
- Soratto, R.P.; Souza-Schlick, G.D.; Fernandes, A.M.; Zanotto, M.D.; Crusciol, C.A.C. Narrow row spacing and high plant population to short height castor genotypes in two cropping seasons. Ind. Crop. Prod. 2012, 35, 244–249. [Google Scholar] [CrossRef]
- Alves, G.D.S.; Tartaglia, F.D.L.; Beltrão, N.E.D.M.; Sampaio, L.R.; Freire, M.A.D.O. Population density and its effect on productivity in the castor bean BRS Energy under irrigated cultivation. Rev. Ciência Agronômica 2015, 46, 546–554. [Google Scholar] [CrossRef] [Green Version]
- de Souza-Schlick, G.D.; Soratto, R.P.; Zanotto, M.D. Optimizing row spacing and plant population arrangement for a new short-height castor genotype in fall-winter. Acta Sci. Agron. 2014, 36, 475. [Google Scholar] [CrossRef] [Green Version]
- de Oliveira, A.B.; Neto, J.F.D.B.; Cardoso, G.D. Growth and yield of castor bean (Ricinus communis L.) CV. ‘Brs energia’ under different spacings. Trop. Subtrop. Agroecosystems 2017, 20, 289–295. [Google Scholar]
- Severino, L.S.; de Moraes, C.R.; de Gondim, T.M.; Cardoso, G.D.; de Beltrão, N.E. Crescimento e produtividade da mamoneira influenciada por plantio em diferentes espaçamentos entre linhas. Rev. Ciência Agronômica 2006, 37, 50–54. [Google Scholar]
- Koutroubas, S.D.; Papakosta, D.K.; Doitsinis, A. Water Requirements for Castor Oil Crop (Ricinus communis L.) in a Mediterranean Climate. J. Agron. Crop. Sci. 2000, 184, 33–41. [Google Scholar] [CrossRef]
- Souza, S.; José, F.; Fernandes, A.; Bosco, J.; Marcus, F. Planting time and irrigation management for castor plant. I–effect on yield components. Eng. Agrícola 2007, 38, 414–421. [Google Scholar]
- Severino, L.S.; Auld, D.L. Seed yield and yield components of castor influenced by irrigation. Ind. Crop. Prod. 2013, 49, 52–60. [Google Scholar] [CrossRef]
- Neves, B.; Santos, M.; Donato, S. Evaluation of Irrigation Levels in the Castor Bean (Ricinus communis L.) in the Brazilian Semiarid Region. Rev. Eng. NA Agric. REVENG 2013, 21, 493–500. [Google Scholar] [CrossRef]
- Yadav, P.; Anjani, K. Assessment of Variation in Castor Genetic Resources for Oil Characteristics. J. Am. Oil Chem. Soc. 2017, 94, 611–617. [Google Scholar] [CrossRef]
- Tsoutsos, T.; Chatzakis, M.; Sarantopoulos, I.; Nikologiannis, A.; Pasadakis, N. Effect of wastewater irrigation on biodiesel quality and productivity from castor and sunflower oil seeds. Renew. Energy 2013, 57, 211–215. [Google Scholar] [CrossRef]
- Abbas, H.; Farid, I.; Soliman, S.; Galal, Y.; Ismail, M.; Kotb, E.; Moslhy, S. Growth and some macronutrients uptake by castor bean irradiated with gamma ray and irrigated with wastewater under sandy soil condition. J. Soil Sci. Agric. Eng. 2015, 6, 433–444. [Google Scholar] [CrossRef] [Green Version]
- Severino, L.S.; Ferreira, G.B.; de Castro, D.A.; Cardoso, G.D. Crescimento e produtividade da mamoneira adubada com macronutrientes e micronutrientes. Pesqui. Agropecuária Bras. 2006, 41, 563–568. [Google Scholar] [CrossRef] [Green Version]
- Reddy, K.R.; Matcha, S.K. Quantifying nitrogen effects on castor bean (Ricinus communis L.) development, growth, and photosynthesis. Ind. Crop. Prod. 2010, 31, 185–191. [Google Scholar] [CrossRef]
- Omotehinse, A.; Igboanugo, A.C. Effects of Fertilizer Application on Growth Capacity of Castor (Ricinus Communis) Shrub. J. Eng. Technol. Appl. Sci. 2019, 4, 19–33. [Google Scholar] [CrossRef]
- Nahar, K.; Pan, W. Urea Fertilization: Effects on Growth, Nutrient Uptake and Root Development of the Biodiesel Plant, Castor Bean (Ricinus communis L.). Am. J. Exp. Agric. 2015, 5, 320–335. [Google Scholar] [CrossRef] [Green Version]
- Polito, L.; Bortolotti, M.; Battelli, M.G.; Calafato, G.; Bolognesi, A. Ricin: An Ancient Story for a Timeless Plant Toxin. Toxins 2019, 11, 324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Copley, M.S.; Bland, H.A.; Rose, P.; Horton, M.; Evershed, R.P. Gas chromatographic, mass spectrometric and stable carbon isotopic investigations of organic residues of plant oils and animal fats employed as illuminants in archaeological lamps from Egypt. Analyst 2005, 130, 860–871. [Google Scholar] [CrossRef]
- Bateni, H.; Karimi, K. Biodiesel production from castor plant integrating ethanol production via a biorefinery approach. Chem. Eng. Res. Des. 2016, 107, 4–12. [Google Scholar] [CrossRef]
- Borg, P.; Le, G.; Lebrun, S.; Pees, B. Example of industrial valorisation of derivative products of Castor oil. Oléagineux Corps Gras Lipides 2009, 16, 211–214. [Google Scholar] [CrossRef] [Green Version]
- Ogunniyi, D.S. Castor oil: A vital industrial raw material. Bioresour. Technol. 2006, 97, 1086–1091. [Google Scholar] [CrossRef]
- Lemos, B.L.; Salomão, E.A.; Viana, M.P.R.; Amorim, R.J. Ethanol and Castor Oil for Two-Stroke and Wankel Engines. SAE Tech. Paper Ser. 2016, 1. [Google Scholar] [CrossRef]
- Singh, A. Castor oil-based lubricant reduces smoke emission in two-stroke engines. Ind. Crop. Prod. 2011, 33, 287–295. [Google Scholar] [CrossRef]
- Cardoso, G.T.; Neto, S.C.; Vecchia, F. Rigid foam polyurethane (PU) derived from castor oil (Ricinus communis) for thermal insulation in roof systems. Front. Arch. Res. 2012, 1, 348–356. [Google Scholar] [CrossRef] [Green Version]
- Uscátegui, Y.L.; Díaz, L.E.; Gómez-Tejedor, J.A.; Vallés-Lluch, A.; Vilariño-Feltrer, G.; Serrano, M.-A.; Valero, M.F. Candidate Polyurethanes Based on Castor Oil (Ricinus communis), with Polycaprolactone Diol and Chitosan Additions, for Use in Biomedical Applications. Molecules 2019, 24, 237. [Google Scholar] [CrossRef] [Green Version]
- de Sousa, T.P.T.; da Costa, M.S.T.; Guilherme, R.; Orcini, W.; Holgado, L.D.A.; Silveira, E.M.V.; Tavano, O.; Magdalena, A.G.; Catanzaro-Guimarães, S.A.; Kinoshita, A. Polyurethane derived from Ricinus Communis as graft for bone defect treatments. Polímeros 2018, 28, 246–255. [Google Scholar] [CrossRef]
- Mubofu, E.B. Castor oil as a potential renewable resource for the production of functional materials. Sustain. Chem. Process. 2016, 4, 1–12. [Google Scholar] [CrossRef]
- Lima, R.L.; Severino, L.S.; Sampaio, L.R.; Sofiatti, V.; Gomes, J.A.; Beltrão, N.E. Blends of castor meal and castor husks for optimized use as organic fertilizer. Ind. Crop. Prod. 2011, 33, 364–368. [Google Scholar] [CrossRef] [Green Version]
- Shrirame, H.Y.; Panwar, N.L.; Bamniya, B.R. Bio Diesel from Castor Oil—A Green Energy Option. Low Carbon Econ. 2011, 2, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.; Antil, R.; Narwal, R. Utilization of deoiled castor cake for crop production. Arch. Agron. Soil Sci. 2004, 50, 389–395. [Google Scholar] [CrossRef]
- Lopes, G.E.M.; Vieira, H.D.; Jasmim, J.M.; Shimoya, A.; Marciano, C.R. Casca do fruto da mamoneira como substrato para as plantas. Rev. Ceres 2011, 58, 350–358. [Google Scholar] [CrossRef] [Green Version]
- De Mello, G.A.B.; De Carvalho, D.F.; O Medici, L.; Silva, A.C.; Gomes, D.; Pinto, M.F. Organic cultivation of onion under castor cake fertilization and irrigation depths. Acta Sci. Agron. 2018, 40, 34993. [Google Scholar] [CrossRef] [Green Version]
- Bateni, H.; Karimi, K.; Zamani, A.; Benakashani, F. Castor plant for biodiesel, biogas, and ethanol production with a biorefinery processing perspective. Appl. Energy 2014, 136, 14–22. [Google Scholar] [CrossRef]
- Kalogiannis, K.G.; Stefanidis, S.D.; Michailof, C.M.; Lappas, A.A. Castor bean cake residues upgrading towards high added value products via fast catalytic pyrolysis. Biomass Bioenergy 2016, 95, 405–415. [Google Scholar] [CrossRef]
- Regulation (EU) No 1009/2019 of 5 June 2019—Laying down Rules on the Making available on the Market of EU Fertilising Products and Amending Regulations (EC) No 1069/2009 and (EC) No 1107/2009 and Repealing Regulation (EC) No 2003. Available online: http://data.europa.eu/eli/reg/2019/1009/oj (accessed on 2 October 2020).
- Nicory, I.M.C.; de Carvalho, G.G.P.; Ribeiro, O.; Santos, S.A.; da Silva, F.F.; Silva, R.R.; Lopes, L.S.C. Productive and metabolic parameters in lambs fed diets with castor seed meal. Livest. Sci. 2015, 181, 171–178. [Google Scholar] [CrossRef]
- McKeon, T.A.; Shim, K.B.; He, X. Reducing the toxicity of castor seed meal through processing treatments. Biocatal. Agric. Biotechnol. 2013, 2, 159–161. [Google Scholar] [CrossRef]
- Akande, T.O.; Odunsi, A.A.; Akinfala, E.O. A review of nutritional and toxicological implications of castor bean (Ricinus communis L.) meal in animal feeding systems. J. Anim. Physiol. Anim. Nutr. 2015, 100, 201–210. [Google Scholar] [CrossRef]
- de Matos, L.H.A.; De Carvalho, G.G.P.; Silva, R.R.; Leite, L.C.; Santos, S.A.; Conceição, C.P.; Santos, L.M.; de Azevêdo, J.A.G.; Santos, A.V.; Pina, D.D.S.; et al. The Use of Castor Meal, a by-Product of the Biodiesel Industry, in a Beef Production System in Tropical Pastures. Ann. Anim. Sci. 2018, 18, 469–482. [Google Scholar] [CrossRef] [Green Version]
- Silva, L.M.; Oliveira, C.H.D.A.; Da Silva, C.M.G.; Silva, A.M.; Fernandes, C.C.L.; Furtado, R.F.; Nunes-Pinheiro, D.C.S.; Guedes, M.I.F.; Rondina, D. Use of castor meal (Ricinus communis L.) as a source of dietary protein in goats during the mating period: Impact on reproductive and metabolic responses. Semin. Ciências Agrárias 2015, 36, 203. [Google Scholar] [CrossRef] [Green Version]
- Marschall, H.R.; Jiang, S.-Y. Tourmaline Isotopes: No Element Left Behind. Elements 2011, 7, 313–319. [Google Scholar] [CrossRef]
- Rossi, G.D.; Santos, C.D.; Alves, D.S.; Pereira, L.L.S.; Carvalho, G.; Rossi, G.D. Biochemical Analysis of a Castor Bean Leaf Extract and its Insecticidal Effects Against Spodoptera frugiperda (Smith) (Lepidoptera: Noctuidae). Neotrop. Ѐntomol. 2012, 41, 503–509. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Kaur, J. Toxicity of Leaf Extracts of Ricinus communis L. (Euphorbiaceace) Against the Third Instar Larvae of Musca domestica L. (Diptera: Muscidae). Am. J. Biosci. 2016, 4, 5. [Google Scholar] [CrossRef]
- Samuel, M.; Zipporah, N.; Philip, N.; Ingonga, J.; Peter, N. Ovicidal and Larvicidal Effects of Ricinus communis L. (Euphorbiaceae) Extracts on Phlebotomus duboscqi. Eur. J. Med. Plants 2016, 11, 1–14. [Google Scholar] [CrossRef]
- Naz, R.; Bano, A. Antimicrobial potential of Ricinus communis leaf extracts in different solvents against pathogenic bacterial and fungal strains. Asian Pac. J. Trop. Biomed. 2012, 2, 944–947. [Google Scholar] [CrossRef] [Green Version]
- Carolina, A.; Herliyana, E.N.; Sulastri, H. Antifungal activity of castor (Ricinus communis L.) leaves methanolic extract on Aspergillus niger. Int. Food Res. J. 2019, 26, 595–598. [Google Scholar]
- Shazia, M.; Imran, K.; Jasmine, F.; Mohd, S.; Huma, M.; Mansoor, S.; Khan, I.; Fatima, J.; Saeed, M.; Mustafa, H. Anti-bacterial, anti-oxidant and cytotoxicity of aqueous and organic extracts of Ricinus communis. Afr. J. Microbiol. Res. 2016, 10, 260–270. [Google Scholar] [CrossRef] [Green Version]
- Sharma, D.K.; Rana, S. Seed-Borne and post-harvest disease of Castor bean (Ricinus Communis Linn.) and their management: A Review. J. Phytol. Res. 2017, 30, 31–45. [Google Scholar]
- Umer, B.; Sori, W.; Getachew, M. Evaluation of castor (Ricinus communis L.) accession as feed for eri-silkworm (Samia cynthia RICINI Boisduval) at Jimma, South West Ethiopia. Sericologia 2016, 56, 219–228. [Google Scholar]
- Zanetti, F.; Chieco, C.; Alexopoulou, E.; Vecchi, A.; Bertazza, G.; Monti, A. Comparison of new castor (Ricinus communis L.) genotypes in the mediterranean area and possible valorization of residual biomass for insect rearing. Ind. Crop. Prod. 2017, 107, 581–587. [Google Scholar] [CrossRef]
- Martins, A.E.; Pereira, M.S.; Jorgetto, A.O.; Martines, M.A.; Silva, R.I.; Saeki, M.J.; Castro, G.R. The reactive surface of Castor leaf (Ricinus communis L.) powder as a green adsorbent for the removal of heavy metals from natural river water. Appl. Surf. Sci. 2013, 276, 24–30. [Google Scholar] [CrossRef]
- Keera, S.; El Sabagh, S.; Taman, A. Castor oil biodiesel production and optimization. Egypt. J. Pet. 2018, 27, 979–984. [Google Scholar] [CrossRef]
- Osorio-Gonzaléz, C.S.; Gómez-Falcon, N.; Sandoval-Salas, F.; Saini, R.; Brar, S.K.; Ramírez, A.A. Production of Biodiesel from Castor Oil: A Review. Energies 2020, 13, 2467. [Google Scholar] [CrossRef]
- Issariyakul, T.; Dalai, A.K. Biodiesel from vegetable oils. Renew. Sustain. Energy Rev. 2014, 31, 446–471. [Google Scholar] [CrossRef]
- Sajjadi, B.; Raman, A.A.A.; Arandiyan, H. A comprehensive review on properties of edible and non-edible vegetable oil-based biodiesel: Composition, specifications and prediction models. Renew. Sustain. Energy Rev. 2016, 63, 62–92. [Google Scholar] [CrossRef]
- Ismail, S.; Abu, S.; Rezaur, R.; Sinin, H. Biodiesel Production from Castor Oil and Its Application in Diesel Engine. ASEAN J. Sci. Technol. Dev. 2014, 31, 90. [Google Scholar] [CrossRef] [Green Version]
- Bello, K.; Airen, F.; Akinola, A.O.; Bello, E.I. A Study of the Lipid Structure of Castor Seed Oil (Ricinus communis L), Biodiesel and Its Characterization. Curr. J. Appl. Sci. Technol. 2020, 38, 1–11. [Google Scholar] [CrossRef]
- Scholz, V.; da Silva, J.N. Prospects and risks of the use of castor oil as a fuel. Biomass Bioenergy 2008, 32, 95–100. [Google Scholar] [CrossRef]
- Berman, P.; Nizri, S.; Wiesman, Z. Castor oil biodiesel and its blends as alternative fuel. Biomass Bioenergy 2011, 35, 2861–2866. [Google Scholar] [CrossRef]
- Silva, N.D.; Santander, C.M.G.; Rueda, S.M.G.; Maciel, M.; Filho, R.M. Characterization of Blend Properties of Castor Biodiesel and Bioethanol. Ind. Eng. Chem. Res. 2013, 52, 15504–15508. [Google Scholar] [CrossRef]
- Demirbas, A.; Bafail, A.; Ahmad, W.; Sheikh, M. Biodiesel production from non-edible plant oils. Renew. Sustain. Energy Rev. 2016, 34, 290–318. [Google Scholar] [CrossRef] [Green Version]
- Meneghetti, S.M.P.; Meneghetti, M.R.; Wolf, C.R.; Silva, E.C.; Lima, G.E.S.; Silva, L.D.L.; Serra, T.M.; Cauduro, F.; De Oliveira, L.G. Biodiesel from Castor Oil: A Comparison of Ethanolysis versus Methanolysis. Energy Fuels 2006, 20, 2262–2265. [Google Scholar] [CrossRef]
- Sánchez, N.; Sánchez, R.; Encinar, J.M.; González, J.F.; Martínez, G. Complete analysis of castor oil methanolysis to obtain biodiesel. Fuel 2015, 147, 95–99. [Google Scholar] [CrossRef]
- Karmakar, B.; Dhawane, S.H.; Halder, G. Optimization of biodiesel production from castor oil by Taguchi design. J. Environ. Chem. Eng. 2018, 6, 2684–2695. [Google Scholar] [CrossRef]
- Ramezani, K.; Rowshanzamir, S.; Eikani, M.H. Castor oil transesterification reaction: A kinetic study and optimization of parameters. Energy 2010, 35, 4142–4148. [Google Scholar] [CrossRef]
- Amouri, M.; Mohellebi, F.; Zaïd, T.A.; Aziza, M. Sustainability assessment of Ricinus communis biodiesel using LCA Approach. Clean Technol. Environ. Policy 2016, 19, 749–760. [Google Scholar] [CrossRef]
- Hoekman, S.K.; Broch, A.; Robbins, C.; Ceniceros, E.; Natarajan, M. Review of biodiesel composition, properties, and specifications. Renew. Sustain. Energy Rev. 2012, 16, 143–169. [Google Scholar] [CrossRef]
- Ijaz, M.; Bahtti, K.H.; Anwar, Z.; Dogar, U.F.; Irshad, M. Production, optimization and quality assessment of biodiesel from Ricinus communis L. oil. J. Radiat. Res. Appl. Sci. 2016, 9, 180–184. [Google Scholar] [CrossRef] [Green Version]
- Dias, J.M.; Araujo, J.M.; Costa, J.; Alvim-Ferraz, M.; Almeida, M. Biodiesel production from raw castor oil. Energy 2013, 53, 58–66. [Google Scholar] [CrossRef]
- Barbosa, D.D.; Serra, T.M.; Meneghetti, S.M.P.; Meneghetti, M.R. Biodiesel production by ethanolysis of mixed castor and soybean oils. Fuel 2010, 89, 3791–3794. [Google Scholar] [CrossRef]
- Oliveira, D.; Di Luccio, M.; Faccio, C.; Rosa, C.D.; Bender, J.P.; Lipke, N.; Amroginski, C.; Dariva, C.; De Oliveira, J.V. Optimization of Alkaline Transesterification of Soybean Oil and Castor Oil for Biodiesel Production. Appl. Biochem. Biotechnol. 2005, 122, 0553–0560. [Google Scholar] [CrossRef]
- Demirbas, A. Biodiesel from sunflower oil in supercritical methanol with calcium oxide. Energy Convers. Manag. 2007, 48, 937–941. [Google Scholar] [CrossRef]
- Mekhilef, S.; Siga, S.; Saidur, R. A review on palm oil biodiesel as a source of renewable fuel. Renew. Sustain. Energy Rev. 2011, 15, 1937–1949. [Google Scholar] [CrossRef]
- Uyumaz, A. Combustion, performance and emission characteristics of a DI diesel engine fueled with mustard oil biodiesel fuel blends at different engine loads. Fuel 2018, 212, 256–267. [Google Scholar] [CrossRef]
- Sanjid, A.; Masjuki, H.; Kalam, M.; Abedin, M.; Rahman, S.A. Experimental Investigation of Mustard Biodiesel Blend Properties, Performance, Exhaust Emission and Noise in an Unmodified Diesel Engine. APCBEE Procedia 2014, 10, 149–153. [Google Scholar] [CrossRef] [Green Version]
- Alam, M.; Rahman, K. Biodiesel from Mustard oil: A Sustainable Engine Fuel Substitute for Bangladesh. Int. J. Renew. Energy Dev. 2013, 2, 141–149. [Google Scholar] [CrossRef]
- Yuvarajan, D.; Munuswamy, D.B.; Nagappan, B.; Pandian, A.K. Performance, combustion and emission analysis of mustard oil biodiesel and octanol blends in diesel engine. Heat Mass Transf. 2018, 54, 1803–1811. [Google Scholar] [CrossRef]
- Rashid, U.; Anwar, F. Production of biodiesel through optimized alkaline-catalyzed transesterification of rapeseed oil. Fuel 2008, 87, 265–273. [Google Scholar] [CrossRef]
- Fagnano, M.; Visconti, D.; Fiorentino, N. Agronomic Approaches for Characterization, Remediation, and Monitoring of Contaminated Sites. Agronomy 2020, 10, 1335. [Google Scholar] [CrossRef]
- Aldhaidhawi, M.; Chiriac, R.; Badescu, V. Ignition delay, combustion and emission characteristics of Diesel engine fueled with rapeseed biodiesel—A literature review. Renew. Sustain. Energy Rev. 2017, 73, 178–186. [Google Scholar] [CrossRef]
- Visconti, D.; Fiorentino, N.; Caporale, A.G.; Stinca, A.; Adamo, P.; Motti, R.; Fagnano, M. Analysis of native vegetation for detailed characterization of a soil contaminated by tannery waste. Environ. Pollut. 2019, 252, 1599–1608. [Google Scholar] [CrossRef]
- Bauddh, K.; Singh, K.; Singh, B.; Singh, R.P. Ricinus communis: A robust plant for bio-energy and phytoremediation of toxic metals from contaminated soil. Ecol. Eng. 2015, 84, 640–652. [Google Scholar] [CrossRef]
- Fagnano, M.; Agrelli, D.; Pascale, A.; Adamo, P.; Fiorentino, N.; Rocco, C.; Pepe, O.; Ventorino, V. Copper accumulation in agricultural soils: Risks for the food chain and soil microbial populations. Sci. Total Environ. 2020, 734, 139434. [Google Scholar] [CrossRef]
- Huang, G.; Rizwan, M.S.; Ren, C.; Guo, G.; Fu, Q.; Zhu, J.; Hu, H. Influence of phosphorous fertilization on copper phytoextraction and antioxidant defenses in castor bean (Ricinus communis L.). Environ. Sci. Pollut. Res. 2016, 25, 115–123. [Google Scholar] [CrossRef]
- Visconti, D.; Fiorentino, N.; Stinca, A.; Di Mola, I.; Fagnano, M. Use of the native vascular flora for risk assessment and management of an industrial contaminated soil. Ital. J. Agron. 2018, 13, 23–33. [Google Scholar]
- Visconti, D.; Álvarez-Robles, M.J.; Fiorentino, N.; Fagnano, M.; Clemente, R. Use of Brassica juncea and Dactylis glomerata for the phytostabilization of mine soils amended with compost or biochar. Chemosphere 2020, 260, 127661. [Google Scholar] [CrossRef] [PubMed]
- Duri, L.G.; Visconti, D.; Fiorentino, N.; Adamo, P.; Fagnano, M.; Caporale, A.G. Health Risk Assessment in Agricultural Soil Potentially Contaminated by Geogenic Thallium: Influence of Plant Species on Metal Mobility in Soil-Plant System. Agronomy 2020, 10, 890. [Google Scholar] [CrossRef]
- Napoli, M.; Cecchi, S.; Grassi, C.; Baldi, A.; Zanchi, C.A.; Orlandini, S. Phytoextraction of copper from a contaminated soil using arable and vegetable crops. Chemosphere 2019, 219, 122–129. [Google Scholar] [CrossRef]
- Khan, M.A.; Khan, S.; Khan, A.; Alam, M. Soil contamination with cadmium, consequences and remediation using organic amendments. Sci. Total. Environ. 2017, 601–602, 1591–1605. [Google Scholar] [CrossRef]
- Fiorentino, N.; Mori, M.; Cenvinzo, V.; Duri, L.G.; Gioia, L.; Visconti, D.; Fagnano, M. Assisted phytoremediation for restoring soil fertility in contaminated and degraded land. Ital. J. Agron. 2018, 13 (Suppl. 1), 34–44. [Google Scholar] [CrossRef]
- Zhang, H.; Guo, Q.; Yang, J.-X.; Ma, J.; Chen, G.; Chen, T.-B.; Zhu, G.; Wang, J.; Zhang, H.; Wang, X.; et al. Comparison of chelates for enhancing Ricinus communis L. phytoremediation of Cd and Pb contaminated soil. Ecotoxicol. Environ. Saf. 2016, 133, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Visconti, D.; Caporale, A.G.; Pontoni, L.; Ventorino, V.; Fagnano, M.; Adamo, P.; Pepe, O.; Woo, S.L.; Fiorentino, N. Securing of an Industrial Soil Using Turfgrass Assisted by Biostimulants and Compost Amendment. Agronomy 2020, 10, 1310. [Google Scholar] [CrossRef]
- Wang, K.; Huang, H.; Zhu, Z.; Li, T.; He, Z.; Yang, X.; Alva, A. Phytoextraction of Metals and Rhizoremediation of PAHs in Co-Contaminated Soil by Co-Planting of Sedum Alfredii with Ryegrass (Lolium Perenne) or Castor (Ricinus Communis). Int. J. Phytoremediation 2013, 15, 283–298. [Google Scholar] [CrossRef]


| Contaminants | Aims of the Research | Reference | Genotype |
|---|---|---|---|
| As | Phytoremediation potential of CB and Helianthus annus | [34] | cv. Guarany |
| As, B, Cu, Fe, Mn, Zn | Phytoremediation potential | [35] | Not specified |
| As, Cd, Pb | Phytoremediation potential co-planting CB with Pteris vitatta with chitosan addition | [36] | Not specified |
| As, Cd, Pb, Zn | Phytoremediation potential of CB and Z. mays with chelates | [37] | Not specified |
| B, Cd, Cr, Cu, Fe, Mn, Ni, Pb, Zn | Effects of organic matter addition | [38] | Not specified |
| Ba | Phytoremediation potential of CB, B. juncea and H. annus | [39] | Not specified |
| Cd | Cd accumulation and drought stress | [40] | Cv. Zibi 5 |
| Cd | Phytoremediation potential | [41] | JX-22 and ZB-9 |
| Cd | Phytoremediation potential | [42] | Zibo 5 and Zibo 8 |
| Cd | Phytoremediation potential | [43] | Cv. Kalpi |
| Cd | Phytoremediation potential of CB and Brassica juncea | [44] | Cv. Kalpi |
| Cd | Phytoremediation potential of CB and Brassica juncea + salinity and drought stress | [17] | Cv. Kalpi |
| Cd | Phytoremediation potential of CB and Brassicaa juncea + Organic and Inorganic amendments | [45] | Cv. Kalpi |
| Cd, Cu, Mn, Ni, Pb, Zn | Crude oil and bioproducts | [46] | Plants established naturally on contaminated site |
| Cd, Cu, Mn, Pb, Zn | Phytoremediation potential | [47] | Plants established naturally on contaminated site |
| Cd, Cu, Ni, Pb, Zn | Phytoremediation potential of fly ash disposal site | [48] | Plants established naturally on contaminated site |
| Cd, DDT | Phytoremediation potential | [49] | Not specified |
| Cd, Ni | Phytoremediation potential with spent mushroom substrate | [50] | Not specified |
| Cd, PAHs, Pb, Zn | Phytoextraction and rhizoremediation by co-planting of Sedum alfredii with CB and Lolium perenne | [36] | Not specified |
| Cd, Pb | Phytoremediation potential | [51] | Cv. Guarany |
| Cd, Zn | Phytoremediation potential co-planting CB with Medicago sativa | [52] | Not specified |
| Cu | Effects of S on toxicity bioavailability of Cu | [53] | Plants established naturally on contaminated site |
| Cu | Influence of nitrogen forms and application rates on phytoextraction | [54] | Not specified |
| Cu | Phytoremediation potential | [55] | Not specified |
| Mineral oil | Phytoremediation potential of mineral oil | [56] | Plants in full production at the municipality of Rio Verde |
| Organochlorine pesticides | Phytoremediation potential of organochlorine pesticides | [57] | Not specified |
| Pb | Phytoremediation potential | [58] | Plants established naturally on contaminated site |
| Pb | Phytoremediation potential | [9] | Not specified |
| Pb | Phytoremediation assisted by mycorrhizal fungi | [59] | Ascession SF7 (previous study from plants established naturally) |
| Pb | Phytoremediation potential | [60] | Not specified |
| Pb | Phytoremediation potential | [61] | DCS-108 |
| Pb | Enhanced phytoremediation with citric acid | [62] | Not specified |
| Country | Site | Seed Yield Mg·ha−1 | Oil Yield Mg·ha−1 | Genotype | Treatment | Reference |
|---|---|---|---|---|---|---|
| Ethiopia | Rift Valley | 1.2–1.4 | 0.6–0.7 | Hiruy | Planting density | [81] |
| Greece | Aliartos | 3.0–3.8 | n.s. | Kaima 93, C-853, C-855, C-856, C-864, C-1002, C-1008 | Genotype evaluation (year 2014) | [82] |
| Italy | Cadriano | 0.7–4.0 | n.s. | C-855, C-856, C-857, C-864, C-1008 | Genotype evaluation (year 2014) | [82] |
| Italy | Ragusa | 0.7–7.3 | 0.3–3.3 | Local 1, Local 2, Brazil, Tunisia | Autumnal sowings | [33] |
| Mexico | Texcoco | 2.6–5.2 | n.s. | Krishna, Rincon | Optimal soil moisture | [83] |
| Colombia | Cordoba | 0.8–1.2 | 0.3–0.6 | Monteira, Cienaga de Oro, Los Cordobas, BRS Nordestina | Planting density | [84] |
| USA | Florida, Citra | 0.7–1.3 | 0.3–0.6 | Birminghan, Hale | Plant growth regulator and harvest aid | [85] |
| USA | Florida, Jay | 0.7–1.2 | 0.3–0.6 | Birminghan, Hale | Plant growth regulator and harvest aid | [85] |
| Italy | Sardinia | 1.4–2.5 | n.s. | Hazera 22, ISCIOR 101 | Irrigation | [86] |
| USA | Texas | 0.2–2.7 | n.s. | BRS Nordestina | Irrigation | [87] |
| Brazil | Carnaubais | 0.1–1.2 | n.s. | BRS Nordestina | Fertilization | [88] |
| Pakistan | Bahawalpur | 1.2–2.4 | n.s. | DS-30 | Fertilization | [89] |
| Feedstock | Seed oil Content | Advantages | Disadvantages | References |
|---|---|---|---|---|
| Castor bean oil | 45–55% | Nonedible, high flash point. high pour and cloud point (useful in winter condition), can grow on marginal and PTEs contaminated soils, miscible in alcohol, easily undergoes transesterification | Low cetane number, high viscosity, ricin content | [110,143,144,145,146,152,158,159,160,161,162] |
| Soybean | 15–20% | Low viscosity, high thermal stability | High production cost, edible, high acid value | [144,145,146,152,158,161,162] |
| Sunflower | 25–35% | Low viscosity | Edible, high acid value, long-term cultivation unsustainable | [144,145,146,152,158,163] |
| Palm | 18–40% | Cheap feedstock, high flashpoint | High cloud point, edible, long-term cultivation unsustainable | [144,145,146,152,158,164] |
| Mustard | 28–32% | High cetane number, cheap feedstock, can grow on soils contaminated with PTEs | High viscosity, low heating value, high cloud point | [145,146,152,165,166,167,168] |
| Rapeseed | 38–46% | High flash point and low cloud point | Effective power and torque decrease at all engine loads, increased NOx emissions up to 15% in most experiments | [145,146,152,158,169,170] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carrino, L.; Visconti, D.; Fiorentino, N.; Fagnano, M. Biofuel Production with Castor Bean: A Win–Win Strategy for Marginal Land. Agronomy 2020, 10, 1690. https://doi.org/10.3390/agronomy10111690
Carrino L, Visconti D, Fiorentino N, Fagnano M. Biofuel Production with Castor Bean: A Win–Win Strategy for Marginal Land. Agronomy. 2020; 10(11):1690. https://doi.org/10.3390/agronomy10111690
Chicago/Turabian StyleCarrino, Linda, Donato Visconti, Nunzio Fiorentino, and Massimo Fagnano. 2020. "Biofuel Production with Castor Bean: A Win–Win Strategy for Marginal Land" Agronomy 10, no. 11: 1690. https://doi.org/10.3390/agronomy10111690
APA StyleCarrino, L., Visconti, D., Fiorentino, N., & Fagnano, M. (2020). Biofuel Production with Castor Bean: A Win–Win Strategy for Marginal Land. Agronomy, 10(11), 1690. https://doi.org/10.3390/agronomy10111690







