Improving Seed Retention and Germination Characteristics of North American Basin Wildrye by Marker-Assisted Gene Introgression
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and SH6-Linked DNA Marker
2.2. Genotyping the Ltc096 Marker
2.3. Measuring Effects of SH6 Seed-Shattering QTL Marker on Seed Yield and Seed Germination Traits
2.4. Statistical Analyses
3. Results
3.1. Marker-Assisted Selection of L57S-491 and L57S-577 Parents and Segregation of Ltc096 Marker Alleles
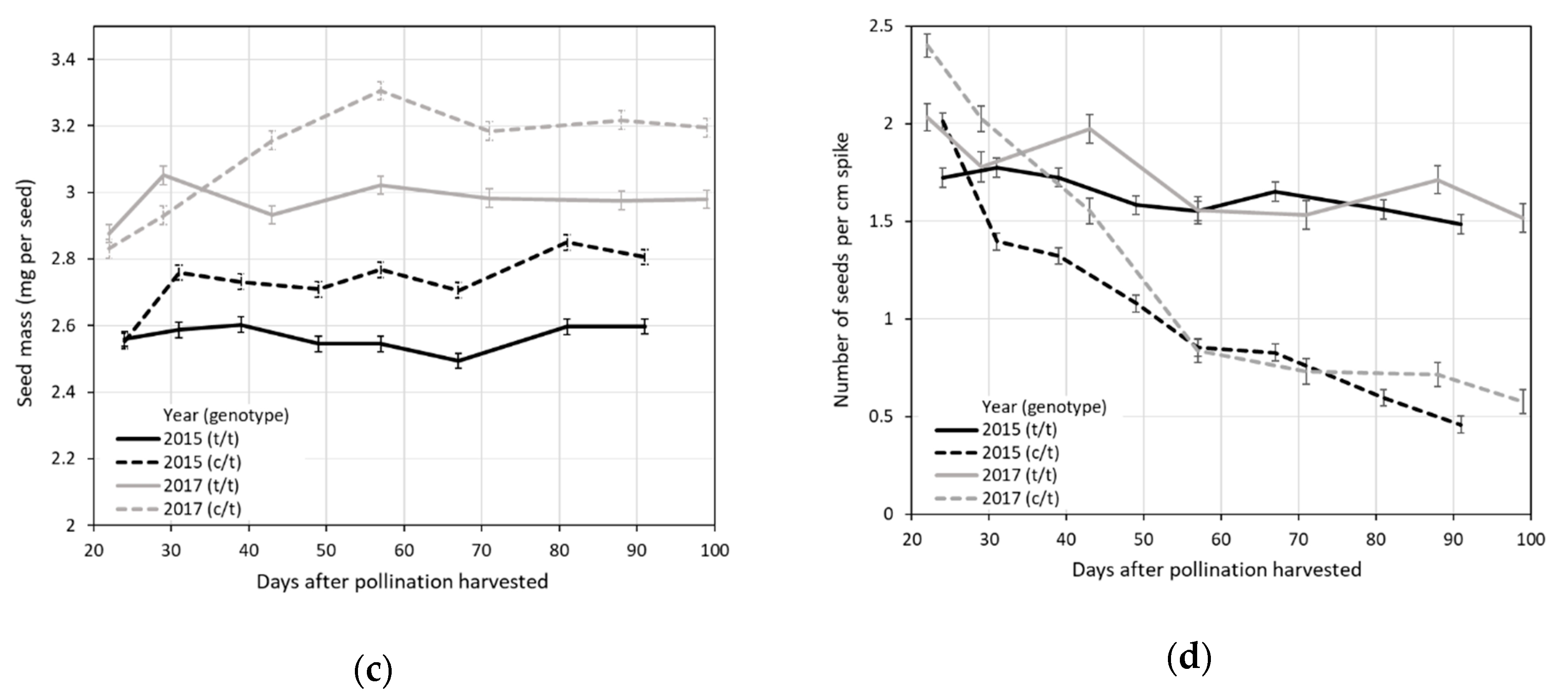
3.2. Average Effects of the Ltc096 Marker on Spike Yield Traits in the L57S-491 × L57S-577 F1 Population
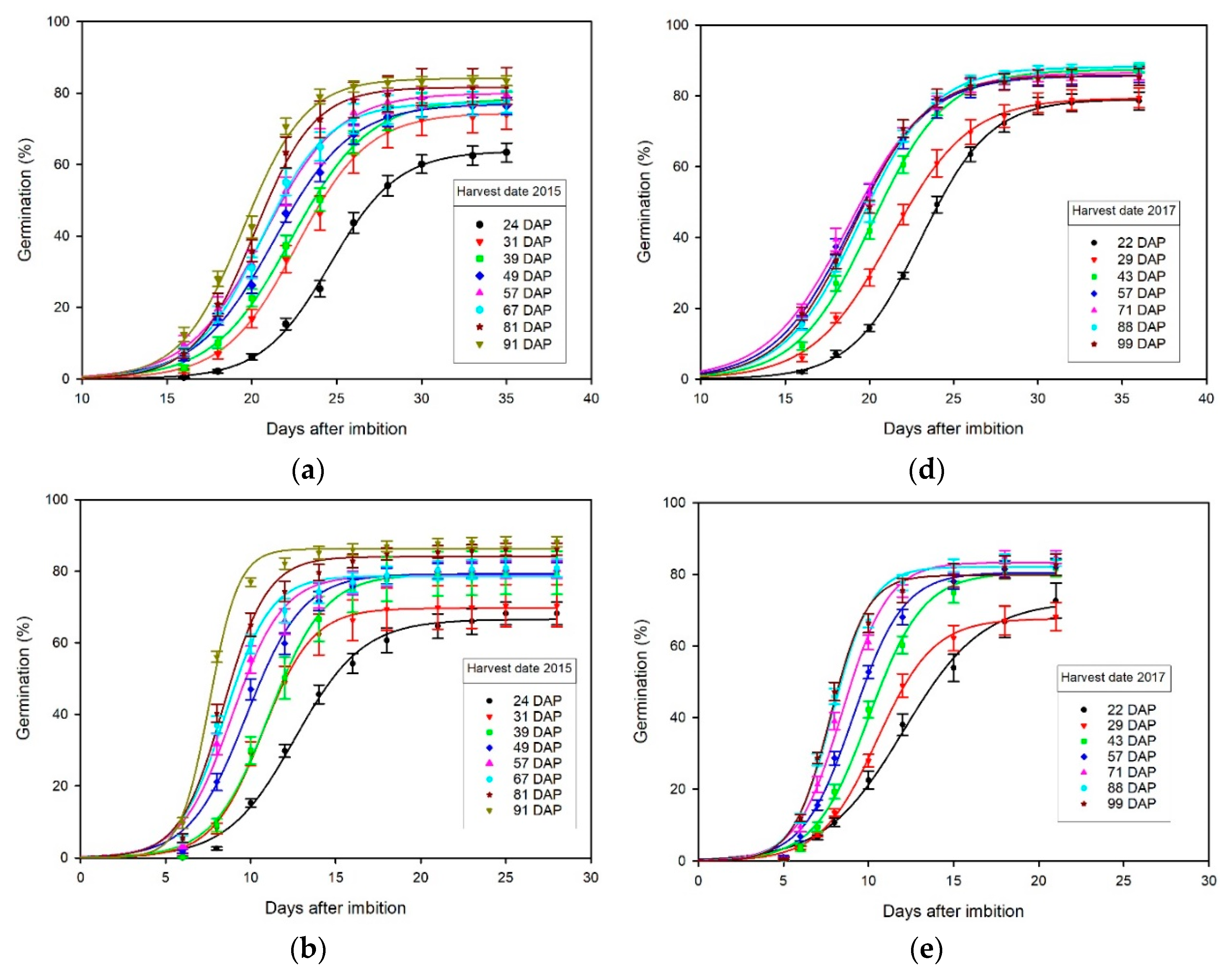
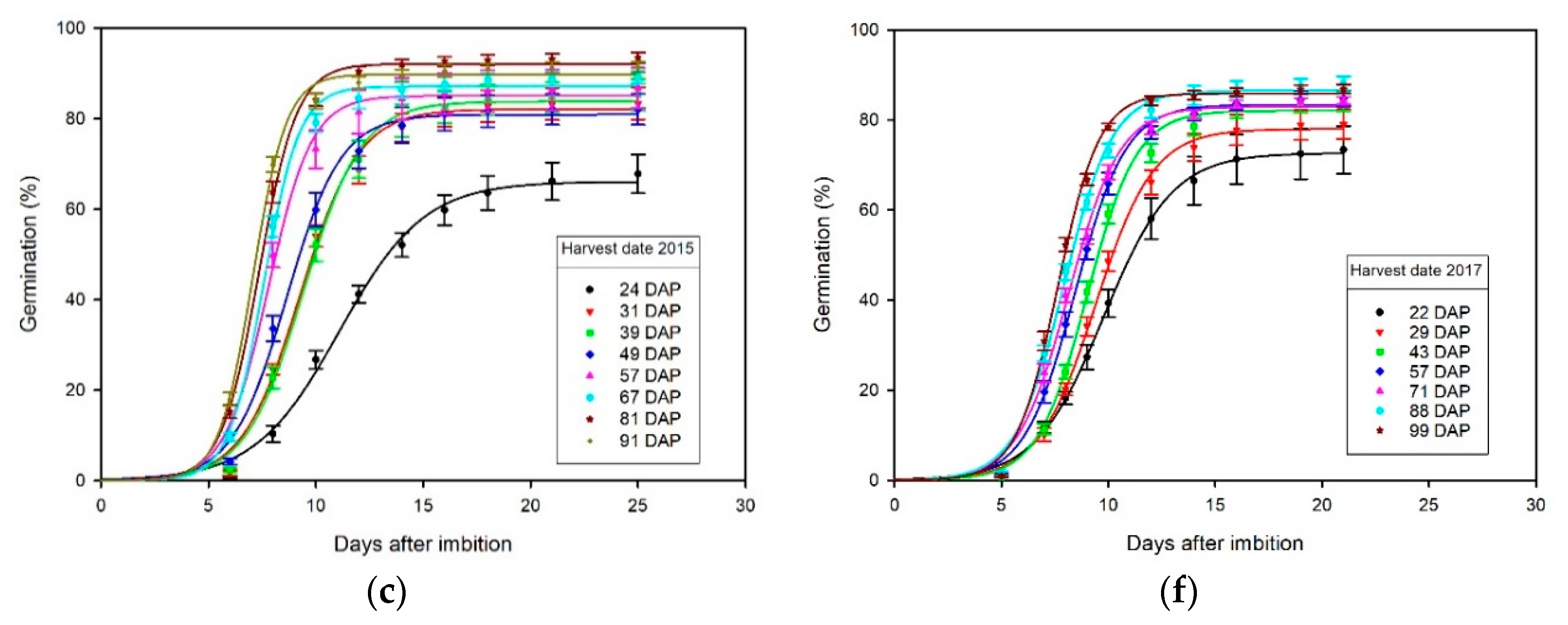
3.3. Effect of Harvest Date on Germination
4. Discussion
4.1. Seed Yield Benefits of the sh6 Seed-Retention Gene
4.2. Germination Effects of Harvest Date
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pilliod, S.D.; Welty, J.L.; Toevs, G.R. Seventy-Five Years of Vegetation Treatments on Public Rangelands in the Great Basin of North. America. Rangelands 2017, 39, 1–9. [Google Scholar] [CrossRef]
- Duniway, C.M.; Palmquist, E.; Miller, M.E. Evaluating rehabilitation efforts following the Milford Flat Fire: Successes, failures, and controlling factors. Ecosphere 2015, 6, 80. [Google Scholar] [CrossRef]
- Peppin, D.; Fulé, P.Z.; Sieg, C.H.; Beyers, J.L.; Hunter, M.E. Post-wildfire seeding in forests of the western United States: An. evidence-based review. Forest Ecol. Manag. 2010, 260, 573–586. [Google Scholar] [CrossRef]
- James, J.; Svejcar, T.; Rinella, M. Demographic processes limiting seedling recruitment in arid grassland restoration. J. Appl. Ecol. 2011, 48, 961–969. [Google Scholar] [CrossRef]
- Larson, J.E.; Sheley, R.L.; Hardegree, S.P.; Doescher, P.S.; James, J.J. Seed and seedling traits affecting critical life stage transitions and recruitment outcomes in dryland grasses. J. Appl. Ecol. 2015, 52, 199–209. [Google Scholar] [CrossRef]
- Leger, E.A.; Atwater, D.Z.; James, J.J. Seed and seedling traits have strong impacts on establishment of a perennial bunchgrass in invaded semi-arid systems. J. Appl. Ecol. 2019, 56, 1343–1354. [Google Scholar] [CrossRef]
- Knutson, K.C.; Pyke, D.A.; Wirth, T.A.; Arkle, R.S.; Pilliod, D.S.; Brooks, M.L.; Chambers, J.C.; Grace, J.B. Long-term effects of seeding after wildfire on vegetation in Great Basin shrubland ecosystems. J. Appl. Ecol. 2014, 51, 1414–1424. [Google Scholar] [CrossRef]
- Madsen, M.D.; Davies, K.W.; Boyd, C.S.; Kerby, J.D.; Svejcar, T.J. Emerging seed enhancement technologies for overcoming barriers to restoration. Restor. Ecol. 2016, 24, S77–S84. [Google Scholar] [CrossRef]
- Rigby, C.W.; Jensen, K.B.; Creech, J.E.; Thacker, E.T.; Waldron, B.L.; Derner, J.D. Establishment and Trends in Persistence of Selected Perennial Cool-Season Grasses in Western United States. Rangel. Ecol. Manag. 2018, 71, 681–690. [Google Scholar] [CrossRef]
- Robins, J.G.; Jensen, K.B.; Jones, T.A.; Waldron, B.L.; Peel, M.D.; Rigby, C.W.; Vogel, K.P.; Mitchell, R.B.; Palazzo, A.J.; Cary, T.J. Stand. establishment and persistence of perennial cool-season grasses in the intermountain west and the central and northern great plains. Rangel. Ecol. Manag. 2013, 66, 181–190. [Google Scholar] [CrossRef][Green Version]
- Brummer, E.C.; Barber, W.T.; Collier, S.M.; Cox, T.S.; Johnson, R.; Murray, S.C.; Olsen, R.T.; Pratt, R.C.; Thro, A.M. Plant breeding for harmony between agriculture and the environment. Front. Ecol. Environ. 2011, 9, 561–568. [Google Scholar] [CrossRef]
- DeHaan, L.R.; van Tassel, D.L.; Cox, T.S. Perennial grain crops: A synthesis of ecology and plant breeding. Renew. Agric. Food Syst. 2007, 20, 5–14. [Google Scholar] [CrossRef]
- Jones, T.A.; Monaco, T.A.; Rigby, C.W. The potential of novel native plant materials for the restoration of novel ecosystems. Elem. Sci. Anth. 2015, 3. [Google Scholar] [CrossRef]
- Cruz, R.; Ganskopp, D. Seasonal preferences of steers for prominent northern great basin grasses. J. Range Manag. 1998, 51, 557. [Google Scholar] [CrossRef]
- Ganskopp, D.; Bohnert, D. Nutritional dynamics of 7 northern great basin grasses. J. Range Manag. 2001, 54. [Google Scholar] [CrossRef]
- Ganskopp, D.; Aguilera, L.; Vavra, M. Livestock Forage Conditioning Among Six Northern Great Basin Grasses. Rangel. Ecol. Manag. 2007, 60, 71–78. [Google Scholar] [CrossRef]
- Ogle, D.G.; Tilley, D.; John, L.S. Plant Guide for Basin Wildrye (Leymus cinereus); USDA NRCS Plant Materials Center: Aberdeen, ID, USA, 2012.
- Culumber, C.M.; Larson, S.R.; Jones, T.A.; Jensen, K.B. Wide-scale population sampling identifies three phylogeographic races of basin wildrye and low-level genetic admixture with creeping wildrye. Crop Sci. 2013, 53, 996–1007. [Google Scholar] [CrossRef]
- Larson, S.R.; Pearson, C.H.; Jensen, K.B.; Jones, T.A.; Mott, I.W.; Robbins, M.D.; Staub, J.E.; Waldron, B.L. Development and testing of cool-season grass species, varieties and hybrids for biomass feedstock production in western North. America. Agronomy 2017, 7, 3. [Google Scholar] [CrossRef]
- Porensky, L.M.; Davison, J.; Leger, E.A.; Miller, W.W.; Goergen, E.M.; Espeland, E.K.; Carroll-Moore, E.M. Grasses for biofuels: A low water-use alternative for cold desert agriculture? Biomass Bioenergy 2014, 66, 133–142. [Google Scholar] [CrossRef]
- Larson, S.R.; Wu, X.; Jones, T.A.; Jensen, K.B.; Chatterton, N.J.; Waldron, B.L.; Robins, J.G.; Bushman, B.S.; Palazzo, A.J. Comparative mapping of growth habit, plant height, and flowering QTLs in two interspecific families of Leymus. Crop Sci. 2006, 46, 2526–2539. [Google Scholar] [CrossRef]
- Larson, S.R.; Kellogg, E.A. Genetic dissection of seed production traits and identification of a major-effect seed retention QTL in hybrid Leymus (Triticeae) wildryes. Crop Sci. 2009, 49, 29–40. [Google Scholar] [CrossRef]
- Young, J.A.; Evans, R.A. Germination of great basin wildrye seeds collected from native stands1. Agron. J. 1981, 73, 917–920. [Google Scholar] [CrossRef]
- Roundy, B.A. Response of basin wildrye and tall wheatgrass seedlings to salination1. Agron. J. 1983, 75, 67–71. [Google Scholar] [CrossRef]
- Roundy, B.A.; Young, J.A.; Evans, R.A. Germination of basin wildrye and tall wheatgrass in relation to osmotic and matric potential1. Agron. J. 1985, 77, 129–135. [Google Scholar] [CrossRef]
- Evans, R.A.; Young, J.A. Magnar basin wildrye: Germination in relation to temperature. J. Range Manag. 1983, 36, 395–398. [Google Scholar] [CrossRef]
- Robins, J.G.; Bushman, B.S.; West, M.S. Effects of Selection for Seedling Vigor on the Genetic Variation in Leymus cinereus. Rangel. Ecol. Manag. 2017, 70, 504–508. [Google Scholar] [CrossRef]
- Jones, T.A.; Parr, S.D.; Winslow, S.R.; Rosales, M.A. Notice of release of ‘Continental’basin wildrye. Nativ. Plants J. 2009, 10, 57–61. [Google Scholar] [CrossRef]
- Berdahl, J.D.; Frank, A.B. Seed maturity in four cool-season forage grasses. Agron. J. 1998, 90, 483–488. [Google Scholar] [CrossRef]
- USDA-NRCS. Conservation Plant Release Brochure for ’Rio’ Beardless Wild Rye (Leymus Triticoides Buckley); USDA NRCS Plant Materials Center: Lockeford, CA, USA, 2014.
- Knapp, A.D.; Wiesner, L.E. Seed dormancy of beardless wildrye (Elymus triticoides Buckl.). J. Seed Technol. 1978, 3, 1–9. [Google Scholar]
- Larson, S.R.; Jensen, K.B.; Robins, J.G.; Waldron, B.L. Genes and quantitative trait. loci controlling biomass yield and forage quality traits in perennial wildrye. Crop Sci. 2014, 54, 111–126. [Google Scholar] [CrossRef]
- Yun, L.; Larson, S.R.; Jensen, K.B.; Staub, J.E.; Grossl, P.R. Quantitative trait loci (QTL) and candidate genes associated with trace element concentrations in perennial grasses grown on phytotoxic soil contaminated with heavy metals. Plant Soil 2015, 396, 277–296. [Google Scholar] [CrossRef]
- Yun, L.; Larson, S.R.; Mott, I.W.; Jensen, K.B.; Staub, J.E. Genetic control of rhizomes and genomic localization of a major-effect growth habit QTL in perennial wildrye. Mol. Genet. Genom. 2014, 289, 383–397. [Google Scholar] [CrossRef]
- Bushman, B.S.; Larson, S.R.; Mott, I.W.; Cliften, P.F.; Wang, R.R.C.; Chatterton, N.J.; Hernandez, A.G.; Ali, S.; Kim, R.W.; Thimmapuram, J.; et al. Development and annotation of perennial Triticeae ESTs and SSR markers. Genome 2008, 51, 779–788. [Google Scholar] [CrossRef]
- Kennard, C.; Phillips, L.; Porter, A. Genetic dissection of seed shattering, agronomic, and color traits in American wildrice (Zizania palustris var. interior L.) with a comparative map. Theor. Appl. Genet. 2002, 105, 1075–1086. [Google Scholar] [CrossRef]
- Larson, S.R.; Kishii, M.; Tsujimoto, H.; Qi, L.; Chen, P.; Lazo, G.R.; Jensen, K.B.; Wang, R.R.C. Leymus EST linkage maps identify 4NsL-5NsL reciprocal translocation, wheat-Leymus chromosome introgressions, and functionally important gene loci. Theor. Appl. Genet. 2012, 124, 189–206. [Google Scholar] [CrossRef]
- Benham, J.J. Genographer Version 1.6.0; Montana State University: Bozeman, MT, USA, 2001. [Google Scholar]
- Larson, S.R.; Kellogg, E.A.; Jensen, K.B. Genes and QTLs Controlling Inflorescence and Stem Branch Architecture in Leymus (Poaceae: Triticeae) Wildrye. J. Hered. 2013, 104, 678–691. [Google Scholar] [CrossRef]
- Konishi, S.; Izawa, T.; Lin, S.Y.; Ebana, K.; Fukuta, Y.; Sasaki, T.; Yano, M.A. SNP caused loss of seed shattering during rice domestication. Science 2006, 312, 1392–1396. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhou, A.; Sang, T. Rice domestication by reducing shattering. Science 2006, 311, 1936–1939. [Google Scholar] [CrossRef]
- Sang, T.; Ge, S. The puzzle of rice domestication (seed shatter disarticulation). J. Integr. Plant Biol. 2008, 49, 760–768. [Google Scholar] [CrossRef]
- Win, K.T.; Yamagata, Y.; Doi, K.; Uyama, K.; Nagai, Y.; Toda, Y.; Kani, T.; Ashikari, M.; Yasui, H.; Yoshimura, A. A single base change explains the independent origin of and selection for the nonshattering gene in African rice domestication. New Phytol. 2017, 213, 1925–1935. [Google Scholar] [CrossRef]
- Yoon, J.; Cho, L.H.; Antt, H.W.; Koh, H.J.; An, G. KNOX Protein OSH15 Induces Grain Shattering by Repressing Lignin Biosynthesis Genes. Plant Physiol. 2017, 174, 312–325. [Google Scholar] [CrossRef]
- Yoon, J.; Cho, L.H.; Kim, S.L.; Choi, H.; Koh, H.J.; An, G. The BEL1-type homeobox gene SH5 induces seed shattering by enhancing abscission-zone development and inhibiting lignin biosynthesis. Plant J. 2014, 79, 717–728. [Google Scholar] [CrossRef]
- Zhou, Y.; Lu, D.; Li, C.; Luo, J.; Zhu, B.F.; Zhu, J.; Shangguan, Y.; Wang, Z.; Sang, T.; Zhou, B.; et al. Genetic control of seed shattering in rice by the APETALA2 transcription factor Shattering Abortion1. Plant Cell 2012, 24, 1034–1048. [Google Scholar] [CrossRef] [PubMed]
- Doebley, J. Unfallen grains: How ancient farmers turned weeds into crops. Science 2006, 312, 1318–1319. [Google Scholar] [CrossRef]
- Li, W.; Gill, B.S. Multiple genetic pathways for seed shattering in the grasses. Funct. Integr. Genom. 2006, 6, 300–309. [Google Scholar] [CrossRef]
- Pourkheirandish, M.; Hensel, G.; Kilian, B.; Senthil, N.; Chen, G.; Sameri, M.; Azhaguvel, P.; Sakuma, S.; Dhanagond, S.; Sharma, R.; et al. Evolution of the grain dispersal system in barley. Cell 2015, 162, 527–539. [Google Scholar] [CrossRef]
- Sang, T. Genes and Mutations Underlying Domestication Transitions in Grasses. Plant Physiol. 2009, 149, 63–70. [Google Scholar] [CrossRef]
- Balanzà, V.; Roig-Villanova, I.; Di Marzo, M.; Masiero, S.; Colombo, L. Seed abscission and fruit dehiscence required for seed dispersal rely on similar genetic networks. Development 2016, 143, 3372–3381. [Google Scholar] [CrossRef]
- Hill, M.J.; Watkin, B.R. Seed production studies on perennial ryegrass, timothy and prairie grass: 2. Changes in physiological components during seed development and time and method of harvesting for maximum seed yield. Grass Forage Sci. 1975, 30, 131–140. [Google Scholar] [CrossRef]
- Steadman, K.J.; Crawford, A.D.; Gallagher, R.S. Dormancy release in Lolium rigidum seeds is a function of thermal after-ripening time and seed water content. Funct. Plant Biol. 2003, 30, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Favier, F.J.; Woods, J.L. The quantification of dormancy loss in barley (Hordeum vulgare L.). Seed Sci. Technol. 1993, 21, 653–674. [Google Scholar]
- Foley, M.E. Temperature and water status of seed affect afterripening in wild oat (Avena fatua). Weed Sci. 1994, 42, 200–204. [Google Scholar] [CrossRef]
- Bauer, M.C.; Meyer, S.E.; Allen, P.S. A simulation model to predict seed dormancy loss in the field for Bromus tectorum L. J. Exp. Bot. 1998, 49, 1235–1244. [Google Scholar]
- Baskin, J.M.; Baskin, C.C. High temperature requirement for afterripening in seeds of winter annuals. New Phytol. 1976, 77, 619–624. [Google Scholar] [CrossRef]
- Baskin, J.M.; Baskin, C.C. Temperature requirements for after-ripening in seeds of nine winter annuals. Weed Res. 1986, 26, 375–380. [Google Scholar] [CrossRef]

| Harvest Date | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| GT 1 | 4PL 2 | 24 DAP | 31 DAP | 39 DAP | 49 DAP | 57 DAP | 67 DAP | 81 DAP | 91 DAP |
| 15_GT1 | 64.2 (2.36) | 75.3 (4.36) | 79.7 (3.46) | 77.6 (2.75) | 79.4 (3.87) | 76.9 (3.61) | 81.2 (4.55) | 82.7 (2.23) | |
| 12.1 (1.26) | 10.5 (1.66) | 9.4 (1.04) | 9.2 (0.83) | 9.5 (1.20) | 10.5 (1.36) | 11.5 (1.86) | 11.02 (0.83) | ||
| 24.6 (0.23) | 22.6 (0.37) | 22.3 (0.29) | 21.3 (0.23) | 20.7 (0.32) | 20.5 (0.29) | 20.2 (0.33) | 19.6 (0.16) | ||
| 0.6 (1.25) | 0.4 (2.77) | 0.2 (2.19) | 0.6 (1.91) | 1.6 (2.84) | 1.2 (2.74) | 1.1 (3.59) | 2.0 (1.81) | ||
| 0.943 | 0.870 | 0.928 | 0.947 | 0.898 | 0.899 | 0.854 | 0.961 | ||
| 15_GT2 | 69.6 (2.69) | 70.9 (4.44) | 81.1 (5.0) | 82.4 (2.4) | 82.2 (3.0) | 81.8 (2.72) | 87.2 (2.21) | 88.0 (1.56) | |
| 5.7 (0.64) | 6.9 (1.46) | 6.4 (1.24) | 5.5 (0.46) | 5.6 (0.59) | 5.8 (0.58) | 6.1 (0.47) | 8.1 (0.50) | ||
| 12.5 (0.26) | 10.6 (0.37) | 10.9 (0.38) | 9.5 (0.17) | 8.7 (0.20) | 8.3 (0.17) | 8.2 (0.13) | 7.5 (0.07) | ||
| 1.0 (1.75) | 0.4 (3.49) | 0.7 (3.77) | 1.7 (1.91) | 2.1 (2.53) | 2.0 (2.35) | 2.0 (1.92) | 1.1 (1.42) | ||
| 0.928 | 0.791 | 0.809 | 0.952 | 0.921 | 0.931 | 0.958 | 0.978 | ||
| 15_GT3 | 69.8 (3.72) | 85.1 (3.00) | 86.1 (3.32) | 83.4 (3.48) | 86.8 (4.23) | 88.5 (1.85) | 93.1 (1.42) | 90.5 (1.49) | |
| 5.1 (0.69) | 6.4 (0.69) | 6.6 (0.79) | 6.5 (0.85) | 7.3 (1.19) | 8.4 (0.63) | 8.0 (0.40) | 8.7 (0.45) | ||
| 11.0 (0.32) | 9.1 (0.18) | 9.3 (0.20) | 8.5 (0.20) | 7.7 (0.21) | 7.5 (0.08) | 7.3 (0.06) | 7.0 (0.06) | ||
| 1.1 (2.28) | 1.5 (2.34) | 0.9 (2.57) | 1.3 (2.83) | 0.8 (3.65) | 0.7 (1.63) | 0.4 (1.27) | 0.3 (1.35) | ||
| 0.904 | 0.937 | 0.925 | 0.913 | 0.878 | 0.975 | 0.986 | 0.983 | ||
| Harvest Date | ||||||||
|---|---|---|---|---|---|---|---|---|
| GT 1 | 4PL 2 | 22 DAP | 29 DAP | 43 DAP | 57 DAP | 71 DAP | 88 DAP | 99 DAP |
| 17_GT1 | 79.2 (2.09) | 80.7 (3.34) | 88.7 (1.86) | 87.2 (2.68) | 87.7 (2.48) | 88.6 (1.90) | 85.6 (2.62) | |
| 11.2 (0.84) | 8.9 (0.91) | 9.0 (0.48) | 8.3 (0.66) | 7.9 (0.58) | 8.9 (0.49) | 9.0 (0.71) | ||
| 23.1 (0.17) | 21.2 (0.28) | 20.1 (0.14) | 18.8 (0.21) | 18.7 (0.19) | 19.4 (0.14) | 19.1 (0.20) | ||
| 1.0 (1.28) | 0.3 (2.29) | 0.1 (1.41) | 0.3 (2.15) | 0.1 (1.97) | 0.8 (1.50) | 1.2 (2.11) | ||
| 0.969 | 0.931 | 0.979 | 0.948 | 0.956 | 0.976 | 0.950 | ||
| 17_GT2 | 72.1 (7.50) | 69.5 (3.20) | 84.4 (2.55) | 83.9 (2.10) | 87.1 (2.06) | 85.8 (1.89) | 83.6 (2.73) | |
| 4.1 (0.64) | 5.8 (0.73) | 5.4 (0.43) | 5.5 (0.37) | 5.7 (0.36) | 6.2 (0.39) | 6.3 (0.60) | ||
| 12.5 (0.63) | 10.6 (0.25) | 10.0 (0.16) | 9.0 (0.13) | 8.3 (0.11) | 7.8 (0.09) | 7.7 (0.13) | ||
| 0.5 (2.04) | 0.6 (1.64) | 0.9 (1.43) | 1.0 (1.36) | 1.8 (1.44) | 2.1 (1.42) | 2.2 (2.08) | ||
| 0.912 | 0.932 | 0.969 | 0.975 | 0.976 | 0.977 | 0.951 | ||
| 17_GT3 | 74.4 (4.46) | 79.7 (2.50) | 83.4 (1.81) | 85.3 (1.84) | 85.2 (1.33) | 88.9 (1.65) | 87.8 (1.11) | |
| 5.8 (0.92) | 6.6 (0.61) | 7.3 (0.49) | 6.8 (0.45) | 6.5 (0.31) | 6.6 (0.38) | 7.6 (0.33) | ||
| 9.8 (0.29) | 9.4 (0.14) | 9.0 (0.09) | 8.4 (0.08) | 8.1 (0.06) | 7.8 (0.07) | 7.6 (0.04) | ||
| 0.3 (2.80) | 0.1 (1.72) | 0.4 (1.33) | 0.6 (1.40) | 0.9 (1.02) | 1.2 (1.29) | 1.1 (0.90) | ||
| 0.858 | 0.949 | 0.972 | 0.972 | 0.985 | 0.979 | 0.989 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Larson, S.R.; Jones, T.A.; Johnson, L.M.; Waldron, B.L. Improving Seed Retention and Germination Characteristics of North American Basin Wildrye by Marker-Assisted Gene Introgression. Agronomy 2020, 10, 1740. https://doi.org/10.3390/agronomy10111740
Larson SR, Jones TA, Johnson LM, Waldron BL. Improving Seed Retention and Germination Characteristics of North American Basin Wildrye by Marker-Assisted Gene Introgression. Agronomy. 2020; 10(11):1740. https://doi.org/10.3390/agronomy10111740
Chicago/Turabian StyleLarson, Steven R., Thomas A. Jones, Linnea M. Johnson, and Blair L. Waldron. 2020. "Improving Seed Retention and Germination Characteristics of North American Basin Wildrye by Marker-Assisted Gene Introgression" Agronomy 10, no. 11: 1740. https://doi.org/10.3390/agronomy10111740
APA StyleLarson, S. R., Jones, T. A., Johnson, L. M., & Waldron, B. L. (2020). Improving Seed Retention and Germination Characteristics of North American Basin Wildrye by Marker-Assisted Gene Introgression. Agronomy, 10(11), 1740. https://doi.org/10.3390/agronomy10111740






