Modeling the Emergence of Echinochloa sp. in Flooded Rice Systems
Abstract
:1. Introduction
2. Materials and Methods
3. Results
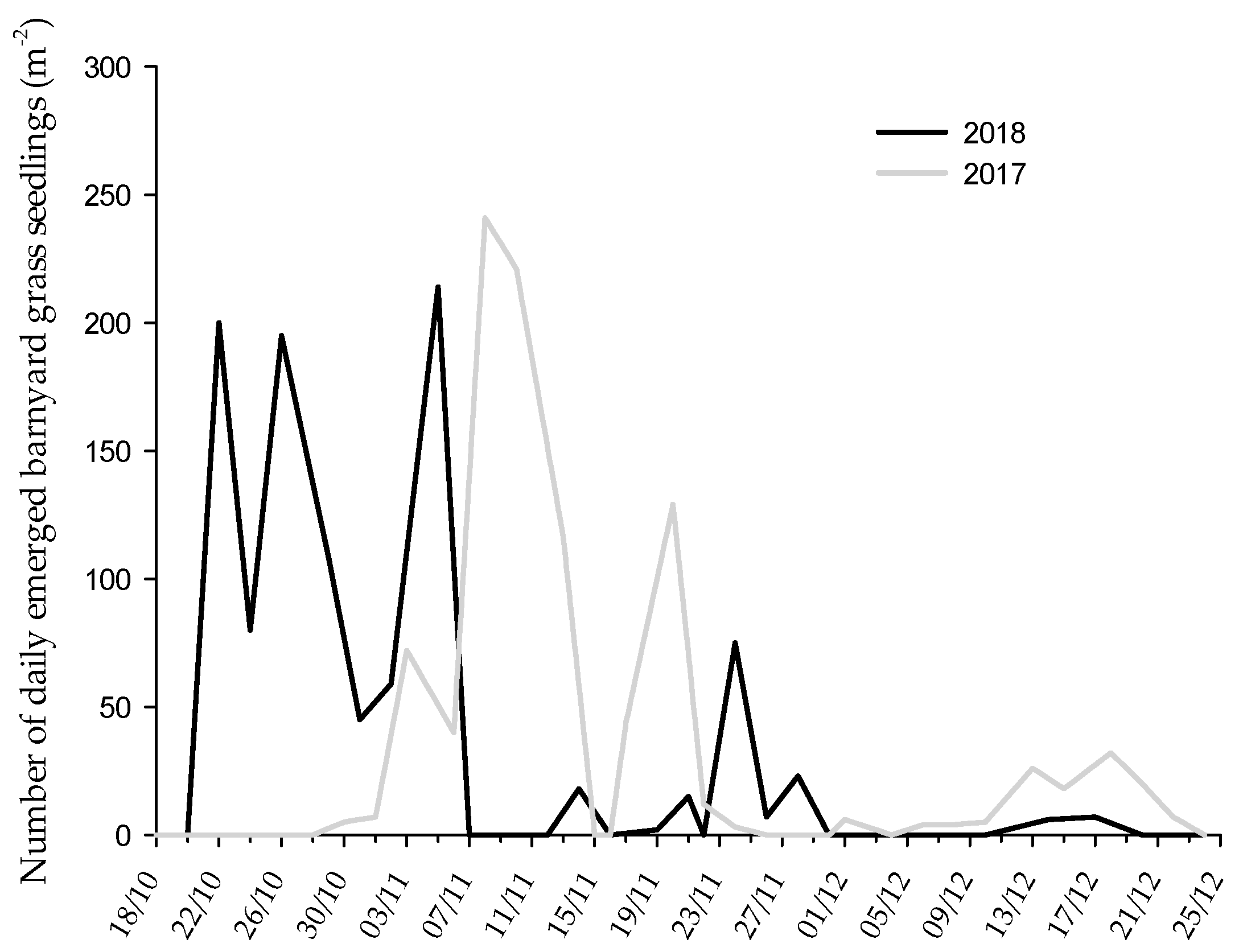
3.1. Barnyard Grass Emergence
3.2. Thermal and Hidrothermal Time Emergence Models
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Holst, N.; Rasmussen, I.A.; Bastiaans, L. Field weed population dynamics: A review of model approaches and applications. Weed Res. 2007, 47, 1–14. [Google Scholar] [CrossRef]
- Masin, R.; Vasileiadis, V.P.; Loddo, D.; Otto, S.; Zanin, G. A single-time survey method to predict the daily weed density for weed control decision-making. Weed Sci. 2011, 59, 270–275. [Google Scholar] [CrossRef]
- Zandoná, R.R.; Agostinetto, D.; Ruchel, Q. Modelagem matemática do fluxo de emergência de plantas daninhas: Ferramenta para decisão no manejo de cultivos. Rev. Bras. Herb. 2018, 17, 3–11. [Google Scholar]
- Loddo, D.; Farshid, G.F.; Zahra, R.; Roberta, M. Base temperatures for germination of selected weed species in Iran. Plant Prot. Sci. 2017, 54, 60–66. [Google Scholar]
- Dorado, J.; Sousa, E.; Calha, I.M.; González-Andújar, J.L.; Fernández-Quintanilla, C. Predicting weed emergence in maize crops under two contrasting climatic conditions. Weed Res. 2009, 49, 251–260. [Google Scholar] [CrossRef]
- Masin, R.; Loddo, D.; Benvenuti, S.; Otto, S.; Zanin, G. Modeling weed emergence in Italian maize fields. Weed Sci. 2012, 60, 254–259. [Google Scholar] [CrossRef] [Green Version]
- Masin, R.; Loddo, D.; Gasparini, V.; Otto, S.; Zanin, G. Evaluation of weed emergence model AlertInf for maize in soybean. Weed Sci. 2014, 62, 360–369. [Google Scholar] [CrossRef]
- Werle, R.; Sandell, L.D.; Buhler, D.D.; Hartzler, R.G.; Lindquist, J.L. Predicting emergence of 23 summer annual weed species. Weed Sci. 2014, 62, 267–279. [Google Scholar] [CrossRef]
- Royo-Esnal, A.; Torra, J.; Conesa, J.A.; Forcella, F.; Recasens, J. Modeling the emergence of three arable bedstraw (Galium) species. Weed Sci. 2010, 58, 10–15. [Google Scholar] [CrossRef]
- Royo-Esnal, A.; Necajeva, J.; Torra, J.; Recasens, J.; Gesch, R.W. Emergence of field pennycress (Thlaspi arvense L.): Comparison of two accessions and modelling. Ind. Crop. Prod. 2015, 66, 161–169. [Google Scholar] [CrossRef] [Green Version]
- Izquierdo, J.; Bastida, F.; Lezaún, J.M.; Del Arco, M.J.S.; Gonzalez-Andujar, J.L. Development and evaluation of a model for predicting Lolium rigidum emergence in winter cereal crops in the Mediterranean area. Weed Res. 2013, 53, 269–278. [Google Scholar] [CrossRef]
- Lundy, M.E.; Hill, J.E.; Van Kessel, C.; Owen, D.A.; Pedroso, R.M.; Boddy, L.G.; Fischer, A.J.; Linquist, B.A. Site-specific, real-time temperatures improve the accuracy of weed emergence predictions in direct-seeded rice systems. Agric. Syst. 2014, 123, 12–21. [Google Scholar] [CrossRef]
- de Lima Fruet, B.; Merotto, A.; da Rosa Ulguim, A. Survey of rice weed management and public and private consultant characteristics in Southern Brazil. Weed Tech. 2020, 34, 351–356. [Google Scholar] [CrossRef]
- Heap, I. International Survey of Herbicide Resistant Weeds. Available online: http://www.weedscience.org/ (accessed on 14 May 2020).
- Zoneamento Agrícola de Risco Climático. Available online: http://www.agricultura.gov.br/assuntos/riscos-seguro/risco-agropecuario/zoneamento-agricola (accessed on 17 June 2017).
- Counce, P.A.; Keisling, T.C.; Mitchell, A.J. A uniform, objective, and adaptative system for expressing rice development. Crop Sci. 2000, 40, 436–443. [Google Scholar] [CrossRef] [Green Version]
- Lorenzi, H. Manual de Identificação e Controle de Plantas Daninhas Plantio Direto e Convencional, 7th ed.; Plantarum: Nova Odessa, Brazil, 2014; p. 384. [Google Scholar]
- Concenço, G.; Parfitt, J.M.B.; Downing, K.; Larue, J.; Silva, J.T. Rice development and water demand under drought stress imposed at distinct growth stages. Afr. J. Agric. Res. 2016, 11, 4147–4156. [Google Scholar]
- Gummerson, R.J. The effect of constant temperatures and osmotic potential on the germination of sugar beet. J. Exp. Bot. 1986, 37, 729–741. [Google Scholar] [CrossRef]
- Chantre, G.R.; Blanco, A.M.; Lodovichi, M.V.; Bandoni, A.J.; Sabbatini, M.R.; López, R.L.; Vigna, M.R.; Gigón, R. Modeling Avena fatua seedling emergence dynamics: An artificial neural network approach. Comp. Electro. Agric. 2012, 88, 95–102. [Google Scholar] [CrossRef]
- Roman, E.S.; Murphy, S.D.; Swanton, C.J. Simulation of Chenopodium album seedling emergence. Weed Sci. 2000, 48, 217–224. [Google Scholar] [CrossRef]
- Fernandez-Quintanilla, C.; Barroso, J.; Recasens, J.; Sans, X.; Torner, C.; Sanchez Del Arco, M.J. Demography of Lolium rigidum in winter barley crops: Analysis of recruitment, survival and reproduction. Weed Res. 2000, 40, 281–291. [Google Scholar] [CrossRef]
- Radosevich, S.R.; Holt, J.S.; Ghersa, C.M. Ecology of Weeds and Invasive Plants: Relationship to Agriculture and Natural Resource Management, 3rd ed.; Wiley-Interscience: Hoboken, NJ, USA, 2007; p. 400. [Google Scholar]
- Masin, R.; Loddo, D.; Benvenuti, S.; Zuin, M.C.; Macchia, M.; Zanin, G. Temperature and water potential as parameters for modeling weed emergence in central-northern Italy. Weed Sci. 2010, 58, 216–222. [Google Scholar] [CrossRef]
- Leguizamon, E.S.; Fernandez-Quintanilla, C.; Barroso, J.; Gonzalez-Andujar, J.L. Using thermal and hydrothermal time to model seedling emergence of Avena sterilis ssp. ludoviciana in Spain. Weed Res. 2005, 45, 149–156. [Google Scholar] [CrossRef]
- Zambrano-Navea, C.; Bastida, F.; Gonzalez-Andujar, J.L. A hydrothermal seedling emergence model for Conyza bonariensis. Weed Res. 2013, 53, 213–220. [Google Scholar] [CrossRef] [Green Version]
- Silva, A.F.; Concenço, G.; Aspiazú, I.; Ferreira, E.A.; Galon, L.; Coelho, A.T.C.P.; Silva, A.A.; Ferreira, F.A. Interferência de plantas daninhas em diferentes densidades no crescimento da soja. Planta Daninha. 2009, 27, 75–84. [Google Scholar] [CrossRef] [Green Version]
- Boddy, L.G.; Bradford, K.J.; Fixcher, A.J. Population based threshold models describe weed germination and emergence patterns across varying temperature, moisture and oxygen conditions. J. Appl. Ecol. 2012, 49, 1225–1236. [Google Scholar] [CrossRef]




| Mod | a 1 | T50 | b | c | R 2 | p-Value | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| A2 | 92.36 | ±1.7 3 | 144.50 | ±3.3 | 127.16 | ±26.1 | 2.22 | ±0.6 | 0.95 | ≤0.0001 * |
| B | 104.44 | ±7.9 | 125.79 | ±8.3 | 86.02 | ±15.7 | 0.88 | ±0.2 | 0.85 | ≤0.0001 * |
| C | 575.08 | - | 2642.95 | - | 4417.44 | - | 0.68 | - | 0.63 | 0.9493 ns |
| D | 547.30 | - | 2681.33 | - | 4539.96 | - | 0.67 | - | 0.62 | 0.8434 ns |
| Mod. | a 1 | T50 | b | c | R 2 | p-Value | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| A 2 | 90.34 | ±2.0 3 | 115.91 | ±2.8 | 128.33 | ±74.4 | 3.50 | ±2.4 | 0.95 | ≤0.0001 * |
| B | 95.24 | ±4.3 | 110.56 | ±5.2 | 83.98 | ±22.4 | 1.45 | ±0.6 | 0.89 | ≤0.0001 * |
| C | 96.91 | ±6.7 | 109.47 | ±8.8 | 71.54 | ±14.3 | 0.93 | ±0.3 | 0.81 | ≤0.0001 * |
| D | 99.26 | ±2.8 | 140.03 | ±4.7 | 127.72 | ±22.5 | 1.91 | ±0.5 | 0.93 | ≤0.0001 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goulart, F.A.P.; Zandoná, R.R.; Schmitz, M.F.; Ulguim, A.R.; Andres, A.; Agostinetto, D. Modeling the Emergence of Echinochloa sp. in Flooded Rice Systems. Agronomy 2020, 10, 1756. https://doi.org/10.3390/agronomy10111756
Goulart FAP, Zandoná RR, Schmitz MF, Ulguim AR, Andres A, Agostinetto D. Modeling the Emergence of Echinochloa sp. in Flooded Rice Systems. Agronomy. 2020; 10(11):1756. https://doi.org/10.3390/agronomy10111756
Chicago/Turabian StyleGoulart, Francisco A. P., Renan R. Zandoná, Maicon F. Schmitz, André R. Ulguim, André Andres, and Dirceu Agostinetto. 2020. "Modeling the Emergence of Echinochloa sp. in Flooded Rice Systems" Agronomy 10, no. 11: 1756. https://doi.org/10.3390/agronomy10111756
APA StyleGoulart, F. A. P., Zandoná, R. R., Schmitz, M. F., Ulguim, A. R., Andres, A., & Agostinetto, D. (2020). Modeling the Emergence of Echinochloa sp. in Flooded Rice Systems. Agronomy, 10(11), 1756. https://doi.org/10.3390/agronomy10111756






