1. Introduction
Sorrel (
Rumex) is a genus of plants in the knotweed family (
Polygonaceae). It is native mainly to the temperate climate zone in the Northern Hemisphere [
1].
Rumex is one of the more popular herbs grown in spring, summer, and autumn [
2]. It is a good source of bioactive compounds and an important food ingredient in various cuisines in Africa, Europe, and Asia. Sorrel leaves have high contents of vitamins (A, C, K, E, H, PP, and B complex), oxalic acid, essential oils and acids, minerals (iron, magnesium, phosphorus, sulfur and silicon), proteins, sugars, tannins, and fiber [
2,
3]. On the other hand, sorrel root, due to its high content of phenolic compounds, is used for various therapeutic purposes as a detoxicant and as a healing agent in liver diseases, skin diseases, and even in malignant or benign tumors [
4]. As a medicinal plant, sorrel is applied in various conditions such as anemia, vitamin deficiency, gastritis, liver diseases, or skin conditions, and for various purposes (i.e., to decrease cholesterol level, to regulate blood pressure, to detoxicate; it is also known for antistress, antioxidant, anticancer and antiviral activity [
5]).
Quality in plant production mainly depends on soil and climate conditions as well as agricultural engineering treatments applied. To standardize the quality of the raw produce, it is necessary to maintain constant conditions in the production process [
6]. The ways to influence the chemical parameters, in particular those related to bioactive compounds include the application of various types of elicitors. Depending on their origin, elicitors can be classified as biotic or abiotic [
7]. The known abiotic elicitors include ozone, which is an allotrope of oxygen. If applied in a correct way, it induces changes in metabolism that further promote an increase in the contents of antioxidants from various chemical groups [
8]. Effectiveness of ozone applied as an elicitor to increase the content of bioactive compounds was confirmed by Xu et al. [
9]. The authors demonstrated that ozone applied short-term at low concentrations to coriander activated antioxidant enzymes as a result of which the cellular level of reactive oxygen species was decreased while negative effects associated with oxidative stress as well as loss of produce quality was eliminated. The main advantage of this type of elicitation is linked with the fact that there are no residues in the plant material after ozone treatment is performed [
10].
The phytotoxic effect appeared not only through necrosis on the morphological parts of the plants, but changes in the intensity of photosynthesis and gas exchange were also observed. It has been shown that long-term exposure in most cases had a negative impact on these parameters. But others showed that short-term exposure to ozone may affect the biochemical balance of plants. So far, mainly fruits have been studied, while there are no reports in the literature on short-term exposure of higher ozone concentrations on the physiological parameters processes of plants. Ozone, especially the tropospheric form, in addition to the beneficial elicitor properties, may also be phytotoxic and may induce considerable changes in the physiological parameters of plants [
11]. Chlorophyll plays an important role in biosynthesis processes taking place in the green parts of plants by enabling transformation of light energy into the energy of chemical bonds in the process of photosynthesis [
12]. Chlorophyll content in plants is undoubtedly characteristic for specific species and varieties [
13], it may, however, be subject to modifications induced by a variety of abiotic stress producing factors [
14] such as gaseous ozone [
15]. So far, research has largely focused on identifying the negative effects of tropospheric ozone on plants [
16,
17]. As a result of exposure to gaseous ozone, chlorophyll content decreases in white clover plants [
18], potato leaves [
19], and black elder [
20]. Additionally, tropospheric ozone causes a decrease in chlorophyll fluorescence in tomato plants, irrespective of the growth phase [
21]. Moreover, effectiveness of photosynthesis decreases in plants exposed to phytotoxic doses of ozone [
22,
23]. Saitanis et al. [
23] investigated the effect of relatively low ozone concentrations on tobacco plants and found that it accelerated the speed of leaf senescence and impaired the mechanism of photosynthesis. Izuta et al. [
24] reported that ozone decreased the intensity of photosynthesis in radish plants. Ladd et al. [
11] observed that chloroses and necroses resulting from chronic exposure of plants to ozone caused a decrease in the intensity of the photosynthesis process. Oliwa et al. [
25] established that the rate of transpiration in
Platycerium bifurcatum decreased in plants exposed to low concentrations of ozone.
The aim of the research was to demonstrate the effect of ozonation on the physiological processes of sorrel plants. It has been hypothesized that ozone under appropriate conditions will positively impact on the physiological processes of plants and the content of selected bioactive compounds. The study was designed to apply an agricultural engineering procedure involving ozone fumigation in order to ultimately obtain raw plant material from red-veined sorrel with increased content of bioactive compounds, while retaining good physiological condition of the plants.
2. Materials and Methods
2.1. Pot Experimental Design
Rumex sanguineus plants were produced in a pot experiment in July 2020 in a greenhouse in triplicate. In the production of red-veined sorrel, applied organic fertilization and water-climatic conditions were as described by Matlok et al. [
26]. The area of one experiment replication was 0.5 m
2 and consisted of 32 pots with a volume of 0.78 dm
3 each. During the experiment standardized seed were utilized (microgreens) possessed by Dutch seed company Pieterpikzonen BV. The production conditions such as temperature and air humidity were controlled. Light-emitting diode LED based technology for lighting of plants during cultivation was utilized (photoperiod used: 12/12 h day/night). The temperature during plant growth in the greenhouse averaged 26/17 °C (day/night) and relative humidity averaged 70% were observed. No plant protection treatments were performed during the experiment. At the final stage of production, the sorrel plants were placed in the ozonation chamber to perform the ozonation treatment.
2.2. Plant Material Characteristics
On the day preceding the treatment involving fumigation with gaseous ozone, the sorrel plants were found with the mean relative chlorophyll contents of 25.32 ± 4.71 and the following parameters of chlorophyll fluorescence: maximum quantum yield of PSII photochemistry 0.830 ± 0.01; maximum quantum yield of primary photochemistry 4.84 ± 0.28; performance index (PI) 4.58 ± 0.81. Additionally, the measurements of the main gas exchange parameters associated with photosynthesis showed the following values: intensity of photosynthesis net (PN) 2.98 ± 0.85 μmol(CO2)m−2s−1; transpiration rate (E) 1.52 ± 0.20 mmol(H2O)m−2s−1; stomatal conductance (gs) 0.17 ± 0.03 mmol m−2s−1; and intercellular CO2 concentration (Ci) 417.14 ± 53.39 mmol L−1.
2.3. Determination of Ozonation Process Phytotoxicity
At the start, tests were performed to determine the phytotoxicity threshold value; for this purpose, red-veined sorrel plants were treated with increasing doses of ozone until the phytotoxicity threshold was achieved. The concentration rates of 1, 3, 5, and 10 ppm (mg m−3) were applied for 1, 3, 5, 7, and 10 min. The phytotoxicity threshold was examined visually based on the leaves’ necrosis content. Based on this trial, the experimental procedure was proposed.
2.4. The Ozone Treatment of the Plant Material
Pots with
Rumex sanguineus plants (30 pcs.) were placed in an ozonation chamber. This device was used in combination with the Ozone Solution TS 30 ozone generator (Ozone Solution, Hull, IA, USA), and the ozone concentration was measured by the 106 M UV-Ozone Solution detector (Ozone Solution, USA), with a measuring range of 0–1000 ppm (mg m
−3) [
27]. The plants were exposed to gaseous ozone at a concentration of 1 ppm for 1, 3, 5, 7 and 10 min, with 10 l min
−1 flow rate of ozone. The ozonation process was carried out at a constant temperature of 25 °C in three replications. The control sample was placed in the gas chamber for 10 min, in the same conditions without the presence of ozone. After the completed process of fumigation with gaseous ozone, the plants were again placed in a greenhouse at a temperature of 25 °C with the soil moisture maintained at a level corresponding to 50% of field capacity. Subsequently, the selected physiological characteristics of the plants were measured one, four, and eight days after the ozone treatment. On the same day, after the measurements were carried out, five pots were picked to represent each variant of the experiment (ozone treatment duration); the overground biomass was harvested from these. Subsequently, the raw leaves of red-veined sorrel were examined for their contents of vitamin C, total polyphenols, and antioxidant potential.
2.5. Measurement of Physiological Parameters
Methodologies described in detail in the
Supplementary Materials (S2; S3; S4) were followed by the measurement of relative chlorophyll content, based on the leaf greenness index (SPAD) method, with the use of SPAD 502 (Konica-Minolta Inc., Osaka, Japan) in the leaves of red-veined sorrel, and in the assessment of the selected parameters of chlorophyll fluorescence (maximum quantum yield of PSII photochemistry (F
v/F
m), the maximum quantum yield of primary photochemistry (F
v/F
0), and the performance index (PI)) as well as the gas exchange (net photosynthetic rate (PN), transpiration rate (E), stomatal conductance (g
s), and intercellular CO
2 concentration (Ci)).
The measurements of the selected physiological parameters of red-veined sorrel leaves were performed immediately before the ozone treatment was applied as well as one, four, and eight days after treatment. The measurements were performed in 20 replications for each ozone treatment duration.
2.6. Content of Bioactive Compounds
The contents of polyphenols in the leaves of
Rumex sanguineus were measured in accordance with the methodology described by Matłok et al. [
28]. Antioxidant potential of red-veined sorrel leaves was determined using the ABTS (2,2-azynobis (3etylobenzotiazolino-6-sulfonian)) method described by Matłok et al. [
29] as well as the DPPH (2,2-difenylo-1-pikrylohydrazyl) method described by Oszmiański et al. [
30].
2.7. Statistical Analysis
To verify the significance of the effect of ozonation time on the quality parameters for each term of analysis, one-way ANOVA (analysis of variance) and the Tukey post-hoc test was used at α = 0.05. The significance of the changes in physiological attributes during the vegetation time after ozonation was analyzed with one-way ANOVA and the Tukey post-hoc test at α = 0.05. These analyses were performed using STATISTICA12.5 PL from StatSoft.
3. Results and Discussion
3.1. Relative Chlorophyll Content (SPAD)
Based on the initial experiments, it was shown that the ozonation process should not apply a concentration exceeding 1 ppm (mg m
−3), and exposition time of not more than 10 min. Longer exposition or higher ozone concentration cause a phytotoxic effect on plants. In this case, the necrosis on leaves was observed. If the critical parameters of the ozonation process were exceeded, 24 h later, necroses and loss of turgor were observed in the red-veined sorrel plants, after a longer duration followed by the plants’ death. Ozone treatment applied to sorrel was in accordance with the procedure proposed in
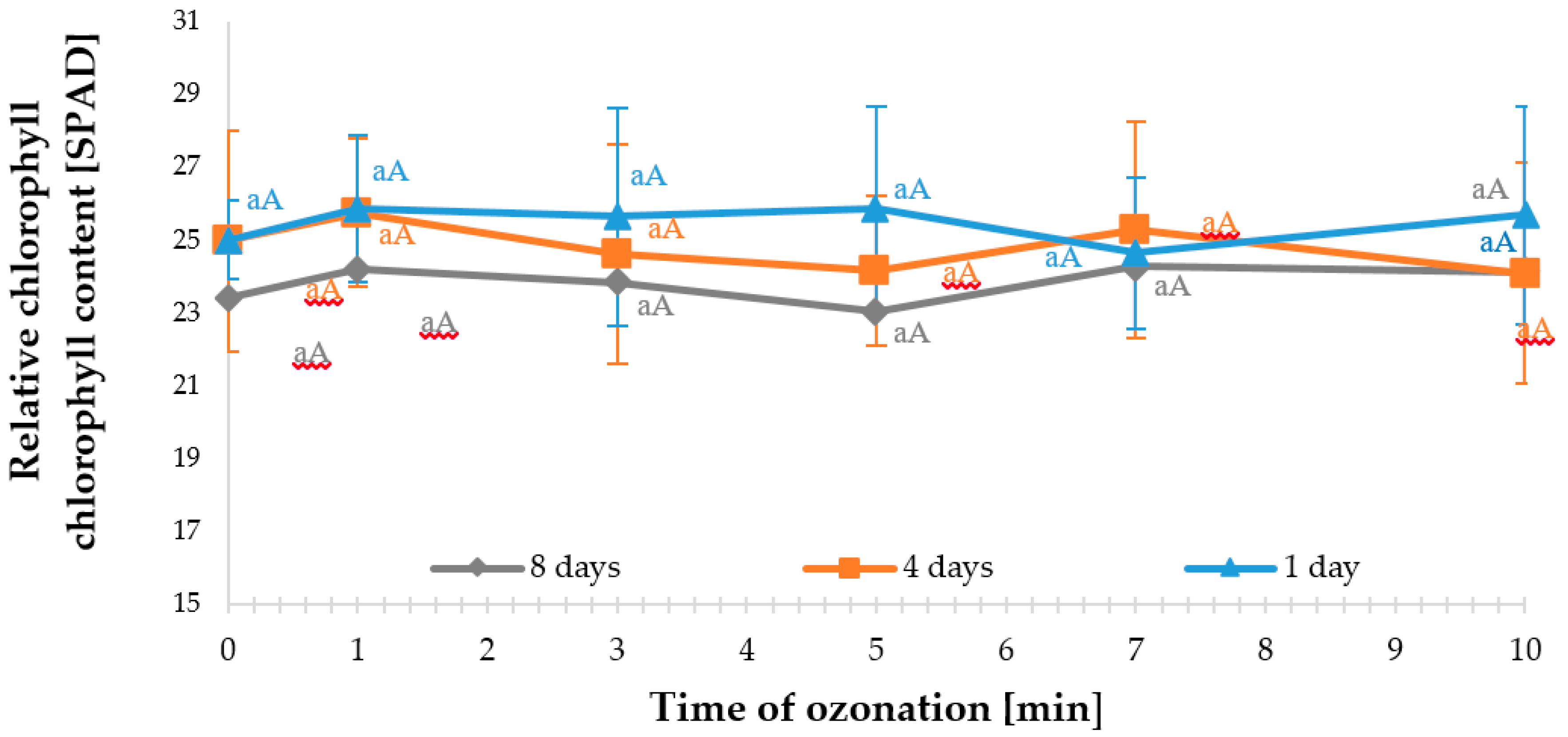
Section 2.4. and did not cause visible damage to the leaves. In order to determine in what way ozone treatment affected the sorrel plants, the relative chlorophyll contents were measured using the SPAD method (
Figure 1).
One day after ozone fumigation was applied to the plants of red-veined sorrel, a slight increase (not significant) in the relative chlorophyll content was found in the leaves of plants exposed to ozone at a 1 ppm (mg m
−3) concentration for 1, 3, 5, and 10 min. The subsequent measurements performed four and eight days after the ozone treatment showed that the sorrel plants subjected to the fumigation for 3, 5, and 10 min responded to ozone, which was reflected by slightly lower (not significant) chlorophyll content in the leaves compared to the control sample. On day 4, this content was lower by 1.6% (3 min), 3.4% (5 min), and 3.7% (10 min). However, on the eighth day, for the exposure time of 5 min after the ozonation treatment, only a 1.5% decrease in the relative content of chlorophyll was recorded compared to the control. This decrease, however, was not significant and no necroses were found on the leaves. In comparison to the chronic exposure of plants to ozone, which takes place in the polluted environment, the response of plants to short-term exposure to ozone follows a different mechanism. However, some plants responded to tropospheric ozone in a similar way. Borowiak et al. [
18] showed that the response of plants to this type of ozone varied, depending on their species and physiological age of the leaves. They identified positive relationships of all chlorophyll forms to the cumulative ozone concentration of 40 ppb (AOT 40) in the case of ozone-sensitive and resistant tobacco, and ozone-sensitive bean and petunia (
Petunia × hybrida L.) exposed to this factor for seven days. The highest chlorophyll contents in all the plant species taken into account were identified after the seventh day of the experiment. Later, the plants usually presented varied responses. Ozone-sensitive tobacco was found with decreased chlorophyll content, in contrast to the resistant tobacco. On the other hand, chlorophyll contents in the leaves of petunia were found to systematically decrease throughout the duration of the experiment.
3.2. Chlorophyll Fluorescence Parameters
Chlorophyll fluorescence measurements in research focusing on plant physiology are non-invasive and they enable the assessment of in vivo photosynthesis, particularly in situations when plants are exposed to various environmental phenomena including abiotic stress producing factors such as gaseous ozone. These may affect maximum quantum yield PSII, which is proportional to the F
v/F
m ratio, reflecting the effectiveness of light in primary reactions of photosynthesis [
31,
32]. The gaseous ozone applied to red-veined sorrel plants produced only a small change (not significant) in this parameter (
Figure 2A). The recorded values of the F
v/F
m ratio in sorrel leaves changed over time (one, four, and eight days following the ozonation), depending on the duration of plant exposure to ozone concentration. On the day following the ozone treatment (day 1), there was a slight increase in PII value in the plants exposed to ozone for all the durations tested, with the exception of the longest 1-min long exposition (a decrease of 0.6% compared to the control). The increase, however, was not significant (1.1%: 1 and 3 min; 0.8%: 5 min; 0.6%: 10 min), and the recorded F
v/F
m values were similar to the values of these parameters in most species of vascular plants (approximately 0.832 of the relative unit) [
33]. On the two subsequent dates of the measurements (four and eight days after the ozone treatment), there was a decrease in F
v/F
m to a value lower than 0.832 in the plants representing all the experimental groups, however, these values were slightly higher in the case of ozone-treated sorrel plants, compared to the controls. The decrease in F
v/F
m observed on these dates potentially could be associated with standard changes in plant physiology. Observed changes in F
v/F
m parameters are not significantly different. A decrease in photosynthesis efficiency of the leaves, relative to the growth phase, was also shown in a study by Michałek [
34].
Analogous effects of the different times (1, 3, 5, 7, and 10 min) during which sorrel plants were exposed to ozone in the present study were observed in measurements of maximum quantum yield of primary photochemistry (F
v/F
0) (
Figure 2B). The ratios F
v/F
0 and F
v/F
m contain the same information, but the former is a more sensitive measure and it reflects the PSII potential activity [
35].
The proposed experimental design did not cause a potentially predicted decrease in the PII parameters measured (F
v/F
m, F
v/F
0). This may have been associated with the short-term exposure of the plants to gaseous ozone due to which their response followed a completely different mechanism than the one resulting from chronic exposure to ozone in the environment. Effects of long-term ozone exposure include a reduced capacity of plants to use photoenergy and consequently to change the processes of photosynthesis [
36]. A study carried out by Wang [
37] showed that chronic exposure of
Quercus Mongolica plants to tropospheric ozone led to a 9% and 28% decrease in the values of F
v/F
m and F
v/F
0, respectively, after 90 days. Similar observations were reported by Nussbaum et al. [
38], who demonstrated the negative effects of ozone on the chlorophyll fluorescence parameters in seven species of herbaceous plants.
Effects of the ozone doses (concentration and duration of exposure) were also investigated in relation to performance index (PI) (
Figure 2C), which describes the effective amount of energy processed by PSII [
39]. The index provides useful information about the condition of the plant by combining information about the number of active reaction centers and initial light stage reactions with data about the flow of electrons through RC [
40]. The experiment carried out in this study showed a difference in PI relative to the duration of sorrel plant fumigation with gaseous ozone at a concentration of 1 ppm (mg m
−3). On the first day after the fumigation treatment, there was a significant increase in PI for all the tested exposure times (27.4%: 1 min; 24.1%: 3 min; 9.9%: 5 min; 28.7%: 7 min; 33.0%: 10 min). However, in the subsequent measurements (four and eight days after the treatment), the PI value decreased in all the experimental objects. The decrease, however, was not significant.
3.3. Gas Exchange Parameters
Plants growing in natural conditions are exposed to a variety of adverse factors, generally described as environmental stresses, which interfere with physiological processes in plants and consequently reduce their growth and yield. The process of photosynthesis is particularly sensitive to many stressful factors including gaseous ozone, and assimilation tissues are frequently damaged.
Negative changes in the plants treated with a gas concentration higher than 1 ppm (mg m
−3) ozone were observed. This concentration is a limiting parameter on the use of ozone on sorrel plants. Usually, a decrease in photosynthetic efficiency is the first symptom of the negative effect of ozone on plants. Imbalance in this phase leads to energy loss of the absorber and to the formation of reactive oxygen species in the chloroplasts [
41,
42].
The effects of ozone treatment applied to red-veined sorrel plants and the physiological response of the plants, particularly changes in photosynthesis process, were investigated by measuring selected gas exchange parameters. These included the intensity of photosynthesis net (PN) (
Figure 3A), whose values depended on the day of the measurement as well as the time during which sorrel plants were exposed to ozone at a concentration of 1 ppm. On the day following the ozone fumigation applied to the plants, there was an increase in PN values for all the exposure durations compared to the control. The second measurement (four days after the treatment) showed an insignificant decrease in PN in the samples exposed for 1, 3, 5, and 7 min. A slight decrease (not significant) in this parameter was also observed on the eighth day following the ozone treatment process for the exposure duration of 3, 5, and 7 min. The initial increase in the PN shown in the measurements was possibly linked with the response of the plants to the stress related to ozone. Seemingly, however, this did not induce the mechanism, which involves the closure of the stomata and synthesis of carotenoids and flavonoids, since no necrotic changes were observed on the leaves. It is likely that ozone entered through the open stomata, which induced the defense effect involving the activation of photosynthesis. Increased amount of energy possibly enabled the activation of other metabolic processes, particularly those that generate secondary metabolites with antioxidant potential. On the other hand, the decrease in PN rapidly observed during the subsequent measurements reflects a state of balance being regained by the plant.
The experimental design made it possible to show that the proposed lengths of time during which the plants were exposed to ozone were significantly related to transpiration rate (E) (
Figure 3B). The first two measurements (one and four days following the fumigation) showed significant increase in E value, coinciding with longer duration of the ozone treatment, compared to the control (no ozone treatment). On the other hand, after eight days, there was a decrease (not significant) in E value (1, 3, 5 min) found in the plants subjected to the ozone treatment, compared to the control, however, the decrease was not significant. It should be emphasized that only a positive effect of this process was observed.
The mechanism of the intensification of gas exchange as well as chlorophyll fluorescence is unknown in the case of short-term exposure to ozone. It is likely that ozone interferes with the biochemical balance of ozonated plants by producing RTFs (reactive oxygen species). To confirm this thesis, future research should take into account the measurement of the level of the superoxide radical, H2O2, and hydroxyl radicals. These factors, in a certain concentration, activate enzymes that remove them and enzymes that synthesize antioxidant substances. These enzymes include superoxide dismutase, catalase, and guaiacol peroxidase. It should be noted that increased gas exchange suggests an influence on metabolism. This thesis should be confirmed in further studies by measuring the change in ATP content (adenosine 5′-triphosphate) and a cell signal transduction marker such as cAMP (adenosine-3′,5′-cyclic monophosphate). Measurement of the change in the content of these factors should explain the mechanism of the observed phenomena.
It was also found that fumigation of the plants with the proposed ozone concentration (1 ppm (mg m
−3)) applied on a short-term basis for a duration of 1 to 10 min, in contrast to chronic exposure to even much lower doses of ozone, also significantly affected the photosynthesis process by increasing net photosynthesis and transpiration (
Figure 3A,B). Oliwa et al. [
26] demonstrated that chronic exposure of
Platycerium bifurcatum plants to ozone concentration of only 150 ppb resulted in a significant decrease in PN and E and an increase in the contents of carotenoids and flavonoids.
3.4. Bioactive Compounds Content
In a water solution (inside the cells), ozone breaks down into reactive oxygen species, whose excessive amount is highly toxic to plants. However, the current findings show that application of ozone in a controlled way may favorably affect the biological value of the plants subjected to the treatment by increased accumulation of bioactive compounds.
The present study showed that ozone treatment carried out in the proposed process conditions significantly affected the levels of antioxidants in sorrel plants, on the specified days after the ozone treatment was applied (
Figure 4A,B). The most beneficial effect was observed four days after fumigation with ozone in the sorrel plants subjected to ozone treatment for one minute. Antioxidant activity of the sorrel plants observed on that day increased by 36.1–41.6% in comparison to the control sample, relative to the measurement method applied. A similar relationship was found in the case of polyphenols (
Figure 4C). Contents of polyphenols increased after this time by ~31%. Higher antioxidant activity was also observed in sorrel plants treated with ozone for 1 min in the measurement carried out eight days after the fumigation, and in sorrel plants treated with ozone for 10 min in the measurement one day after the treatment.
Increased contents of polyphenols following ozone treatment were observed in a number of earlier studies. Piechowiak and Balawejder [
43] investigated the effects of ozone on the levels of oxidative stress and small-molecule antioxidants in raspberries stored at room temperature. The authors found that ozone treatment where the gas was applied to the fruit at a concentration of 10 ppm for 30 min activated defense mechanisms against oxidative stress (i.e., increases activity of antioxidant enzymes as well as enzymes involved in biosynthesis of polyphenols), which was reflected by increased antioxidant activity. Similar associations were observed by Chen et al. [
44], who demonstrated that increased polyphenol contents in strawberries treated with 5 ppm ozone concentration for 10 h increased expression of the genes encoding phenylalanine ammonia lyases. Furthermore, Xu et al. [
9] showed that treatment of coriander with ozone for 10 min at a concentration of 0.68 mg L
−1 activated antioxidant enzymes, leading to a decrease in the cellular content of reactive oxygen species and at the same time eliminating the negative effects of oxidative stress and preventing loss of quality in the raw produce.
Increased biosynthesis of these compounds may be associated with increased plant metabolism, as shown by the measurement of gas exchange intensity. However, without in-depth studies of the biochemical mechanisms of this process, this thesis will remain a hypothesis.