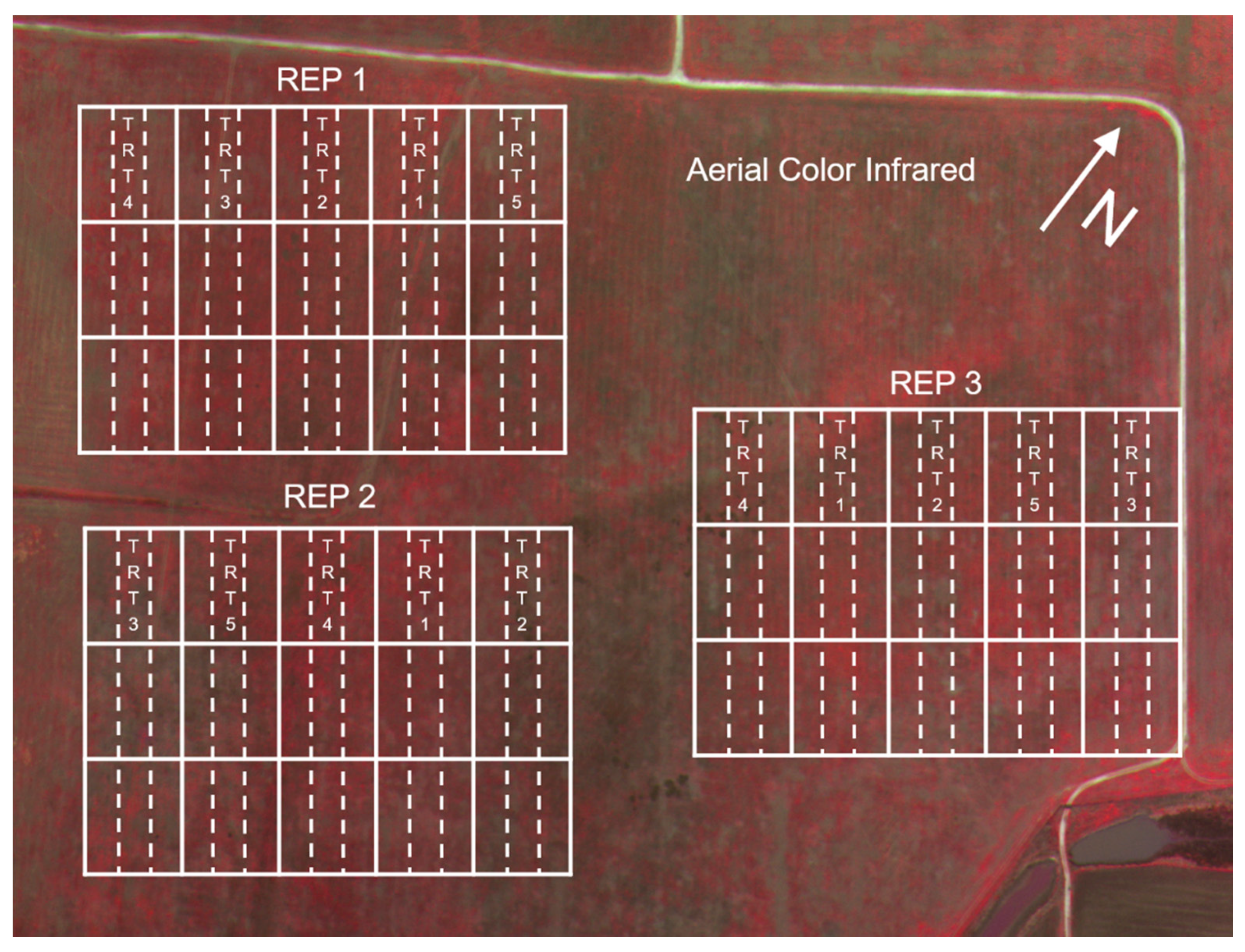
3.1. Deposit Measurements on Mylar Cards and Weed Foliage
During year one, the deposition of dye tracer on Mylar plates was significantly higher in CP nozzle treatment than that in the ES charged on treatment. Both treatments received significantly higher deposits compared to the untreated control (
Table 4). Similarly, during year two, the CP nozzle resulted in significantly higher deposition on Mylar plate samplers compared to ES charged off and on nozzles. Deposition from the rotary atomizer was significantly less than that from the CP nozzle. Both the ES on and off nozzles provided the least deposition on Mylar plates. Similar to that on Mylar samplers, the deposition of dye tracer on weed foliage significantly varied between treatments during the study (
Table 4). Deposition on weed leaves for the CP and rotary nozzles was statistically similar in year one, but in year two, the CP nozzle received significantly higher deposition than the ES on and off nozzles. Deposition on the weed canopy for the CP and the rotary atomizer was statistically comparable.
Using the Mylar data as a standard deposition rate, the percent of deposit collected by the leaves appears to elicit a functional relationship between the deposition and the droplet size spectrum (D
v0.5) portfolio of 200, 250, and 350 µm expected for the ES nozzle, the rotary atomizer, and the CP nozzle, respectively. During year one, the spray deposition rates of the ES nozzle and the CP nozzle averaged 45.4% and 16.8%, respectively. During year two, the spray deposition rates averaged 96.1 and 86.9% for the ES nozzle, off and on, respectively, and the deposition rates were 49.7 and 42.3% for the rotary and the CP nozzles, respectively. These spray deposition data suggest that the nozzle which produced a smaller droplet spectrum tended to have higher deposits compared to the one with a larger droplet spectrum. Kirk et al. [
7] found a significant correlation (r = 0.54) between spray deposits on Mylar and yellow foxtail grass,
Setaria glauca L., but they cautioned that the coefficient of determination was too low for reliably predicting deposits on plant surfaces from that measured on artificial collectors. Artificial collectors such as Mylar plates have a rigid surface and are monotonously alike in geometry, while leaf surfaces are morphologically diverse with widely varying topography. Given that Kirk et al. [
7] studied deposition on laboratory-grown foxtail plant, it is expected that in a weed-infested field with multiple species with widely varying leaf canopies, the correlation coefficient for deposition between Mylar cards and plant canopy would be probably even lower than r = 0.54.
3.2. Deposition on Water Sensitive Paper
The aerial spray nozzles significantly influenced spray application rate (
Table 5). The CP nozzle increased spray rate by 36% over the rotary atomizer nozzle (16.33 vs. 12.01 L·ha
−1). The volume of spray delivered by the ES on nozzle was comparable to that for the ES off nozzle (4.40 vs. 2.65 L·ha
−1) and was significantly lower than those for the CP and rotary nozzles. The CP nozzle significantly increased spray coverage by 26% over the rotary atomizer nozzle (4.23 vs. 3.36%) and 238 and 446% over the electrostatic charged on and off nozzles, respectively. Spray coverage did not significantly vary between ES charged on and off nozzles (1.25 vs. 0.78%). Overall, the ES charged on nozzle treatment received nearly 61% more spray coverage (%) on an artificial collector than the ES charged off nozzle. Additionally, the volumetric deposition of spray solutions was 66% more for the ES charged on nozzle than that for the ES charged off nozzle. The rotary atomizer nozzle produced significantly greater droplet density compared to the CP nozzle (62.5 vs. 46.8 droplets/cm
2). Droplet density was 33.5% more for the rotary atomizer than that for the CP nozzle.
This result was expected given that the CP and rotary nozzle treatments were applied at the same spray rate, with the rotary treatments having a VMD that was 100 µm smaller than that for the CP, which would have created a larger overall number of spray droplets at the time of spray application. Similar results were reported by Thompson et al. [
34] who found that the rotary nozzle produced larger droplet density compared to the hydraulic nozzle (34.8 vs. 20.8 droplets/cm
2) when glyphosate was aerially applied using a helicopter on forestry weeds in Canada. Although the ES charged on nozzle received almost 48% more droplets per unit area than the ES uncharged nozzle, they were not significantly different (29.3 vs. 19.8 droplets/cm
2), likely due to the small sample size. The VMDs were significantly larger for the CP nozzle than those for the rotary atomizer nozzles. The VMDs for the ES charged on nozzles were statistically comparable to those for the ES off nozzles.
The CP nozzle treatment based on a targeted Dv0.5 of 350 μm produced droplets ranging in size from 311.1 to 345.6 μm in each of the three replications on WSP samplers. These values represent 89 to 99% of the targeted droplet size. The rotary atomizer nozzle based on a targeted Dv0.5 of 250 μm produced droplets ranging in size from 243 to 253 μm and represent 97 to 100% of the targeted size. Unlike the ES charged on nozzles, which produced droplets ranging in size from 230.4 to 249.7 μm, the droplet size for the CP and rotary atomizer nozzles appeared to be relatively constant and was in reasonable agreement with the targeted droplet size.
3.3. Efficacy
Figure 7 shows the least square mean plot for the NDVI values obtained during year one of the study. A significant difference occurred between treatments at DAT 1 (
F = 3.39;
p < 0.035; df = 2, 312) with the mean NDVI value for the control being significantly higher than that for the ES charged on nozzle treatment, but was comparable to that for the CP nozzle treatment. However, these treatment differences became well pronounced and deviated from one another as the season progressed. The NDVI values differed significantly between treatments at DAT 5 and thereafter throughout the sampling periods (
F values were 48.50, 121.74, 94.38, 118.95, and 98.89, respectively, for DAT 5, 7, 9, 11, and 14 with
p < 0.0001 at 2, 326 df). The CP nozzle at 28.1 L·ha
−1 spray rate caused a significantly greater reduction in NDVI compared to the ES charged on nozzle at 9.4 L·ha
−1 spray rate. The mean NDVI values decreased 56.9% in the CP nozzle treatment compared to 15.1% in the ES charged on treatment from DAT 1 to 14.
Figure 8 shows the percent change in NDVI values from DAT 5 to 14, calculated from the NDVI data at DAT 1 as the baseline measurement. The differences between treatments during each of the sampling dates were highly significant (
F values were 95.30, 145.82, 108.79, 140.52, and 121.11, respectively, for DAT 5, 7, 9, 11, and 14 with
p < 0.0001 at 2, 326 df). The CP nozzle significantly reduced weed vigor throughout the sampling period compared to the control and the ES charged on nozzle did not perform as effectively as the CP nozzle in reducing weed vigor. The decline in weed health was as much as 3-fold greater in the CP nozzle treatment compared to the ES charged on treatment at DAT 14. The control plots exhibited positive vegetative growth throughout the sampling period, except that there was a small decline (−2.38%) in weed vigor at DAT 14.
During year two, the NDVI values for ground-based remote sensing showed that all treatments were significantly different from the control at DAT 17 (
F = 33.1;
p < 0.0001; df = 4, 145).
Figure 9 showed that the decline in weed health was comparable between the ES charged on, the CP and the rotary atomizer nozzles. Similarly, the ES charged on nozzle reduced NDVI significantly compared to the ES charged off nozzle.
Figure 10 shows that the percent change in NDVI values at 17 DAT was significantly different between treatments (
F = 9.6;
p < 0.0001; df = 4, 145). The test results were similar to NDVI data except that the difference between ES charged on and off was not significantly different, although the ES charged on nozzle caused a 36% reduction in weed vigor compared to 15.4% for the ES charged off nozzle.
Several researchers have reported that electrostatically charged glyphosate significantly improved weed control. For instance, Franz et al. [
35] reported that electrostatically charged glyphosate significantly improved control of ryegrass compared to uncharged glyphosate under calm wind conditions, while under windy conditions with gusts up to 4.5 m·s
−1, a large proportion of electrostatically charged droplets drifted away from the target site and thus, depressed the efficacy of ES charging. Wolf et al. [
36] reported that electrostatically charged glyphosate increased deposition on the weed canopy 4-fold and caused a 2-fold increase in weed control compared to the uncharged glyphosate. Martin and Latheef [
37] reported that ryegrass health declined 80% faster by charging the glyphosate spray solution compared to the uncharged spray. Additionally, charged glyphosate significantly decreased percent of spray volume with spray droplets <100 µm compared to uncharged glyphosate [
37]. This would likely cause the spray droplets to reach the target site faster as the proportion of larger droplets predominates in the spray cloud. Additionally, Martin and Carlton [
38] reported that electrostatic nozzles which produced smaller droplets had higher charge-to-mass ratios, which tend to increase the attraction between the droplets and the target. Conversely to these findings, Zhang et al. [
10] reported that aerially applied glyphosate using conventional flat-fan nozzles and the rotary atomizers controlled weeds in fallow farmlands better than the ES charged off and on nozzles. It is important to note that strong wind prevailed during this study in year one (
Table 3) with in-wind speeds varying from 3.3 to 5.0 m·s
−1 and that this could have likely resulted in off-target drift of smaller droplets, as reported by Franz et al. [
35], and thus, likely depressed glyphosate efficacy. It is noteworthy, however, that Zhang et al. [
10] did not provide meteorological data and it is, therefore, not possible to assess whether or not wind velocity played a role in the results reported by them.
Figure 11 shows that the NDVI values obtained from aerial imaging at 16 DAT significantly varied between treatments (
F = 15.34;
p < 0.0003; df = 4, 10). Weed vigor was significantly depressed in all aerial nozzle treatments compared to the control. Additionally, the ES charged on nozzle improved suppression of weeds significantly compared to the control but was comparable to the uncharged glyphosate. Additionally, the rotary atomizer nozzles reduced weed vigor comparable to the ES charged on and ES charged off nozzles. However, the CP nozzles did better than the ES charged off nozzle in controlling weeds.
Figure 12 shows that the CP and the rotary atomizer nozzles were the only treatments which significantly depressed weed vigor compared to the control (
F = 8.52;
p < 0.0029; df = 4, 10). The ES charged on and ES charged off nozzles provided statistically comparable weed control. Vegetative growth continued unabated in the untreated check plots. Weed species occur as patches with mixtures of many species and cause spatial heterogeneity in their distribution [
39]. The reflectance values also vary between bare soil, weed-free, and weed-infested areas [
40]. Other types of land covers, such as soil, residues, rocks, etc., form part of an individual pixel and collectively become mixed pixels [
40]. The aerial imagery data with mixed pixel values are likely factors that could increase the variations between treatments and make statistical separation tenuous. To date, neither an analytical technique nor an algorithm is available to separate the mixed pixel components from an individual pixel and Chang et al. [
40] discussed the difficulties involved in separating the individual components in a mixed pixel.
When large samples of the aerial imagery data composed of 33K pixel values per experimental unit were analyzed, all aerial nozzle treatments significantly diverged from the control (
Figure 13). The rotary atomizer nozzle treatment provided the best control, followed by the CP and the ES charged on nozzles. The ES charged on nozzle treatment reduced weed vigor significantly more than the ES charged off nozzle. The decline in percent change in NDVI computed from DAT 0 as the baseline period averaged 662, 715, 840, and 949% for the ES charged off and ES charged on nozzles, the CP nozzle, and the rotary atomizer nozzle, respectively, when compared to the control. The negatively linear reduction in weed health for the aerial nozzles shown in
Figure 13 indicates that the treatment efficacy conformed to an descending order with the rotary atomizer nozzle predominating in effectively controlling weeds compared to other aerial nozzle technologies.
The visual estimates of weed control at 16 DAT indicate that all the treatments were significantly different from the control (
Table 6,
F = 32.1;
p < 0.0001; df = 4, 145). Weed mortality in the untreated check was the lowest and barely exceeded 6%. Weed mortality was comparable between the CP, the rotary atomizer, and the ES charged on nozzles. The percentage of weeds dying was two-fold greater for the ES charged on nozzle than that for the ES uncharged nozzle. Noticeably, the ES charged on nozzle received 60.7 and 45.9% greater deposition than the ES charged off nozzle on Mylar cards and weed foliage, respectively (
Table 4). Furthermore, the percent area spray coverage on WSPs was nearly 61% more for the ES charged on nozzle than that for the ES charged off nozzle (
Table 5). Additionally, the volumetric deposition of spray solutions was 66% more for the ES charged on nozzle than that for the ES charged off nozzle. These data suggest that the difference in weed mortality between ES charged on and ES charged off treatments (64 vs. 39%), as shown by visual estimates, was likely due to the increased spray deposition from electrically charging the spray. Several authors have reported that electrostatic charging of spray solutions did increase deposition on plant canopy [
12,
41,
42].
3.4. Droplet Spectrum vs. Efficacy
The D
V0.5 spray droplets were smaller for the rotary atomizer than those for the CP nozzles. Likewise, the D
V0.5 droplets for the ES charged on nozzle were comparable to those for the rotary atomizer nozzle. This could have contributed to a greater foliar absorption of glyphosate by the weed species as several studies demonstrate that smaller droplets with higher concentration of glyphosate increased phytotoxicity against weeds [
43,
44,
45]. However, others have reported that spray droplet size did not affect glyphosate efficacy as coarse sprays appear to provide good herbicide efficacy across a wide array of modes of action, and help mitigate spray drift potential compared to finer droplets [
46,
47]. Liu et al. [
48] reported that although the absorption of glyphosate on
Populus tremuloides Michx. increased with droplet size, herbicide concentration was more important than either the droplet size or the droplet density. Ramsdale et al. [
49] found that increased herbicide concentration maximized glyphosate efficacy through reduction in the amount of antagonistic salts in the carrier volume. This was further confirmed by more efficacious low volume spray applications at 23 or 47 L·ha
−1 compared to higher spray rates. However, the performance of herbicides relative to droplet size also varied with weed species [
50,
51,
52].
The rotary atomizer nozzles had an average of 34.3% more droplets on WSP cards than the CP nozzles (638 vs. 475). Thus, droplet density was significantly higher for the rotary nozzles than that for the CP nozzles (62.5 vs. 46.8 droplets/cm
2). Indeed, Behrens [
53] showed that droplet spacing of about 3.1 mm equivalent to 464 droplets/cm
2 for 2,4,5-T herbicide increased control of weeds on cotton and mesquite. Additionally, Phillips et al. [
54] obtained better weed control in field trials with more droplets per unit area, either by reducing droplet size to 135 μm or by increasing the spray volume to 60 L·ha
−1. The factors which influence spray droplet spectra for the AU5000 rotary atomizer are the rotation speed (rpm) of the rotating wire gauze cylinder, aircraft speed, and nozzle flow rate [
17,
55]. At higher flow rate and lower airspeed, the rotation velocity slows down and causes coarser spray droplets. Higher airspeed causes more air shear across the atomizer and produces finer spray droplets. These data indicate that the spray droplet spectra generated by the rotary atomizers could be manipulated by the aerial applicators to achieve effective weed control. Additionally, Hoffmann et al. [
56] reported that the aerial applicators could add spray adjuvants to the tank mix and obtain a desirable droplet spectrum when using rotary atomizers.
A large body of research data on efficacy of glyphosate against weed populations cited above indicates that a multiplicity of factors is involved in enhancing deposition, adhesion, absorption, and translocation within the plant vascular system. Many factors such as weed foliage architecture, surfactants, droplet size, spray rates, and application hardware interact with one another and influence the suppression of weed populations. Studies have shown that the time of application of glyphosate significantly influenced its efficacy as well [
57,
58,
59], probably because of interactions between temperature, dew, diurnal leaf movement, and botanical characteristics of weed species. Butts et al. [
47] studied a range of droplet sizes using a dicamba-glyphosate mixture and found that the optimum droplet sizes within each year varied with weed species, geographic location, weather conditions, and herbicide resistance. Research data that reported on droplet spectra effects on glyphosate efficacy involved a wide variety of crops and environmental conditions, but none of them addressed the effect of aerially applied herbicides on weed mortality. It is noteworthy that the ES charged on nozzle with one-third of the spray application rate of the rotary and the CP nozzles performed equally well during year two and warrants further study. Further research is required to elucidate the relationship between herbicide efficacy and application hardware, application heights, airspeed, weather factors, and aerial platforms.