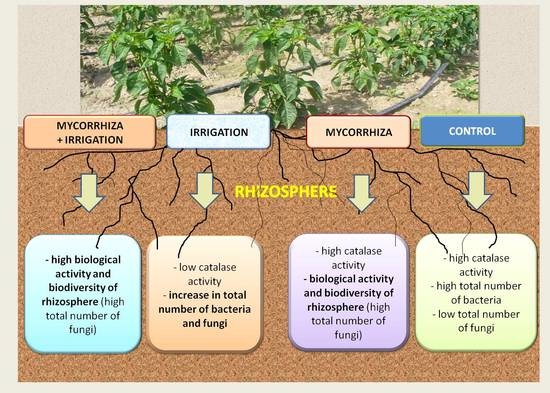
Effect of Mycorrhizal Inoculation and Irrigation on Biological Properties of Sweet Pepper Rhizosphere in Organic Field Cultivation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Determination of Soil Catalase Activity
2.3. Analysis of Microbial Community in the Rhizosphere
Analysis of Rhizosphere Microorganism Abundance
2.4. Extraction of Total DNA, PCR Reaction, and Next-Generation Sequencing
2.5. Analysis of Biodiversity of Soil Using Biolog EcoPlates Methods
2.6. Statistical Analysis
3. Results
3.1. Soil Catalase Activity
3.2. Communities of Rhizosphere Microorganisms
3.3. Biodiversity of Fungi Colonizing the Rhizosphere
3.4. Soil DNA Analysis
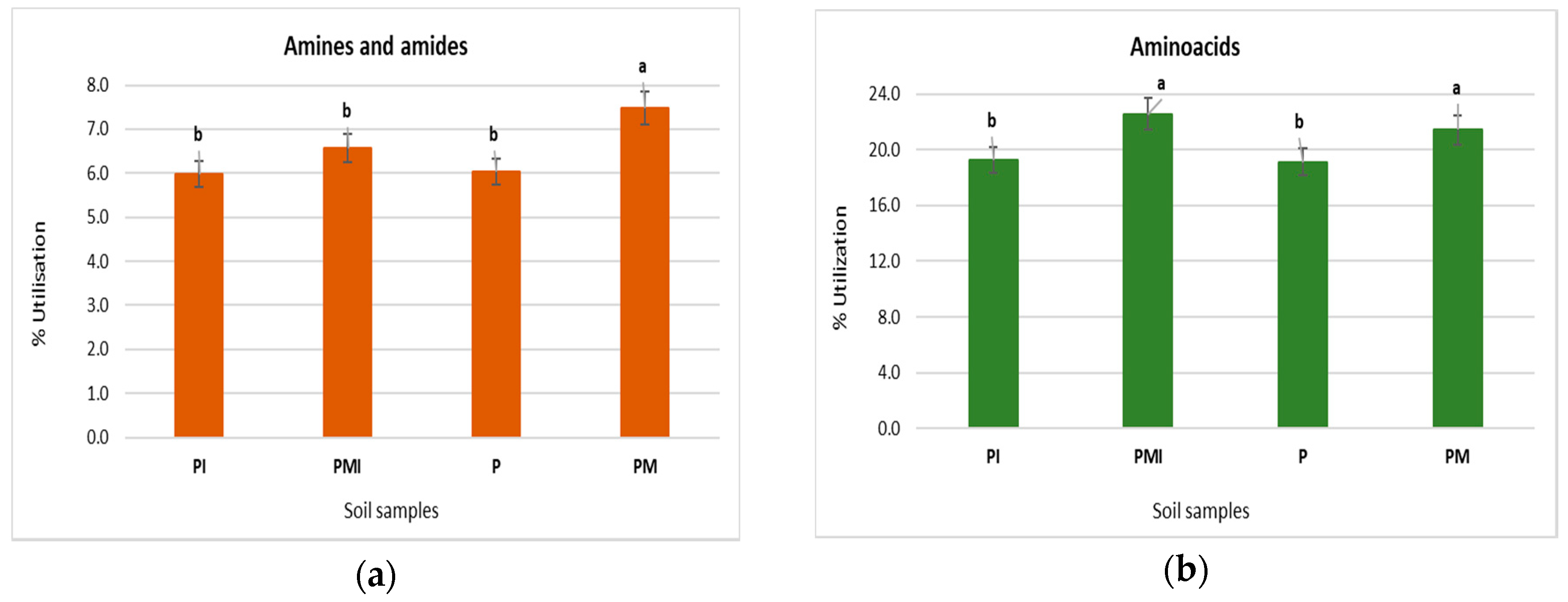
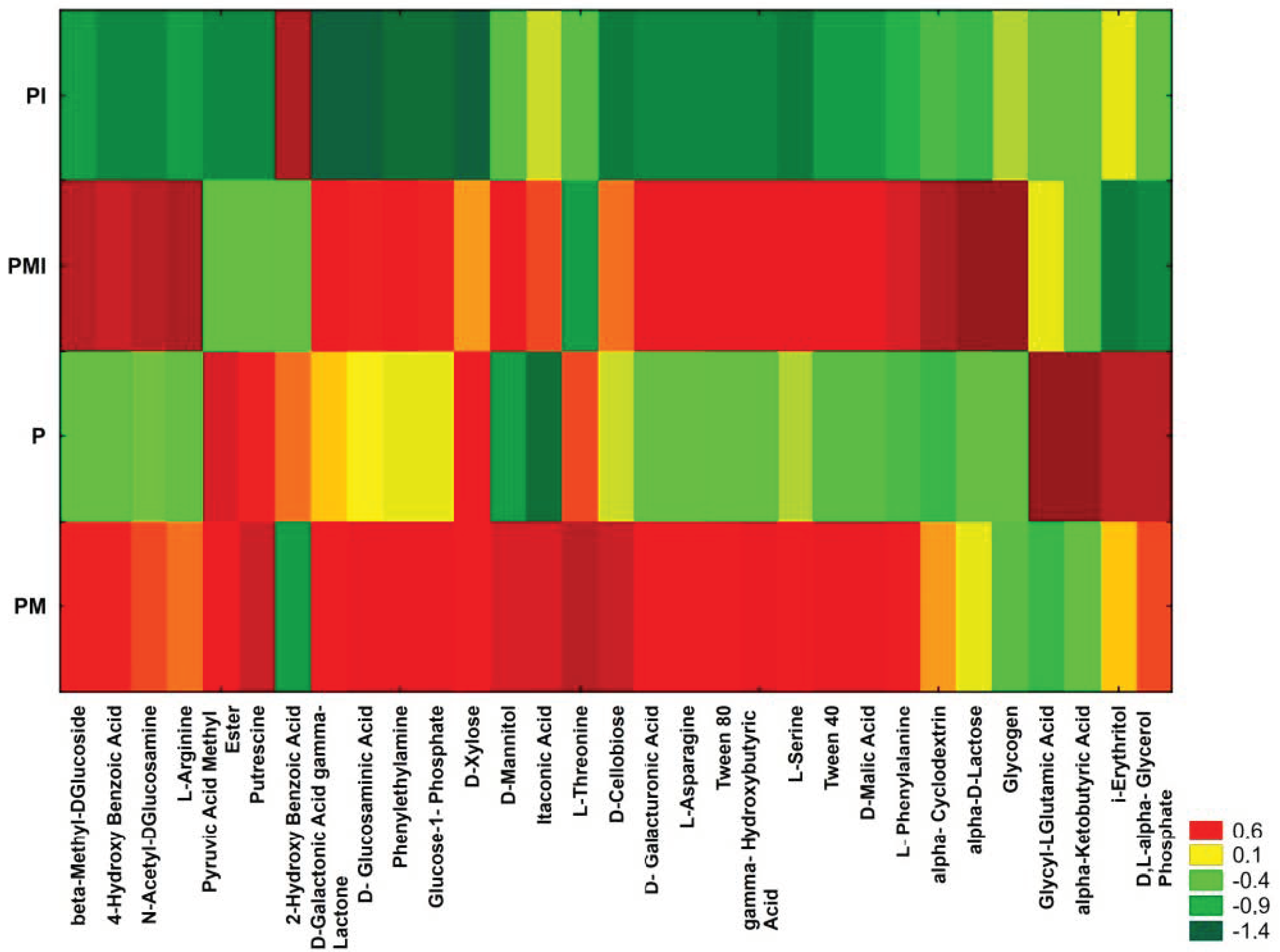
3.5. Biolog EcoPlates Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Anderson, T.A.; Guthrie, E.A.; Walton, B.T. Bioremediation in the rhizosphere. Environ. Sci. Technol. 1993, 27, 2630–2636. [Google Scholar] [CrossRef]
- Leśniak, A. Zmiany aktywności mikrobiologicznej i enzymatycznej w glebie skażonej węglowodorami aromatycznymi pod wpływem szczepienia kostrzewy łąkowej Pseudomonas stutzeri i Azospirillum spp. Zeszyty Naukowe Akademii Rolniczej im. Hugona Kołłątaja Krakowie 2007, 93, 463–472. [Google Scholar]
- Wallenstein, M.D.; Weintraub, M.N. Emerging tools for measuring and modeling thein situ activity of soil extracellular enzymes. Soil Biol. Biochem. 2008, 40, 2098–2106. [Google Scholar] [CrossRef]
- Woźniak, M.; Gałązka, A. The rhizosphere microbiome and its beneficial effects on plants—Current knowledge and perspectives. Adv. Microbiol. 2019, 58, 59–69. [Google Scholar] [CrossRef] [Green Version]
- Brzezińska, M.; Włodarczyk, T. Enzymy wewnątrzkomórkowych przemian redoks (oksydoreduktazy). Acta Agrophysica 2005, 120, 11–22. [Google Scholar]
- Martyn, W.; Skwaryło-Bednarz, B. Właściwości biologiczne gleb lekkich występujących w rejonie Roztoczańskiego Parku Narodowego. Acta Agrophysica 2005, 5, 695–704. [Google Scholar]
- Turski, M.; Wyczółkowski, A. Wpływ zróżnicowanego użytkowania gleb wytworzonych z lessu na aktywność respiracyjną i dehydrogenaz. Acta Agrophysica 2008, 12, 801–811. [Google Scholar]
- Skwaryło-Bednarz, B. Influence of contamination of soil with copper on the activity of dehydrogenases in areas where amaranthus is cultivated. Ecol. Chem. Eng. A 2012, 19, 155–160. [Google Scholar]
- Lemanowicz, J.; Koper, J. Aktywność fosfatazy i zawartość fosforu w glebie spod wybranych roślin uprawnych nawożonych gnojowicą. Proc. ECOpole 2012, 6, 239–243. [Google Scholar] [CrossRef]
- Bartkowiak, A.; Lemanowicz, J. Właściwości chemiczne wybranych profili glebowych Basenu Unisławskiego na tle aktywności enzymatycznej. Nauka Przyr. Technol. 2012, 6, 43–54. [Google Scholar]
- Garcıa-Gil, J.C.; Plaza, C.; Soler-Rovira, P.; Polo, A. Long-term effects of municipal solid waste compost application on soil enzyme activities and microbial biomass. Soil Biol. Biochem. 2000, 32, 1907–1913. [Google Scholar] [CrossRef]
- Oudah, H.K.; Jawad, M.M.; Abed, E.H. Role of enzymes catalase, peroxidase and amino acid (proline) in Raphanus sativus and Lepidium sativus in exposure levels different water pollution of ion lead. Iraqi J. Sci. 2017, 58, 592–599. [Google Scholar]
- Skwaryło-Bednarz, B.; Krzepiłko, A. Effect of different fertilization on enzyme activity in rhizosphere and non-rhizosphere of amaranth. Int. Agrophys. 2009, 23, 409–412. [Google Scholar]
- Epangesti, N.; Pineda, A.; Pieterse, C.M.J.; Dicke, M.; Van Loon, J. Two-way plant mediated interactions between root-associated microbes and insects: From ecology to mechanisms. Front. Plant Sci. 2013, 4, 414. [Google Scholar] [CrossRef] [Green Version]
- Vázquez, M.M.; César, S.; Azcón, R.; Barea, J.M. Interactions between arbuscular mycorrhizal fungi and other microbial inoculants (Azospirillum, Pseudomonas, Trichoderma) and their effects on microbial population and enzyme activities in the rhizosphere of maize plants. Appl. Soil Ecol. 2000, 15, 261–272. [Google Scholar] [CrossRef]
- Woźniak, M.; Gałązka, A.; Grządziel, J.; Głodowska, M. The identification and genetic diversity of endophytic bacteria isolated from selected crops. J. Agric. Sci. 2018, 156, 547–556. [Google Scholar] [CrossRef]
- Monther, M.T.; Kamaruzaman, S.; Tahat, M.M.; Sijam, K. Arbuscular mycorrhizal fungi and plant root exudates bio-communications in the rhizosphere. Afr. J. Microbiol. Res. 2012, 6, 7295–7301. [Google Scholar] [CrossRef] [Green Version]
- Łyszcz, M.; Gałązka, A. Wybrane metody molekularne wykorzystywane w ocenie bioróżnorodności mikroorganizmów glebowych. Post. Mikrobiol. 2016, 55, 309–319. [Google Scholar]
- Łyszcz, M.; Gałązka, A. Genetyczne metody różnicowania mikroorganizmów w systemie gleba-roślina. Post. Mikrobiol. 2017, 56, 341–352. [Google Scholar] [CrossRef]
- Jamiołkowska, A.; Buczkowska, H.; Thanoon, A.H. Effect of biological preparations on health state of pepper fruits and content of saccharides. Acta Sci. Pol. Hortorum Cultus 2016, 15, 95–107. [Google Scholar]
- Jamiołkowska, A.; Księżniak, A.; Hetman, B.; Kopacki, M.; Skwaryło-Bednarz, B.; Gałązka, A.; Thanoon, A.H. Interactions of arbuscular mycorrhizal fungi with plants and soil microflora. Acta Sci. Pol. Hortorum Cultus 2017, 16, 89–95. [Google Scholar] [CrossRef]
- Jamiołkowska, A.; Thanoon, A.H.; Patkowska, E.; Grządziel, J. Impact of AMF Claroideoglomus etunicatum on the structure of fungal communities in the tomato rhizosphere. Acta Mycol. 2019, 54, 1–7. [Google Scholar] [CrossRef]
- Jakobsen, I.; Smith, S.E.; Smith, F.A. Function and diversity of arbuscular mycorrhizae in carbon and min-eral nutrition. In Mycorrhizal Ecology; Van der Heijden, M.G.A., Sanders, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2003; pp. 75–92. [Google Scholar]
- Buczkowska, H. Badania Nad Modyfikacją Mikroklimatu w Polowej Uprawie Papryki Słodkiej (Capsicum Annuum L.) Ser; Wyd.—Rozp. Nauk; Wydawnictwa Akademii Rolniczej: Lublin, Poland, 1996; p. 73. [Google Scholar]
- Johnson, J.L.; Temple, K.L. Some Variables Affecting the Measurement of “Catalase Activity” in Soil. Soil Sci. Soc. Am. J. 1964, 28, 207–209. [Google Scholar] [CrossRef]
- Patkowska, E.; Błażewicz-Woźniak, M. The microorganisms communities in the soil under the cultivation of carrot (Daucus carota L.). Acta Sci. Pol. Hortorum Cultus 2014, 13, 103–115. [Google Scholar]
- Patkowska, E.; Konopiński, M. Antagonistic bacteria in the soil after cover crops cultivation. Plant Soil Environ. 2014, 60, 69–73. [Google Scholar] [CrossRef] [Green Version]
- Martyniuk, S.; Masiak, D.; Stachyra, A.; Myśków, W. Populations of the root zone microorganisms of various grasses and their antagonism towards Gaeumannomyces graminis var. tritici. Pamiętnik Puławski 1991, 98, 139–144. [Google Scholar]
- Czaban, J.; Gajda, A.; Wróblewska, B. The mobility of bacteria from rhizosphere and different zones of winter wheat roots. Pol. J. Environ. Stud. 2007, 16, 301–308. [Google Scholar]
- Patkowska, E.; Krawiec, M. Yielding and healthiness of pea cv. ‘Sześciotygodniowy TOR’ after applying biotechnical preparations. Acta Sci. Pol. Hortorum Cultus 2016, 15, 143–156. [Google Scholar]
- Schmidt, P.-A.; Bálint, M.; Greshake, B.; Bandow, C.; Römbke, J.; Schmitt, I. Illumina metabarcoding of a soil fungal community. Soil Biol. Biochem. 2013, 65, 128–132. [Google Scholar] [CrossRef]
- Weber, K.P.; Legge, R.L. One-dimensional metric for tracking bacterial communitydivergence using sole carbon source utilization patterns. J. Microbiol. Methods 2009, 79, 55–61. [Google Scholar] [CrossRef]
- Kolde, R. Pheatmap: Pretty Heatmaps. R Package Version 1.0.12. 2019. Available online: https://CRAN.R-project.org/package=pheatmap (accessed on 28 September 2020).
- R Core Team. R: A Language and Environment for Statistical Computing. 2018. Available online: https://www.r-project.org/ (accessed on 13 February 2012).
- Rivers, A.; Weber, K.C.; Gardner, T.G.; Liu, S.; Armstrong, S.D. ITSxpress: Software to rapidly trim internally transcribed spacer sequences with quality scores for marker gene analysis. F1000Research 2018, 7, 1418. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; A Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [Green Version]
- Nilsson, R.H.; Larsson, K.-H.; Taylor, A.F.S.; Bengtsson-Palme, J.; Jeppesen, T.S.; Schigel, D.; Kennedy, P.; Picard, K.; Glöckner, F.O.; Tedersoo, L.; et al. The UNITE database for molecular identification of fungi: Handling dark taxa and parallel taxonomic classifications. Nucleic Acids Res. 2018, 47, D259–D264. [Google Scholar] [CrossRef] [PubMed]
- Murali, A.; Bhargava, A.; Wright, E.S. IDTAXA: A novel approach for accurate taxonomic classification of microbiome sequences. Microbiome 2018, 6, 1–14. [Google Scholar] [CrossRef]
- McMurdie, P.J.; Holmes, S. phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [Green Version]
- Hammer, O.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Doran, J.W.; Zeiss, M.R. Soil health and sustainability: Managing the biotic component of soil quality. Appl. Soil Ecol. 2000, 15, 3–11. [Google Scholar] [CrossRef] [Green Version]
- Nannipieri, P.; Ascher, J.; Ceccherini, M.T.; Landi, L.; Pietramellara, G.; Renella, G. Microbial diversity and soil functions. Eur. J. Soil Sci. 2003, 54, 655–670. [Google Scholar] [CrossRef]
- Qiu, L.; Bi, Y.; Jiang, B.; Wang, Z.; Zhang, Y.; Zhakypbek, Y. Arbuscular mycorrhizal fungi ameliorate the chemical properties and enzyme activities of rhizosphere soil in reclaimed mining subsidence in northwestern China. J. Arid. Land 2018, 11, 135–147. [Google Scholar] [CrossRef] [Green Version]
- He, X.; Li, Y.; Zhao, L. Dynamics of arbuscular mycorrhizal fungi and glomalin in the rhizosphere of Artemisia ordosica Krasch. in Mu Us sandland, China. Soil Biol. Biochem. 2010, 42, 1313–1319. [Google Scholar] [CrossRef]
- Wielgosz, E.; Szember, A. Wpływ wybranych roślin na liczebność i aktywność drobnoustrojów glebowych. Ann. UMCS Sec. E 2006, 61, 107–119. [Google Scholar]
- Lamb, C.J.; Dixon, R.A. Molecular communication in interactions between plants and microbial pathogens. Plant Physiol. Plant Mol. Biol. 1990, 41, 339–367. [Google Scholar]
- Bielińska, E.J. Aktywność enzymów glebowych w ryzosferze mniszka lekarskiego jako wskaźnik stanu ekochemicznego gleb miejskich. J. Res. Appl. Agric. Eng. 2007, 52, 10–14. [Google Scholar]
- Romanowicz, A.; Krzepiłko, A. Porównanie aktywności katalazy w różnych organach maliny powtarzającej Rubus idaeus L. odmiany Polana oraz w glebie pod jej uprawą, oznaczanej metodą wolumetryczną. Pol. J. Agron. 2013, 15, 49–53. [Google Scholar]
- Orwin, K.H.; Kirschbaum, M.U.F.; John, M.G.S.; Dickie, I.A. Organic nutrient uptake by mycorrhizal fungi enhances ecosystem carbon storage: A model-based assessment. Ecol. Lett. 2011, 14, 493–502. [Google Scholar] [CrossRef]
- Mayer, Z. Impact of arbuscular mycorrhizal fungi on some defense enzyme activities at an early stage of maize (zea mays. L.) under different abiotic stresses. Appl. Ecol. Environ. Res. 2019, 17, 6241–6253. [Google Scholar] [CrossRef]
- Szymczak, J.; Kłódka, D.; Smolik, B.; Pawlica, M. Wpływ soli kadm u na aktywność enzymów stresu oksydacyjnego w glebie i kukurydzy (Zea mays var. Saccharata). Ochr. Środ. Zasob. Natur. 2011, 48, 210–215. [Google Scholar]
- Barea, J.; Pozo, M.J.; Azcón, R.; Azcón-Aguilar, C. Microbial co-operation in the rhizosphere. J. Exp. Bot. 2005, 56, 1761–1778. [Google Scholar] [CrossRef] [Green Version]
- Nihorimbere, V.; Ongena, M.; Smargiassi, M.; Thonart, P. Bene-ficial effect of the rhizosphere microbial community for plant growth and health. Biotechnol. Agron. Société et Environ. 2011, 15, 327–337. [Google Scholar]
- Mitter, B.; Pfaffenbichler, N.; Sessitsch, A. Plant–microbe partnerships in 2020. Microb. Biotechnol. 2016, 9, 635–640. [Google Scholar] [CrossRef]
- Jaroszuk-Ściseł, J.; Kurek, E.; Rodzik, B.; Winiarczyk, K. Interactions between rye (Secale cereale) root border cells (RBCs) and pathogenic and nonpathogenic rhizosphere strains of Fusarium culmorum. Mycol. Res. 2009, 113, 1053–1061. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, A.M.; Bauer, W.D.; Bird, D.M.; Cullimore, J.; Tyler, B.; Yoder, J.I. Molecular signals and receptors: Controlling rhizosphere interactions between plants and other organisms. Ecology 2003, 84, 858–868. [Google Scholar] [CrossRef] [Green Version]
- Bais, H.P.; Park, S.-W.; Weir, T.L.; Callaway, R.M.; Vivanco, J.M. How plants communicate using the underground information superhighway. Trends Plant Sci. 2004, 9, 26–32. [Google Scholar] [CrossRef]
- Tamas, L.; Šimonovičová, M.; Huttová, J. Mistríki Changes in the composition of cell wall proteins in barley roots during germination and growth in aluminium presence. Plant Soil Environ. 2011, 49, 327–331. [Google Scholar] [CrossRef] [Green Version]
- Bücking, H.; Abubaker, J.; Govindarajulu, M.; Tala, M.; Pfeffer, P.E.; Nagahashi, G.; Lammers, P.; Shachar-Hill, Y. Root exudates stimulate the uptake and metabolism of organic carbon in germinating spores ofGlomus intraradices. New Phytol. 2008, 180, 684–695. [Google Scholar] [CrossRef]
- Joseph, P.J.; Sivaprasad, P. The Potential of Arbuscular Mycorrhizal Associations for Biocontrol of Soil-Born Diseases; Springer Science and Business Media LLC: New York, NY, USA, 2000; pp. 139–153. [Google Scholar]
- Leelavathi, M.S.; Vani, L.; Reena, P. Antimicrobial activity of Trichoderma harzianum against bacteria and fungi. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 96–103. [Google Scholar]
- Reddy, B.N.; Saritha, K.V.; Hindumathi, A. In vitro screening for antagonistic potential of seven species of Trichoderma against different plant pathogenic fungi. Res. J. Biol. 2014, 2, 29–36. [Google Scholar]
- Elżbieta, P.; Agnieszka, J.; Marzena, B.-W. Antagonistic activity of selected fungi of the soil environment of carrot. Plant, Soil Environ. 2018, 64, 58–63. [Google Scholar] [CrossRef] [Green Version]
- Adhikari, A.; Dutta, S.; Nandi, S.; Bhattacharya, I.; De Roy, M.; Sarkar, G.; Mandal, T. Antagonistic potentiality of native rhizobacterial isolates against root rot disease of okra, incited by Rhizoctonia solani. Afr. J. Agric. Res. 2013, 8, 405–412. [Google Scholar] [CrossRef]
- Ritika, B.; Utpal, D. An overview of fungal and bacterial biopesticides to control plant pathogens/diseases. Afr. J. Microbiol. Res. 2014, 8, 1749–1762. [Google Scholar] [CrossRef] [Green Version]
- Kumar, M.M.; Singh, R.; Tomer, A. In vitro evaluation of antagonistic activity of Pseudomonas fluorescens against fungal pathogen. J. Biopestic. 2014, 7, 43–46. [Google Scholar]
- Sarma, B.K.; Yadav, S.K.; Patel, J.S.; Singh, H.B. Molecular Mechanisms of Interactions of Trichoderma with other Fungal Species. Open Mycol. J. 2014, 8, 140–147. [Google Scholar] [CrossRef] [Green Version]
- Smitha, C.; Finosh, G.T.; Rajesh, R.; Abraham, P.K. Induction of hydrolytic enzymes of phytopathogenic fungi in response to Trichoderma viride influence biocontrol activity. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 1207–1217. [Google Scholar]
- Manwar, A.V.; Vaiganker, P.D.; Bhonge, L.S.; Chincholkar, S.B. In vitro suppression of plant pathogens by siderophores of fluorescent Pseudomonas. Ind. J. Microbiol. 2000, 40, 109–112. [Google Scholar]
- Dehestani, A.; Kazemitabar, K.; Ahmadian, G.; Jelodar, N.B.; Salmanian, A.H.; Seyedi, M.; Rahimian, H.; Ghasemi, S. Chitinolytic and antifungal activity of a Bacillus pumilus chitinase expressed in Arabidopsis. Biotechnol. Lett. 2009, 32, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, A.L.; Ekelund, F.; Johansen, A.; Winding, A. Interaction of bacteria-feeding soil flagellates and Pseudomonas spp. Biol. Fertil. Soils 2009, 46, 151–158. [Google Scholar] [CrossRef]
- Frąc, M.; Hannula, S.E.; Bełka, M.; Jędryczka, M. Fungal Biodiversity and Their Role in Soil Health. Front. Microbiol. 2018, 9, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Anna, G.; Karolina, G.; Jarosław, G.; Jerzy, K. Effect of different agricultural management practices on soil biological parameters including glomalin fraction. Plant Soil Environ. 2017, 63, 300–306. [Google Scholar] [CrossRef] [Green Version]
- Gałązka, A.; Grządziel, J.; Gałązka, R.; Gawryjołek, K.; Ukalska-Jaruga, A.; Smreczak, B. Fungal Community, Metabolic Diversity, and Glomalin-Related Soil Proteins (GRSP) Content in Soil Contaminated With Crude Oil After Long-Term Natural Bioremediation. Front. Microbiol. 2020, 11, 572314. [Google Scholar] [CrossRef]
- Zhang, X.; Ye, L.; Kang, Z.; Zou, J.; Li, X. Mycorrhization of Quercus acutissima with Chinese black truffle significantly altered the host physiology and root-associated microbiomes. PeerJ 2019, 7, e6421. [Google Scholar] [CrossRef] [Green Version]





| Year | Experimental Treatment | Soil Zone | Plant Development Phase | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2016 | 2017 | 2018 | P | PM | PMI | PI | Rhizosphere | Non-Rhizosphere | BBCH 22 | BBCH 72–73 |
| 0.0366 a | 0.0243 b | 0.0281 b | 0.0389 a | 0.0301 b | 0.0226 c | 0.0271 bc | 0.0439 a | 0.0175 b | 0.0265 b | 0.0328 a |
| LSDα ≤ 0.05 = 0.0042 | LSDα ≤ 0.05 = 0.0052 | LSDα ≤ 0.05 = 0.0020 | LSDα ≤ 0.05 = 0.0029 | |||||||
| F value = 25.24735 | F value = 23.2059 | F value = 686.8324 | F value = 17.5118 | |||||||
| Experimental Treatment | Total CFU of Bacteria (106∙g−1 DW of Soil) | CFU of Bacillus spp. (106∙g−1 DW of Soil) | CFU of Pseudomonas spp. (106∙g−1 DW of Soil) | Total CFU of Fungi (103∙g−1 DW of Soil) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2016 | 2017 | 2018 | Mean | 2016 | 2017 | 2018 | Mean | 2016 | 2017 | 2018 | Mean | 2016 | 2017 | 2018 | Mean | |
| P | 22.66 c | 20.00 c | 24.63 d | 22.43 d | 7.82 c | 7.19 c | 7.37 c | 7.46 d | 10.74 d | 10.15 d | 11.34 d | 10.74 d | 9.97 a | 9.22 b | 10.05 b | 9.75 b |
| PM | 17.63 b | 17.03 b | 18.93 b | 17.86 b | 3.68 b | 2.09 a | 3.62 a | 3.13 b | 5.12 b | 3.52 b | 5.26 b | 4.63 b | 8.47 a | 7.34 a | 7.94 a | 7.92 a |
| PI | 18.84 b | 18.94 b | 21.92 c | 19.90 c | 6.34 c | 5.39 b | 5.99 b | 5.91 c | 8.22 c | 7.56 c | 8.09 c | 7.96 c | 22.18 c | 19.98 d | 20.73 d | 20.96 d |
| PMI | 14.66 a | 14.01 a | 15.61 a | 14.76 a | 1.71 a | 1.10 a | 2.29 a | 1.70 a | 2.67 a | 1.82 a | 2.39 a | 2.29 a | 16.41 b | 14.03 c | 16.38 c | 15.61 c |
| ITS NGS | ||||
|---|---|---|---|---|
| H | Chao1 | Simpson | Fisher | |
| P | 2.447 | 57 | 0.8385 | 8.784 |
| PM | 2.661 | 60 | 0.8936 | 9.086 |
| PI | 2.984 | 69 | 0.9119 | 10.94 |
| PMI | 3.009 | 74 | 0.9178 | 11.69 |
| Experimental Treatment | Average Well Color Development | Shannon-Wiener (H’) | Evenness (E) | Richness (R) |
|---|---|---|---|---|
| P | 1.880 b | 3.418 a | 0.995 b | 31.00 a |
| PM | 2.028 a | 3.405 a | 0.991 a | 31.00 a |
| PI | 1.702 b | 3.409 b | 0.993 a | 31.00 a |
| PMI | 2.006 a | 3.394 a | 0.991 a | 30.67 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jamiołkowska, A.; Skwaryło-Bednarz, B.; Patkowska, E.; Buczkowska, H.; Gałązka, A.; Grządziel, J.; Kopacki, M. Effect of Mycorrhizal Inoculation and Irrigation on Biological Properties of Sweet Pepper Rhizosphere in Organic Field Cultivation. Agronomy 2020, 10, 1693. https://doi.org/10.3390/agronomy10111693
Jamiołkowska A, Skwaryło-Bednarz B, Patkowska E, Buczkowska H, Gałązka A, Grządziel J, Kopacki M. Effect of Mycorrhizal Inoculation and Irrigation on Biological Properties of Sweet Pepper Rhizosphere in Organic Field Cultivation. Agronomy. 2020; 10(11):1693. https://doi.org/10.3390/agronomy10111693
Chicago/Turabian StyleJamiołkowska, Agnieszka, Barbara Skwaryło-Bednarz, Elżbieta Patkowska, Halina Buczkowska, Anna Gałązka, Jarosław Grządziel, and Marek Kopacki. 2020. "Effect of Mycorrhizal Inoculation and Irrigation on Biological Properties of Sweet Pepper Rhizosphere in Organic Field Cultivation" Agronomy 10, no. 11: 1693. https://doi.org/10.3390/agronomy10111693