Kinetics of C Mineralization of Biochars in Three Excessive Compost-Fertilized Soils: Effects of Feedstocks and Soil Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Characterization of Soils and Compost
2.2. Preparation and Characterization of Biochar
2.3. Extracting Water-Soluble Biochar C and Analyses
2.4. Soil–Biochar Incubation Experiment
2.5. Data Analysis and Statistics
3. Results
3.1. Biochar Characterization
3.2. Water Soluble Extracts of Biochar
3.3. Carbon Mineralization
3.4. Kinetics of Carbon Mineralization
3.5. Nutrient Availability
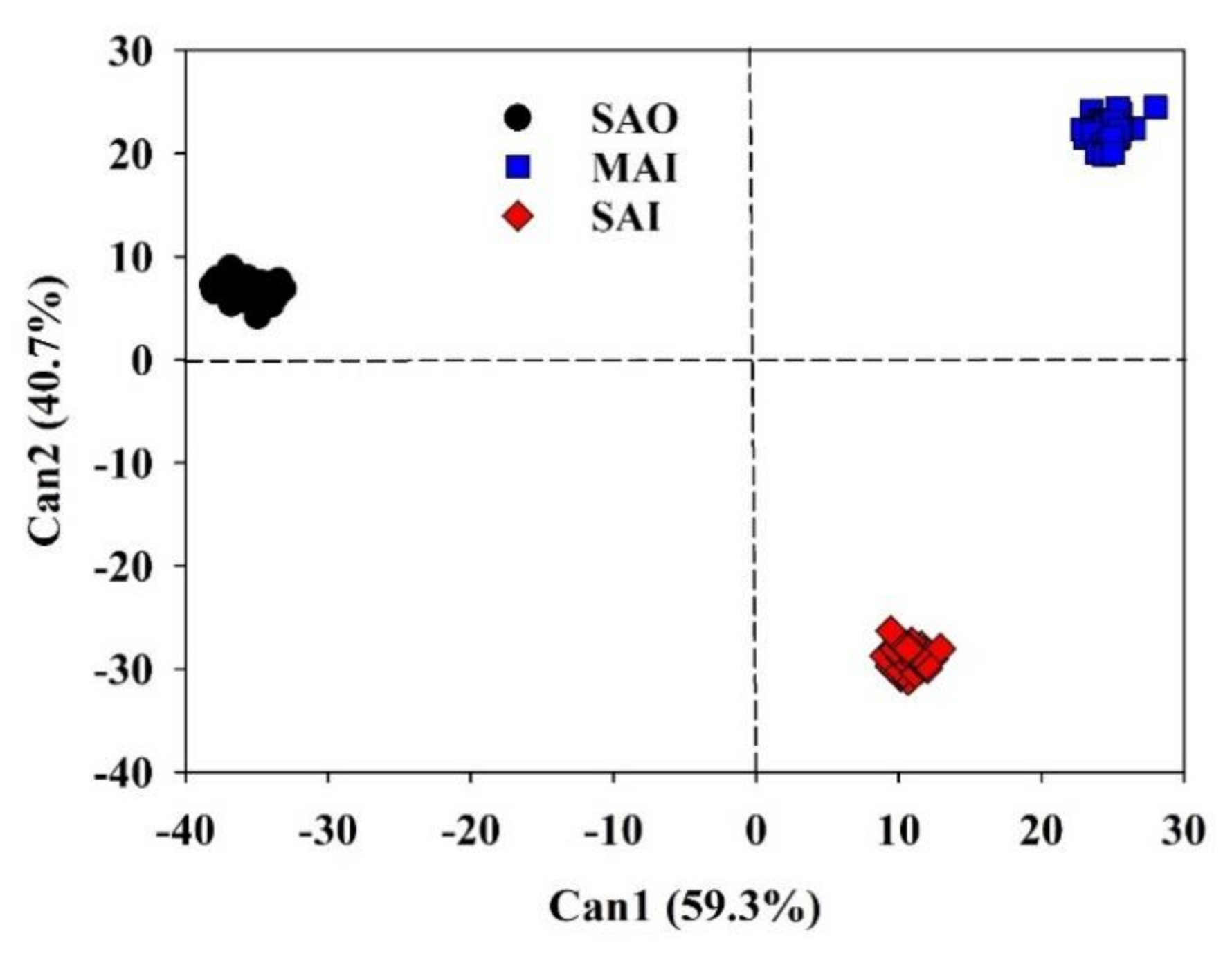
3.6. Canonical Discriminant Analysis
4. Discussion
4.1. CO2 Emissions as Affected by Biochar
4.2. C Sequestration and Nutrient Availability as Affected by Biochar
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Qayyum, M.F.; Steffens, D.; Reisenauer, H.P.; Schubert, S. Kinetics of carbon mineralization of biochars compared with wheat straw in three soils. J. Environ. Qual. 2012, 41, 1210–1220. [Google Scholar] [CrossRef] [PubMed]
- Glaser, B.; Lehmann, J.; Zech, W. Ameliorating physical and chemical properties of highly weathered soils in the tropics with charcoal—A review. Biol. Fertil. Soils 2002, 35, 219–230. [Google Scholar] [CrossRef]
- Keith, A.; Singh, B.; Singh, B.P. Interactive priming of biochar and labile organic matter mineralization in a smectite-rich soil. Environ. Sci. Technol. 2011, 45, 9611–9618. [Google Scholar] [CrossRef] [PubMed]
- Plaza, C.; Giannetta, B.; Fernández, J.M.; López-de-Sá, E.G.; Polo, A.; Gascó, G.; Méndez, A.; Zaccone, C. Response of different soil organic matter pools to biochar and organic fertilizers. Agric. Ecosyst. Environ. 2016, 225, 150–159. [Google Scholar] [CrossRef]
- Liang, B.; Lehmann, J.; Solomon, D.; Sohi, S.; Thies, J.E.; Skjemstad, J.O.; Luizão, F.J.; Engelhard, M.H.; Neves, E.G.; Wirick, S. Stability of biomass-derived black carbon in soils. Geochim. Cosmochim. Acta 2008, 72, 1598–1610. [Google Scholar] [CrossRef]
- Rogovska, N.; Laird, D.; Cruse, R.; Fleming, P.; Parkin, T.; Meek, D. Impact of biochar on manure carbon stabilization and greenhouse gas emissin. Soil Sci. Soc. Am. J. 2011, 75, 871–879. [Google Scholar] [CrossRef] [Green Version]
- Zimmerman, A.R.; Gao, B.; Ahn, M.-Y. Positive and negative carbon mineralization priming effects among a variety of biochar-amended soils. Soil Biol. Biochem. 2011, 43, 1169–1179. [Google Scholar] [CrossRef]
- Biederman, L.A.; Harpole, S. Biochar and its effects on plant productivity and nutrient cycling: A meta-analysis. Glob. Chang. Biol. 2013, 5, 202–214. [Google Scholar] [CrossRef]
- Windeatt, J.H.; Ross, A.B.; Williams, P.T.; Forster, P.M.; Nahil, M.A.; Singh, S. Characteristics of biochars from crop residues: Potential for carbon sequestration and soil amendment. J. Environ. Manag. 2014, 146, 189–197. [Google Scholar] [CrossRef]
- Ippolito, J.A.; Stromberger, M.E.; Lentz, R.D.; Dungan, R.S. Hardwood biochar and manure co-application to a calcareous soil. Chemosphere 2016, 142, 84–91. [Google Scholar] [CrossRef]
- Tsai, C.-C.; Chang, Y.-F. Carbon dynamics and fertility in biochar-amended soils with excessive compost application. Agronomy 2019, 9, 511. [Google Scholar] [CrossRef] [Green Version]
- Soil Survey Staff. Soil Survey Manual; United States Department of Agriculture Handbook No. 18; U.S. Government Printing Office: Washington, DC, USA, 2017.
- Hwang, G.S.; Yu, H.Y.; Toba, A. Effects of carbonization temperatures in an earthen kiln on the true density and electric resistivity of Makino bamboo charcoal. Taiwan J. For. Sci. 2004, 19, 237–245. [Google Scholar] [CrossRef]
- Hwang, G.S.; Ho, C.L.; Yu, H.Y.; Su, Y.C. Bamboo vinegar collected during charcoal making with an earthen kiln and its basic properties. Taiwan J. For. Sci. 2006, 21, 547–557, (In Chinese, with English abstract). [Google Scholar]
- Hwang, G.-S. Evaluation of the carbon retention benefits of charcoal derived from lead tree (Leucaena leucocephala (Lam.) de. Wit). For. Res. Prof. Newsl. 2009, 16, 33–35. (In Chinese) [Google Scholar] [CrossRef]
- Hwang, G.-S. Charcoal production and product development of lead tree (Leucaena leucocephala (Lam.) de. Wit). For. Res. Prof. Newsl. 2012, 19, 30–32. (In Chinese) [Google Scholar] [CrossRef]
- Hwang, G.S.; Lee, C.M.; Ho, C.L.; Yu, H.Y.; Wang, C.H.; Yu, H.M. Study on earthen kiln charcoal making with branches of Fraxinus Formosana pruned from plain afforestation. Q. J. Chin. For. 2013, 46, 63–72, (In Chinese, with English abstract). [Google Scholar]
- Tsai, C.C.; Hu, T.E.; Chen, Z.S. Estimation of soil organic carbon stocks in plantation forest soils of Northern Taiwan. Taiwan J. For. Sci. 2009, 24, 103–115. [Google Scholar] [CrossRef]
- Tsai, C.-C.; Chang, Y.-F. Effects of biochar to excessive compost-fertilized soils on the nutrient status. Agronomy 2020, 10, 683. [Google Scholar] [CrossRef]
- Hernandez-Mena, L.; Pécora, A.; Beraldo, A. Slow pyrolysis of bamboo biomass: Analysis of biochar properties. Chem. Eng. Trans. 2014, 37, 115–120. [Google Scholar] [CrossRef]
- Luo, Y.; Durenkamp, Y.; De Nobili, M.; Lin, Q.; Brookes, P.C. Short term soil priming effects and the mineralisation of biochar following its incorporation to soils of different pH. Soil Biol. Biochem. 2011, 43, 2304–2314. [Google Scholar] [CrossRef]
- Tsai, C.-C.; Chang, Y.-F. Nitrogen Availability in Biochar-Amended Soils with Excessive Compost Application. Agronomy 2020, 10, 444. [Google Scholar] [CrossRef] [Green Version]
- Zibilske, L.M. Carbon Mineralization. In Methods of Soil Analysis, Part 2, Microbiological and Biochemical Properties; Weaver, R.W., Angle, J.S., Bottomly, P., Eds.; Soil Science of America Inc.: Madison, WI, USA, 1994; pp. 835–863. [Google Scholar]
- Díez, J.A.; Polo, A.; Guerrero, F. Effect of sewage sludge on nitrogen availability in peat. Biol. Fertil. Soils 1992, 13, 248–251. [Google Scholar] [CrossRef] [Green Version]
- Méndez, A.; Gómez, A.; Paz-Ferreiro, J.; Gascó, G. Effects of sewage sludge biochar on plant metal availability after application to a Mediterranean soil. Chemosphere 2012, 89, 1354–1359. [Google Scholar] [CrossRef] [PubMed]
- Hailegnaw, N.S.; Mercl, F.; Pračke, K.; Száková, J.; Tlustoš, P. High temperature-produced biochar can be efficient in nitrate loss prevention and carbon sequestration. Geoderma 2019, 338, 48–55. [Google Scholar] [CrossRef]
- Bai, M.; Wilske, B.; Buegger, F.; Esperschütz, J.; Kammann, C.I.; Eckhardt, C.; Koestler, M.; Kraft, P.; Bach, M.; Frede, H.G.; et al. Degradation kinetics of biochar from pyrolysis and hydrothermal carbonization in temperate soils. Plant Soil 2013, 372, 375–387. [Google Scholar] [CrossRef]
- Cruz-Castillo, J.G.; Ganeshanandam, S.; MacKay, B.R.; Lawes, G.S.; Lawoko, C.R.O.; Woolley, D.J. Applications of canonical discriminant analysis in horticultural research. HortScience 1994, 29, 1115–1119. [Google Scholar] [CrossRef] [Green Version]
- Rinklebe, J.; Shaheen, S.M.; Yu, K. Release of As, Ba, Cd, Cu, Pb, and Sr under pre-definite redox conditions in different rice paddy soils originating from the USA and Asia. Geoderma 2016, 270, 21–32. [Google Scholar] [CrossRef]
- Liang, B.; Lehmann, J.; Sohi, S.P.; Thies, J.E.; O’Neill, B.; Trujillo, L.; Gaunt, J.; Solomon, D.; Grossman, J.; Neves, E.G.; et al. Black carbon affects the cycling of non-black carbon in soil. Org. Geochem. 2010, 41, 206–213. [Google Scholar] [CrossRef]
- Novak, J.M.; Busscher, W.J.; Watts, D.W.; Laird, D.A.; Ahmedna, M.A.; Niandou, M.A.S. Short-term CO2 mineralization after additions of biochar and switchgrass to a Typic Kandiudult. Geoderma 2010, 154, 281–288. [Google Scholar] [CrossRef]
- Mukome, F.N.D.; Six, J.; Parikh, S.J. The effects of walnut shell and wood feedstock biochar amendments on greenhouse gas emissions from a fertile soil. Geoderma 2013, 200–201, 90–98. [Google Scholar] [CrossRef]
- Cheng, C.H.; Lehmann, J.; Engelhard, M.H. Natural oxidation of black carbon in soils: Changes in molecular form and surface charge along a climosequence. Geochim. Cosmochim. Acta 2008, 72, 1598–1610. [Google Scholar] [CrossRef]
- Aciego Pietri, J.C.; Brookes, P.C. Relationships between soil pH and microbial properties in a UK arable soil. Soil Biol. Biochem. 2008, 40, 1856–1861. [Google Scholar] [CrossRef]
- Watanabe, T. Significance of active aluminum and iron on organic carbon preservation and phosphate sorption/release in tropical soils. In Soils, Ecosystem Processes, and Agricultural Development; Funakawa, S., Ed.; Springer: Tokyo, Japan, 2017; pp. 103–125. [Google Scholar] [CrossRef]
- Malhi, S.J.; Nyborg, M.; Harapiak, J.T. Effects of long-term N fertilizer-induced acidification and liming on micronutrients in soil and in bromegrass hay. Soil Tillage Res. 1998, 8, 91–100. [Google Scholar] [CrossRef]
- Gaskin, J.W.; Steiner, C.; Harris, K.; Das, K.C.; Bibens, B. Effect of low-temperature pyrolysis conditions on biochar for agricultural use. Trans ASABE 2008, 51, 2061–2069. [Google Scholar] [CrossRef]
- Pariyar, P.; Kumari, K.; Jain, M.K.; Jadhao, P.S. Evaluation of change in biochar properties derived from different feedstock and pyrolysis temperature for environmental and agricultural application. Sci. Total Environ. 2020, 713, 136433. [Google Scholar] [CrossRef]
- Prasad, M.; Tzortzakis, N.; McDaniel, N. Chemical characterization of biochar and assessment of the nutrient dynamics by means of preliminary plant growth tests. J. Environ. Manag. 2018, 216, 89–95. [Google Scholar] [CrossRef]
- Smith, J.L.; Collins, H.P.; Bailey, V.L. The effect of young biochar on soil respiration. Soil Biol. Biochem. 2010, 42, 2345–2347. [Google Scholar] [CrossRef]
- Aller, M.F. Biochar properties: Transport, fate, and impact. Crit. Rev. Environ. Sci. Technol. 2016, 46, 1183–1296. [Google Scholar] [CrossRef]
- Demirbas, A. Combustion characteristics of different biomass fuels. Prog. Energy Combust. Sci. 2004, 30, 219–230. [Google Scholar] [CrossRef]
- Spokas, K.A. Review of the stability of biochar in soils: Predictability of O:C molar ratios. Carbon Manag. 2010, 1, 289–303. [Google Scholar] [CrossRef] [Green Version]
- Zhao, S.X.; Ta, N.; Wang, X.D. Effect of temperature on the structural and physicochemical properties of biochar with apple tree branches as feedstock material. Energies 2017, 10, 1293. [Google Scholar] [CrossRef] [Green Version]
- Kim, W.K.; Shim, T.; Kim, Y.S.; Hyun, S.; Ryu, C.; Park, Y.K.; Jung, J. Characterization of cadmium removal from aqueous solution by biochar produced from a giant Miscanthus at different pyrolytic temperatures. Bioresour. Technol. 2013, 138, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Hammes, K.; Smernik, R.J.; Skjemstad, J.O.; Herzog, A.; Vogt, U.F.; Schmidt, M.W.I. Synthesis and characterisation of laboratory charred grass straw (Oryza sativa) and chestnut wood (Castanea sativa) as reference materials for black carbon quantification. Org. Geochem. 2006, 37, 1629–1633. [Google Scholar] [CrossRef]
- Schimmelpfennig, S.; Glaser, B. One step forward toward characterization: Some important material properties to distinguish biochars. J. Environ. Qual. 2012, 41, 1001–1013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agegnehu, G.; Bass, A.M.; Nelson, P.N.; Bird, M.I. Benefits of biochar, compost and biochar–compost for soil quality, maize yield and greenhouse gas emissions in a tropical agricultural soil. Sci. Total Environ. 2016, 543, 295–306. [Google Scholar] [CrossRef]
- Schulz, H.; Glaser, B. Effects of biochar compared to organic and inorganic fertilizers on soil quality and plant growth in a greenhouse experiment. J. Plant Nutr. Soil Sci. 2012, 175, 410–422. [Google Scholar] [CrossRef]
- Berek, A.K.; Hue, N.V.; Radovich, T.J.K.; Ahmad, A.A. Biochars improve nutrient phyto-availability of Hawaii’s highly weathered soils. Agronomy 2018, 8, 203. [Google Scholar] [CrossRef] [Green Version]
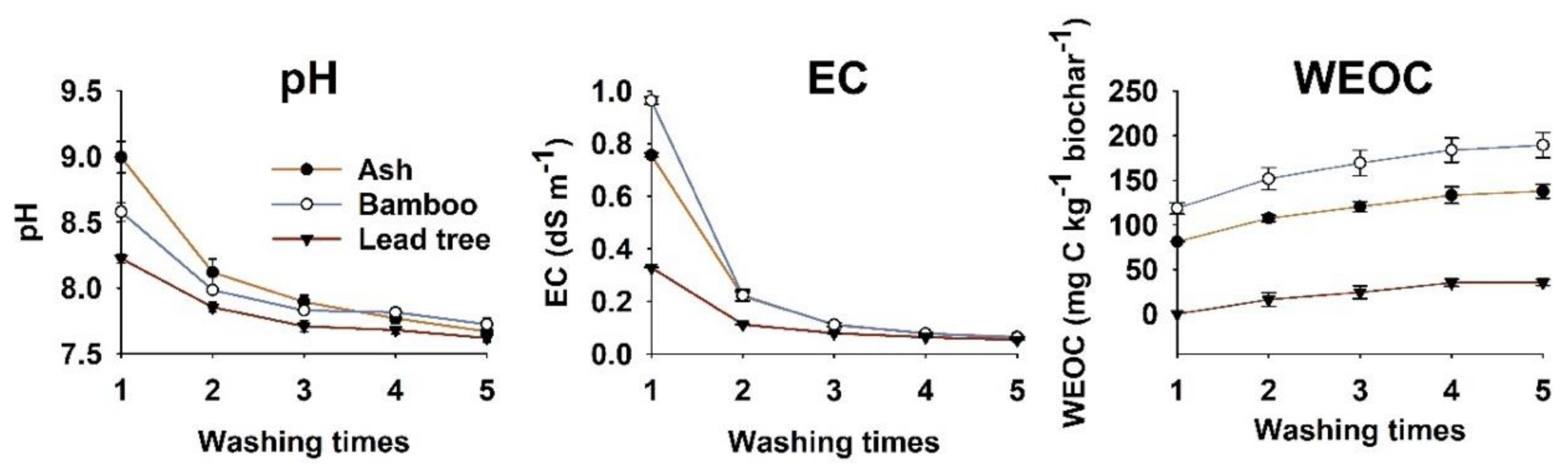
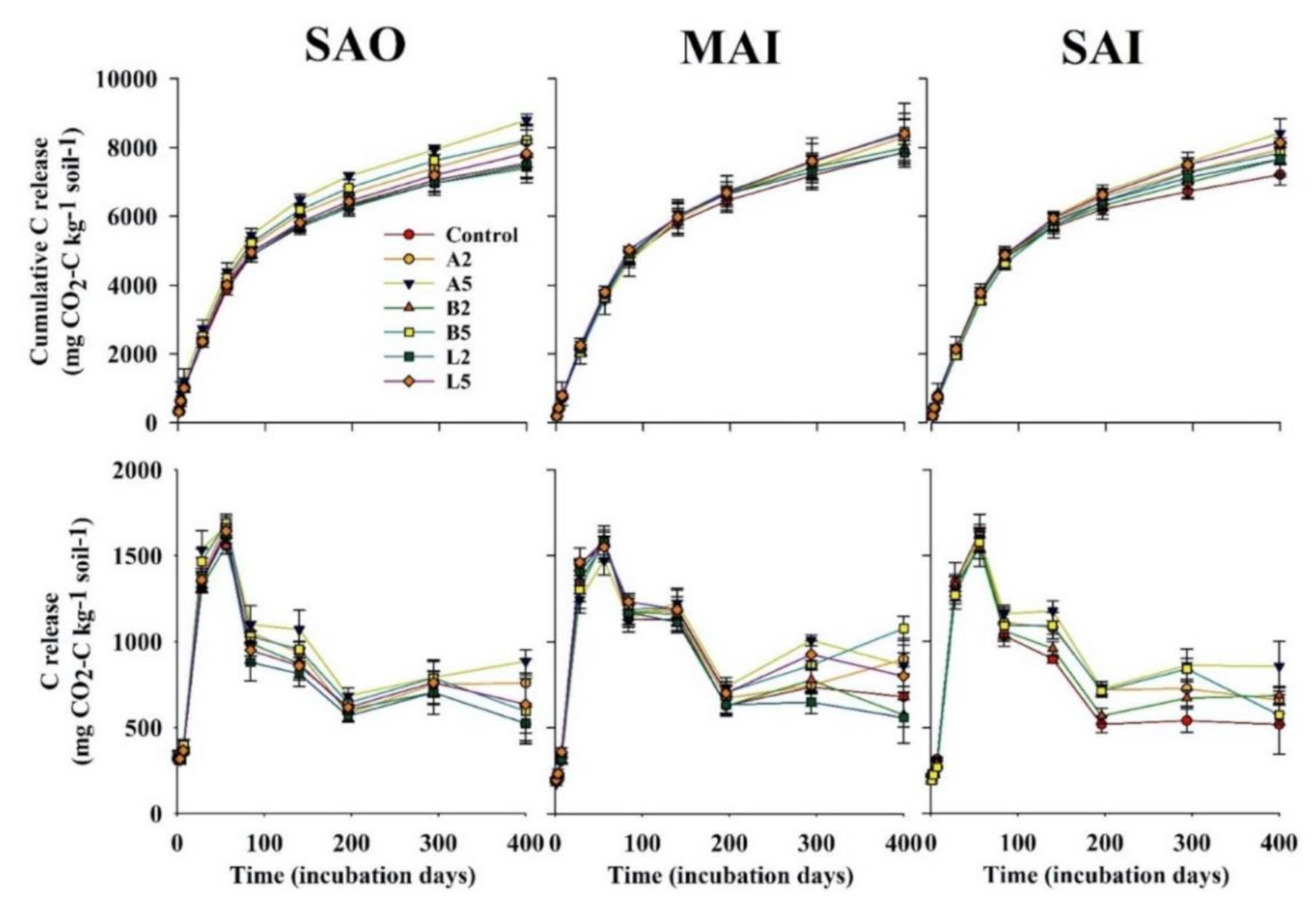
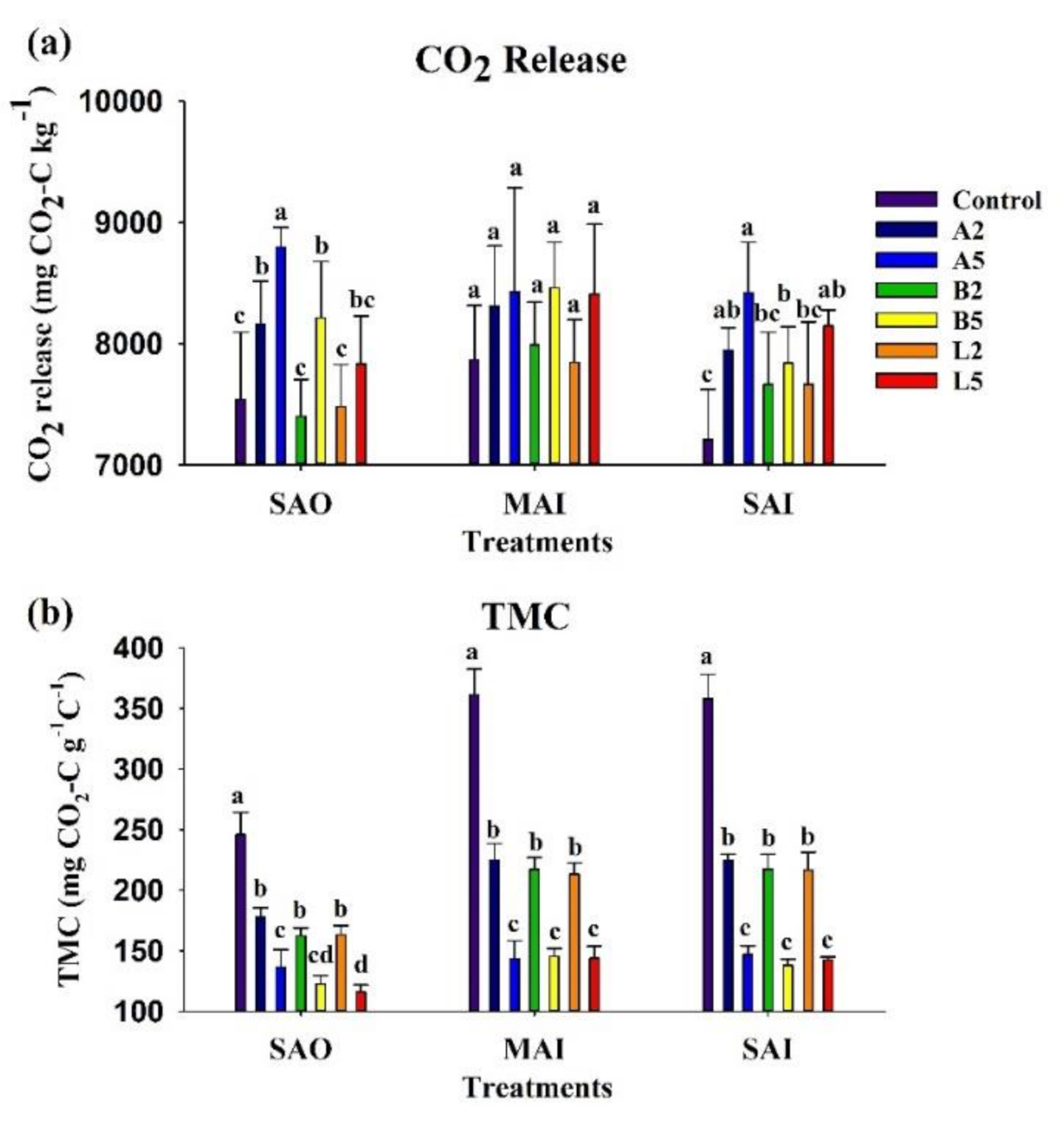
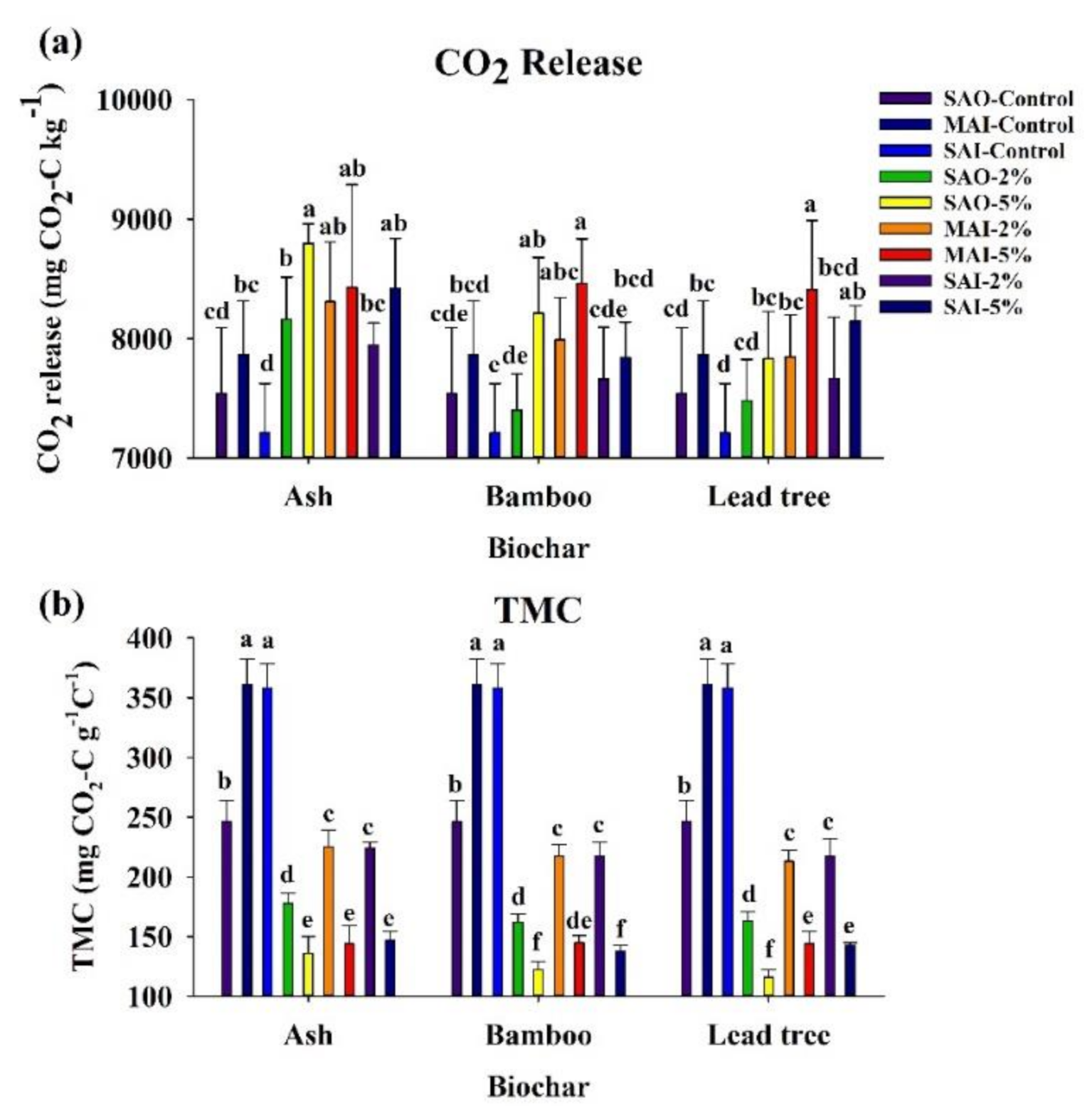
| Characteristics | Ash Biochar | Bamboo Biochar | Lead Tree Biochar |
|---|---|---|---|
| pH 1 | 10.3 ± 0.03 | 10.6 ± 0.04 | 9.9 ± 0.03 |
| Electrical Conductivity (EC) (dS m−1) | 1.97 ± 0.03 1/2.63 ± 0.04 2 | 2.16 ± 0.08 1/3.06 ± 0.09 2 | 0.77 ± 0.03 1/1.36 ± 0.02 2 |
| Cation exchangeable capacity (CEC) (cmol(+) kg−1 soil) | 8.46 ± 1.35 | 15.5 ± 6.64 | 5.20 ± 0.53 |
| Exchangeable K (cmol(+) kg−1 soil) | 7.22 ± 0.94 | 6.02 ± 1.72 | 1.91 ± 0.15 |
| Exchangeable Na (cmol(+) kg−1 soil) | 1.40 ± 0.05 | 1.17 ± 0.02 | 1.26 ± 0.01 |
| Exchangeable Ca (cmol(+) kg−1 soil) | 2.16 ± 0.27 | 0.30 ± 0.08 | 3.62 ± 0.32 |
| Exchangeable Mg (cmol(+) kg−1 soil) | 0.68 ± 0.05 | 0.21 ± 0.07 | 0.40 ± 0.02 |
| Base saturation (BS) (%) | 100 (119~155) | 54.0 ± 17.9 | 100 (102~163) |
| Mehlich 3-P (mg kg−1) | 872 ± 12.7 | 487 ± 29.1 | 96.6 ± 3.13 |
| Mehlich 3-K (mg kg−1) | 2389 ± 4.92 | 2886 ± 259 | 616 ± 41.8 |
| Mchlich 3-Ca (mg kg−1) | 3650 ± 115 | 465 ± 4.53 | 4093 ± 447 |
| Mehlich 3-Mg (mg kg−1) | 422 ± 8.29 | 218 ± 25.6 | 278 ± 8.17 |
| Mehlich 3-Fe (mg kg−1) | 88.4 ± 0.14 | 40.6 ± 0.35 | 65.5 ± 4.12 |
| Mehlich 3-Mn (mg kg−1) | 42.9 ± 0.05 | 44.4 ± 2.93 | 20.9 ± 0.90 |
| Mehlich 3-Cu (mg kg−1) | 7.92 ± 0.74 | 0.53 ± 0.01 | 0.02 ± 0.03 |
| Mehclich 3-Pb (mg kg−1) | ND 3 | 0.04 | ND 3 |
| Mehlich 3-Zn (mg kg−1) | 6.24 ± 1.32 | 19.9 ± 1.90 | 0.35 ± 0.06 |
| Total P (g kg−1) | 1.81 ± 0.07 | 1.86 ± 0.04 | 0.55 ± 0.01 |
| Total K (g kg−1) | 1.59 ± 0.05 | 2.18 ± 0.03 | 0.63 ± 0.02 |
| Total Na (g kg−1) | 0.39 ± 0.01 | 0.34 ± 0.01 | 0.36 ± 0.01 |
| Total Ca (g kg−1) | 4.96 ± 0.18 | 0.51 ± 0.001 | 7.19 ± 0.40 |
| Total Mg (g kg−1) | 0.48 ± 0.02 | 0.30 ± 0.004 | 0.32 ± 0.02 |
| Total Cu (mg kg−1) | 13.7 ± 0.70 | 2.80 ± 0.08 | 1.36 ± 0.04 |
| Total Pb (mg kg−1) | 0.83 ± 0.36 | 0.83 ± 0.16 | 0.41 ± 0.02 |
| Total Zn (mg kg−1) | 9.40 ± 1.76 | 27.2 ± 0.50 | 1.73 ± 0.16 |
| Elemental analysis | |||
| C% | 83.0 ± 0.40 | 81.8 ± 0.37 | 82.5 ± 0.91 |
| H% | 1.49 ± 0.04 | 1.60 ± 0.12 | 1.50 ± 0.01 |
| N% | 0.54 ± 0.01 | 0.64 ± 0.05 | 0.70 ± 0.01 |
| O% | 12.6 ± 0.04 | 13.3 ± 0.13 | 12.0 ± 0.11 |
| C/N ratio | 154 | 128 | 118 |
| (O + N)/C atomic ratio | 0.114 | 0.129 | 0.116 |
| O/C atomic ratio | 0.108 | 0.122 | 0.109 |
| H/C atomic ratio | 0.215 | 0.234 | 0.219 |
| Soil | Treatment | Labile C Pool | Resistant C Pool | Rsqr | Adj Rsqr | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cl (%) | kl (% d−1) | t1/2 (d) | MRT (d) | Cr (%) | kr (% d−1) | t1/2 (yr) | MRT (yr) | ||||
| SAO | Control | 23.1 | 0.0174 | 40 | 57 | 76.9 | 0.0003 | 6 | 9 | 0.9330 | 0.9260 |
| A2 | 14.2 | 0.0163 | 43 | 61 | 85.8 | 0.0002 | 9 | 14 | 0.9751 | 0.9725 | |
| A5 | 9.00 | 0.0170 | 41 | 59 | 91.0 | 0.0001 | 19 | 27 | 0.9950 | 0.9944 | |
| B2 | 14.3 | 0.0168 | 41 | 60 | 85.7 | 0.0002 | 9 | 14 | 0.9642 | 0.9605 | |
| B5 | 9.32 | 0.0158 | 44 | 63 | 90.7 | 0.0001 | 19 | 27 | 0.9911 | 0.9902 | |
| L2 | 14.4 | 0.0169 | 41 | 59 | 85.6 | 0.0002 | 9 | 14 | 0.9631 | 0.9593 | |
| L5 | 8.79 | 0.0176 | 39 | 57 | 91.2 | 0.0001 | 19 | 27 | 0.9805 | 0.9785 | |
| MAI | Control | 23.1 | 0.0174 | 40 | 57 | 76.9 | 0.0003 | 6 | 9 | 0.9330 | 0.9260 |
| A2 | 15.0 | 0.0156 | 44 | 64 | 85.0 | 0.0002 | 9 | 14 | 0.9993 | 0.9989 | |
| A5 | 9.30 | 0.0150 | 46 | 67 | 90.7 | 0.0001 | 19 | 27 | 0.9995 | 0.9992 | |
| B2 | 16.7 | 0.0143 | 48 | 70 | 83.3 | 0.0002 | 9 | 14 | 0.9995 | 0.9992 | |
| B5 | 9.60 | 0.0151 | 46 | 66 | 90.4 | 0.0001 | 19 | 27 | 0.9993 | 0.9989 | |
| L2 | 16.6 | 0.0149 | 47 | 67 | 83.4 | 0.0001 | 19 | 27 | 0.9995 | 0.9993 | |
| L5 | 9.10 | 0.0178 | 39 | 56 | 90.9 | 0.0001 | 19 | 27 | 0.9993 | 0.9990 | |
| SAI | Control | 27.8 | 0.0168 | 41 | 60 | 72.2 | 0.0003 | 6 | 9 | 0.9990 | 0.9986 |
| A2 | 16.2 | 0.0149 | 47 | 67 | 83.8 | 0.0002 | 9 | 14 | 0.9994 | 0.9991 | |
| A5 | 9.63 | 0.0153 | 45 | 65 | 90.4 | 0.0001 | 19 | 27 | 0.9993 | 0.9991 | |
| B2 | 15.0 | 0.0178 | 39 | 56 | 85.0 | 0.0002 | 9 | 14 | 0.9993 | 0.9991 | |
| B5 | 10.2 | 0.0140 | 50 | 71 | 89.8 | 0.0001 | 19 | 27 | 0.9994 | 0.9992 | |
| L2 | 15.9 | 0.0168 | 41 | 60 | 84.1 | 0.0002 | 9 | 14 | 0.9992 | 0.9989 | |
| L5 | 9.82 | 0.0158 | 44 | 63 | 90.2 | 0.0001 | 19 | 27 | 0.9994 | 0.9991 | |
| Source | df 1 | TC | TN | TP | P | K | Ca | Mg | Fe | Al | Mn | Cu | Pb | Zn |
| Soil | 2 | *** 2 | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** |
| Biochar | 2 | ns | *** | * | *** | *** | ns | * | *** | *** | ns | *** | *** | *** |
| Rate | 2 | *** | ns | ns | ns | *** | ns | ** | ** | *** | ns | ** | *** | ns |
| Soil × Biochar | 4 | ns | *** | *** | *** | *** | ns | *** | *** | *** | *** | *** | *** | *** |
| Biochar × Rate | 4 | ns | ns | ns | ns | *** | * | ns | ns | ns | ns | ** | ns | ns |
| Soil × Rate | 4 | ns | ns | ns | ns | ns | * | ns | ns | ns | ** | *** | *** | ns |
| Soil × Biochar × Rate | 8 | ns | ns | ns | ** | ** | ns | ns | * | ns | ns | * | * | * |
| Source | n | TC | TN | TP | P | K | Ca | Mg | Fe | Al | Mn | Cu | Pb | Zn |
| Soil | ||||||||||||||
| SAO | 35 | A 3 | A | A | B | C | C | C | B | A | C | A | A | A |
| MAI | 35 | B | B | B | A | A | A | B | C | C | A | B | B | B |
| SAI | 35 | C | C | B | C | B | B | A | A | B | B | C | C | C |
| Biochar | ||||||||||||||
| Ash | 30 | A | B | B | A | B | B | B | A | B | AB | B | B | B |
| Bamboo | 30 | B | A | A | A | A | A | A | A | A | A | A | A | A |
| Lead tree | 30 | AB | A | B | B | C | AB | A | B | B | B | B | C | C |
| Rate | ||||||||||||||
| 0% | 15 | C | A | A | B | C | A | A | A | A | A | A | A | A |
| 2% | 45 | B | A | A | A | B | A | A | A | B | A | A | A | A |
| 5% | 45 | A | A | A | AB | A | A | B | B | C | A | B | B | A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, C.-C.; Chang, Y.-F. Kinetics of C Mineralization of Biochars in Three Excessive Compost-Fertilized Soils: Effects of Feedstocks and Soil Properties. Agronomy 2020, 10, 1749. https://doi.org/10.3390/agronomy10111749
Tsai C-C, Chang Y-F. Kinetics of C Mineralization of Biochars in Three Excessive Compost-Fertilized Soils: Effects of Feedstocks and Soil Properties. Agronomy. 2020; 10(11):1749. https://doi.org/10.3390/agronomy10111749
Chicago/Turabian StyleTsai, Chen-Chi, and Yu-Fang Chang. 2020. "Kinetics of C Mineralization of Biochars in Three Excessive Compost-Fertilized Soils: Effects of Feedstocks and Soil Properties" Agronomy 10, no. 11: 1749. https://doi.org/10.3390/agronomy10111749
APA StyleTsai, C.-C., & Chang, Y.-F. (2020). Kinetics of C Mineralization of Biochars in Three Excessive Compost-Fertilized Soils: Effects of Feedstocks and Soil Properties. Agronomy, 10(11), 1749. https://doi.org/10.3390/agronomy10111749






