Microorganisms and Biological Pest Control: An Analysis Based on a Bibliometric Review
Abstract
:1. Introduction
2. Materials and Methods
Content Analysis
3. Results
3.1. Performance Analysis
3.2. Science Mapping
4. Discussion
4.1. Biological Control
4.2. Biopesticides
4.3. Rhizobacteria
4.4. Trichoderma
4.5. Bacillus subtilis
4.6. Bacillus thuringiensis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Compant, S.; Duffy, B.; Nowak, J.; Clément, C.; Barka, E.A. Use of plant growth-promoting bacteria for biocontrol of plant diseases: Principles, mechanisms of action, and future prospects. Appl. Environ. Microbiol. 2005, 71, 4951–4959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bravo, A.; Likitvivatanavong, S.; Gill, S.S.; Soberón, M. Bacillus thuringiensis: A story of a successful bioinsecticide. Insect Biochem. Mol. Biol. 2011, 41, 423–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montesinos, E. Development, registration and commercialization of microbial pesticides for plant protection. Int. Microbiol. 2003, 6, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Matthews, S.; Rao, V.S.; Durvasula, R.V. Modeling horizontal gene transfer (HGT) in the gut of the Chagas disease vector Rhodnius prolixus. Parasites Vectors 2011, 4, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Hurwitz, I.; Hillesland, H.; Fieck, A.; Das, P.; Durvasula, R. The paratransgenic sand fly: A platform for control of Leishmania transmission. Parasites Vectors 2011, 4, 82. [Google Scholar] [CrossRef] [Green Version]
- Dunstand-Guzmán, E.; Peña-Chora, G.; Hallal-Calleros, C.; Pérez-Martínez, M.; Hernández-Velazquez, V.M.; Morales-Montor, J.; Flores-Pérez, F.I. Acaricidal effect and histological damage induced by Bacillus thuringiensis protein extracts on the mite Psoroptes cuniculi. Parasites Vectors 2015, 8, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Sanahuja, G.; Banakar, R.; Twyman, R.M.; Capell, T.; Christou, P. Bacillus thuringiensis: A century of research, development and commercial applications. Plant Biotechnol. J. 2011, 9, 283–300. [Google Scholar] [CrossRef] [Green Version]
- Peña, G.; Miranda-Rios, J.; De La Riva, G.; Pardo-López, L.; Soberón, M.; Bravo, A. A Bacillus thurigiensis S-layer protein involved in toxicity against Epilachna varivestis (Coleoptera: Coccinellidae). Appl. Environ. Microbiol. 2006, 72, 353–360. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Zhang, N.; Yong, X.; Yang, X.; Shen, Q. Biocontrol of Rhizoctonia solani damping-off disease in cucumber with Bacillus pumilus SQR-N43. Microbiol. Res. 2012, 167, 135–143. [Google Scholar] [CrossRef]
- Kaya, H.K.; Gaugler, R. Entomopathogenic nematodes. Annu. Rev. Entomol. 1993, 38, 181–206. [Google Scholar] [CrossRef]
- Szewczyk, B.; Hoyos-Carvajal, L.; Paluszek, M.; Skrzecz, I.; Lobo De Souza, M. Baculoviruses—Re-emerging biopesticides. Biotechnol. Adv. 2006, 24, 143–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simón, O.; Williams, T.; Possee, R.D.; López-Ferber, M.; Caballero, P. Stability of a Spodoptera frugiperda nucleopolyhedrovirus deletion recombinant during serial passage in insects. Appl. Environ. Microbiol. 2010, 76, 803–809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Babalola, O.O. Beneficial bacteria of agricultural importance. Biotechnol. Lett. 2010, 32, 1559–1570. [Google Scholar] [CrossRef] [PubMed]
- Hariprasad, P.; Chandrashekar, S.; Singh, S.B.; Niranjana, S.R. Mechanisms of plant growth promotion and disease suppression by Pseudomonas aeruginosa strain 2apa. J. Basic Microbiol. 2014, 54, 792–801. [Google Scholar] [CrossRef]
- Bashan, Y.; Holguin, G.; De-Bashan, L.E. Azospirillum-plant relationships: Physiological, molecular, agricultural, and environmental advances (1997–2003). Can. J. Microbiol. 2004, 50, 521–577. [Google Scholar] [CrossRef] [Green Version]
- Ruiza, D.; Agaras, B.; de Werrab, P.; Wall, L.G.; Valverde, C. Characterization and screening of plant probiotic traits of bacteria isolated from rice seeds cultivated in Argentina. J. Microbiol. 2011, 49, 902–912. [Google Scholar] [CrossRef]
- Camargo Dos Santos, P.J.; Savi, D.C.; Rodrigues Gomes, R.; Goulin, E.H.; Da Costa Senkiv, C.; Ossamu Tanaka, F.A.; Rodrigues Almeida, A.M.; Galli-Terasawa, L.; Kava, V.; Glienke, C. Diaporthe endophytica and D. terebinthifolii from medicinal plants for biological control of Phyllosticta citricarpa. Microbiol. Res. 2016, 186–187, 153–160. [Google Scholar] [CrossRef]
- Dillon, R.J.; Dillon, V.M. The gut bacteria of insects: Nonpathogenic interactions. Annu. Rev. Entomol. 2004, 49, 71–92. [Google Scholar] [CrossRef]
- Sevim, A.; Gökçe, C.; Erbaş, Z.; Özkan, F. Bacteria from Ips sexdentatus (Coleoptera: Curculionidae) and their biocontrol potential. J. Basic Microbiol. 2012, 52, 695–704. [Google Scholar] [CrossRef]
- Sansinenea, E.; Vázquez, C.; Ortiz, A. Genetic manipulation in Bacillus thuringiensis for strain improvement. Biotechnol. Lett. 2010, 32, 1549–1557. [Google Scholar] [CrossRef]
- Seidl, V.; Song, L.; Lindquist, E.; Gruber, S.; Koptchinskiy, A.; Zeilinger, S.; Schmoll, M.; Martínez, P.; Sun, J.; Grigoriev, I.; et al. Transcriptomic response of the mycoparasitic fungus Trichoderma atroviride to the presence of a fungal prey. BMC Genom. 2009, 10. [Google Scholar] [CrossRef] [Green Version]
- Ferré, J.; Van Rie, J. Biochemistry and genetics of insect resistance to Bacillus thuringiensis. Annu. Rev. Entomol. 2002, 47, 501–533. [Google Scholar] [CrossRef]
- Vitorino, L.C.; Bessa, L.A. Technological microbiology: Development and applications. Front. Microbiol. 2017, 8, 827. [Google Scholar] [CrossRef] [PubMed]
- Fravel, D.R. Commercialization and implementation of biocontrol. Annu. Rev. Phytopathol. 2005, 43, 337–359. [Google Scholar] [CrossRef] [PubMed]
- Sandoval-Coronado, C.F.; Luna-Olvera, H.A.; Arévalo-Niño, K.; Jackson, M.A.; Poprawski, T.J.; Galán-Wong, L.J. Drying and formulation of blastospores of Paecilomyces fumosoroseus (Hyphomycetes) produced in two different liquid media. World J. Microbiol. Biotechnol. 2001, 17, 423–428. [Google Scholar] [CrossRef]
- Hernández-Rosas, F.; García-Pacheco, L.A.; Figueroa-Rodríguez, K.A.; Figueroa-Sandoval, B.; Salinas Ruiz, J.; Sangerman-Jarquín, D.M.; Díaz-Sánchez, E.L. Análisis de las investigaciones sobre Metarhizium anisopliae en los últimos 40 años. Rev. Mex. Cienc. Agrícolas 2019, 10, 155–166. [Google Scholar] [CrossRef] [Green Version]
- Bridge, P.D.; Prior, C.; Sagbohan, J.; Lomer, C.J.; Carey, M.; Buddie, A. Molecular characterization of isolates of Metarhizium from locusts and grasshoppers. Biodivers. Conserv. 1997, 6, 177–189. [Google Scholar] [CrossRef]
- Blanford, S.; Thomas, M.B.; Langewald, J. Behavioural fever in the Senegalese grasshopper, Oedaleus senegalensis, and its implications for biological control using pathogens. Ecol. Entomol. 1998, 23, 9–14. [Google Scholar] [CrossRef]
- Thomas, M.B.; Blandford, S.; Gbongboui, C.; Lomer, C.J. Experimental studies to evaluate spray applications of a mycoinsecticide against the rice grasshopper, Hieroglyphus daganensis, in northern Benin. Entomol. Exp. Appl. 1998, 87, 93–102. [Google Scholar] [CrossRef]
- Gillespie, J.P.; Bateman, R.; Charnley, A.K. Role of cuticle-degrading proteases in the virulence of Metarhizium spp. for the desert locust, Schistocerca gregaria. J. Invertebr. Pathol. 1998, 71, 128–137. [Google Scholar] [CrossRef]
- Kpindou, O.-K.D.; Shah, P.A.; Langewald, J.; Lomer, C.J.; Van Der Paauw, H.; Sidibe, A.; Daffé, C.O. Essais sur l’utilisation d’un biopesticide à base des conidies de Metarhizium flavoviride pour le contrôle des sauteriaux au Mali. J. Appl. Entomol. 1997, 121, 285–291. [Google Scholar] [CrossRef]
- Bateman, R. Methods of application of microbial pesticide formulations for the control of grasshoppers and locusts. Mem. Entomol. Soc. Can. 1997, 129, 69–81. [Google Scholar] [CrossRef]
- Bateman, R.P.; Douro-Kpindou, O.K.; Kooyman, C.; Lomer, C.; Ouambama, Z. Some observations on the dose transfer of mycoinsecticide sprays to desert locusts. Crop Protect. 1998, 17, 151–158. [Google Scholar] [CrossRef]
- Hong, T.D.; Jenkins, N.E.; Ellis, R.H.; Moore, D. Limits to the negative logarithmic relationship between moisture content and longevity in conidia of Metarhizium flavoviride. Ann. Bot. 1998, 81, 625–630. [Google Scholar] [CrossRef] [Green Version]
- Jenkins, N.E.; Goettel, M.S. Methods for mass-production of microbial control agents of grasshoppers and locusts. Mem. Entomol. Soc. Can. 1997, 129, 37–48. [Google Scholar] [CrossRef]
- Sharma, S.; Shera, P.S.; Kaur, R.; Sangha, K.S. Evaluation of augmentative biological control strategy against major borer insect pests of sugarcane—A large-scale field appraisal. Egypt. J. Biol. Pest Control 2020, 30. [Google Scholar] [CrossRef]
- Vianna, M.F.; Pelizza, S.; Russo, M.L.; Toledo, A.; Mourelos, C.; Scorsetti, A.C. ISSR markers to explore entomopathogenic fungi genetic diversity: Implications for biological control of tobacco pests. J. Biosci. (Bangalore) 2020, 45. [Google Scholar] [CrossRef]
- Rojas, E.C.; Jensen, B.; Jørgensen, H.J.L.; Latz, M.A.C.; Esteban, P.; Ding, Y.; Collinge, D.B. Selection of fungal endophytes with biocontrol potential against Fusarium head blight in wheat. Biol. Control 2020, 144. [Google Scholar] [CrossRef]
- Abd-Elgawad, M.M.M. Biological control agents in the integrated nematode management of potato in Egypt. Egypt. J. Biol. Pest Control 2020, 30. [Google Scholar] [CrossRef]
- Mathews, A.A.; Basha, S.T.; Eswara Reddy, N.P. Fungicide compatible Trichoderma fasiculatum and Trichoderma koningii as bioagents against mango anthracnose. Asian J. Microbiol. Biotechnol. Environ. Sci. 2010, 12, 505–509. [Google Scholar]
- Barra-Bucarei, L.; González, M.G.; Iglesias, A.F.; Aguayo, G.S.; Peñalosa, M.G.; Vera, P.V. Beauveria bassiana multifunction as an endophyte: Growth promotion and biologic control of Trialeurodes vaporariorum, (westwood) (hemiptera: Aleyrodidae) in tomato. Insects 2020, 11, 591. [Google Scholar] [CrossRef] [PubMed]
- Dedej, S.; Delaplane, K.S.; Scherm, H. Effectiveness of honey bees in delivering the biocontrol agent Bacillus subtilis to blueberry flowers to suppress mummy berry disease. Biol. Control 2004, 31, 422–427. [Google Scholar] [CrossRef]
- Chergui, S.; Boudjemaa, K.; Benzehra, A.; Karaca, I. Pathogenicity of indigenous Beauveria bassiana (Balsamo) against Ceratitis capitata Wiedemann (Diptera: Tephritidae) under laboratory conditions. Egypt. J. Biol. Pest Control 2020, 30. [Google Scholar] [CrossRef]
- Ismoilov, K.; Wang, M.; Jalilov, A.; Zhang, X.; Lu, Z.; Saidov, A.; Sun, X.; Han, P. First report using a native lacewing species to control Tuta absoluta: From laboratory trials to field assessment. Insects 2020, 11, 286. [Google Scholar] [CrossRef] [PubMed]
- El Arnaouty, S.A.; El-Heneidy, A.H.; Afifi, A.I.; Heikal, I.H.; Kortam, M.N. Comparative study between biological and chemical control programs of certain sweet pepper pests in greenhouses. Egypt. J. Biol. Pest Control 2020, 30. [Google Scholar] [CrossRef]
- Costa, M.I.S.; Faria, L.B. Integrated pest management: Theoretical insights from a threshold policy. Neotrop. Entomol. 2010, 39, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larkin, R.P. Biological control of soilborne diseases in organic potato production using hypovirulent strains of Rhizoctonia solani. Biol. Agric. Hortic. 2020, 36, 119–129. [Google Scholar] [CrossRef]
- Tang, M.; Liao, H.; Wan, Z.; Herrera-Viedma, E.; Rosen, M. Ten years of sustainability (2009 to 2018): A bibliometric overview. Sustainability 2018, 10, 1655. [Google Scholar] [CrossRef]
- Hood, W.W.; Wilson, C.S. The literature of bibliometrics, scientometrics, and informetrics. Scientometrics 2001, 52, 291–314. [Google Scholar] [CrossRef]
- Centre for Science and Technology Studies. VOSviewer; Leiden University: Leiden, The Netherlands, 2018. [Google Scholar]
- Heersmink, R.; van den Hoven, J.; van Eck, N.J.; van de Berg, J. Bibliometric mapping of computer and information ethics. Ethics Inf. Technol. 2011, 13, 241–249. [Google Scholar] [CrossRef] [Green Version]
- Sweileh, W.M. Global research trends of World Health Organization’s top eight emerging pathogens. Glob. Health 2017, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Eck, N.J.; Waltman, L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics 2010, 84, 523–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kan Yeung, A.W.; Goto, T.K.; Leung, W.K. The changing landscape of neuroscience research, 2006–2015: A bibliometric study. Front. Neurosci. 2017, 11, 1–10. [Google Scholar] [CrossRef]
- Harman, G.E.; Howell, C.R.; Viterbo, A.; Chet, I.; Lorito, M. Trichoderma species-Opportunistic, avirulent plant symbionts. Nat. Rev. Microbiol. 2004, 2, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Lugtenberg, B.; Kamilova, F. Plant-growth-promoting rhizobacteria. Annu. Rev. Microbiol. 2009, 63, 541–556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haas, D.; Défago, G. Biological control of soil-borne pathogens by fluorescent pseudomonads. Nat. Rev. Microbiol. 2005, 3, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Verschuere, L.; Rombaut, G.; Sorgeloos, P.; Verstraete, W. Probiotic bacteria as biological control agents in aquaculture. Microbiol. Mol. Biol. Rev. 2000, 64, 655–671. [Google Scholar] [CrossRef] [Green Version]
- Whipps, J.M. Microbial interactions and biocontrol in the rhizosphere. J. Exp. Bot. 2001, 52, 487–511. [Google Scholar] [CrossRef] [PubMed]
- Hallmann, J.; Quadt-Hallmann, A.; Mahaffee, W.F.; Kloepper, J.W. Bacterial endophytes in agricultural crops. Can. J. Microbiol. 1997, 43, 895–914. [Google Scholar] [CrossRef]
- Stouthamer, R.; Breeuwer, J.A.J.; Hurst, G.D.D. Wolbachia pipientis: Microbial manipulator of arthropod reproduction. Annu. Rev. Microbiol. 1999, 53, 71–102. [Google Scholar] [CrossRef]
- Gatesoupe, F.J. The use of probiotics in aquaculture. Aquaculture 1999, 180, 147–165. [Google Scholar] [CrossRef]
- Berg, G. Plant-microbe interactions promoting plant growth and health: Perspectives for controlled use of microorganisms in agriculture. Appl. Microbiol. Biotechnol. 2009, 84, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Bravo, A.; Gill, S.S.; Soberón, M. Mode of action of Bacillus thuringiensis Cry and Cyt toxins and their potential for insect control. Toxicon 2007, 49, 423–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoitink, H.A.J.; Boehm, M.J. Biocontrol within the context of soil microbial communities: A substrate-dependent phenomenon. Annu. Rev. Phytopathol. 1999, 37, 427–446. [Google Scholar] [CrossRef]
- Vorholt, J.A. Microbial life in the phyllosphere. Nat. Rev. Microbiol. 2012, 10, 828–840. [Google Scholar] [CrossRef]
- Wall, D.H.; Nielsen, U.N.; Six, J. Soil biodiversity and human health. Nature 2015, 528, 69–76. [Google Scholar] [CrossRef]
- Gerhardson, B. Biological substitutes for pesticides. Trends Biotechnol. 2002, 20, 338–343. [Google Scholar] [CrossRef]
- Glare, T.; Caradus, J.; Gelernter, W.; Jackson, T.; Keyhani, N.; Köhl, J.; Marrone, P.; Morin, L.; Stewart, A. Have biopesticides come of age? Trends Biotechnol. 2012, 30, 250–258. [Google Scholar] [CrossRef]
- Tamez Guerra, P.; Galán Wong, L.J.; Medrano Roldán, H.; García Gutiérrez, C.; Rodríguez Padilla, C.; Gómez Flores, R.A.; Tamez Guerra, R.S. Bioinsecticidas: Su empleo, producción y comercialización en México. Ciencia UANL 2001, IV, 143–152. [Google Scholar]
- Quiroz-Castañeda, R.E.; Mendoza-Mejía, A.; Obregón-Barboza, V.; Martínez-Ocampo, F.; Hernández-Mendoza, A.; Martínez-Garduño, F.; Guillén-Solís, G.; Sánchez-Rodríguez, F.; Peña-Chora, G.; Ortíz-Hernández, L.; et al. Identification of a new Alcaligenes faecalis strain MOR02 and assessment of its toxicity and pathogenicity to insects. BioMed Res. Int. 2015, 2015, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Lu, L.; Cheng, B.; Du, D.; Hu, X.; Peng, A.; Pu, Z.; Zhang, X.; Huang, Z.; Chen, G. Morphological, molecular and virulence characterization of three Lecanicillium species infecting Asian citrus psyllids in Huangyan citrus groves. J. Invertebr. Pathol. 2015, 125, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Urquiza, A.; Luo, Z.; Keyhani, N.O. Improving mycoinsecticides for insect biological control. Appl. Microbiol. Biotechnol. 2014, 99, 1057–1068. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Feng, M.G. Advances in fundamental and applied studies in China of fungal biocontrol agents for use against arthropod pests. Biol. Control 2014, 68, 129–135. [Google Scholar] [CrossRef]
- Rezende, J.M.; Zanardo, A.B.R.; da Silva Lopes, M.; Delalibera, I.; Rehner, S.A. Phylogenetic diversity of Brazilian Metarhizium associated with sugarcane agriculture. BioControl 2015, 60, 495–505. [Google Scholar] [CrossRef]
- De Faria, M.R.; Wraight, S.P. Mycoinsecticides and Mycoacaricides: A comprehensive list with worldwide coverage and international classification of formulation types. Biol. Control 2007, 43, 237–256. [Google Scholar] [CrossRef]
- Paulitz, T.C.; Bélanger, R.R. Biological control in greenhouse systems. Annu. Rev. Phytopathol. 2001, 39, 103–133. [Google Scholar] [CrossRef]
- Vos, C.M.F.; De Cremer, K.; Cammue, B.P.A.; De Coninck, B. The toolbox of Trichoderma spp. in the biocontrol of Botrytis cinerea disease. Mol. Plant Pathol. 2015, 16, 400–412. [Google Scholar] [CrossRef] [Green Version]
- Kunst, F.; Ogasawara, N.; Moszer, I.; Albertini, A.M.; Alloni, G.; Azevedo, V.; Bertero, M.G.; Bessières, P.; Bolotin, A.; Borchert, S.; et al. The complete genome sequence of the gram-positive bacterium Bacillus subtilis. Nature 1997, 390, 249–256. [Google Scholar] [CrossRef] [Green Version]
- Bais, H.P.; Fall, R.; Vivanco, J.M. Biocontrol of Bacillus subtilis against infection of Arabidopsis roots by Pseudomonas syringae is facilitated by biofilm formation and surfactin production. Plant Physiol. 2004, 134, 307–319. [Google Scholar] [CrossRef] [Green Version]
- Padilla, C.; Pardo-López, L.; De La Riva, G.; Gómez, I.; Sánchez, J.; Hernandez, G.; Nuñez, M.E.; Carey, M.P.; Dean, D.H.; Alzate, O.; et al. Role of tryptophan residues in toxicity of Cry1Ab toxin from Bacillus thuringiensis. Appl. Environ. Microbiol. 2006, 72, 901–907. [Google Scholar] [CrossRef] [Green Version]
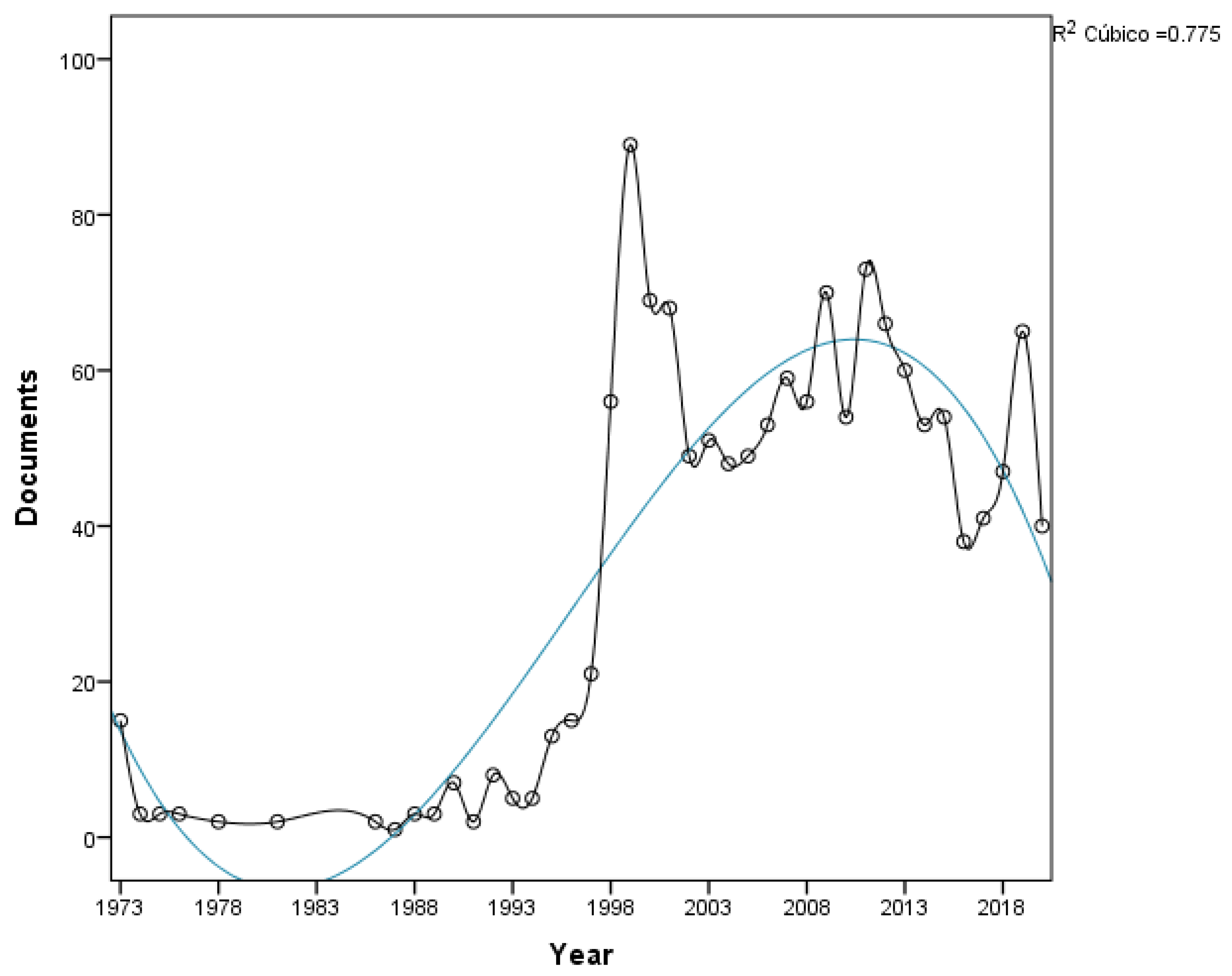
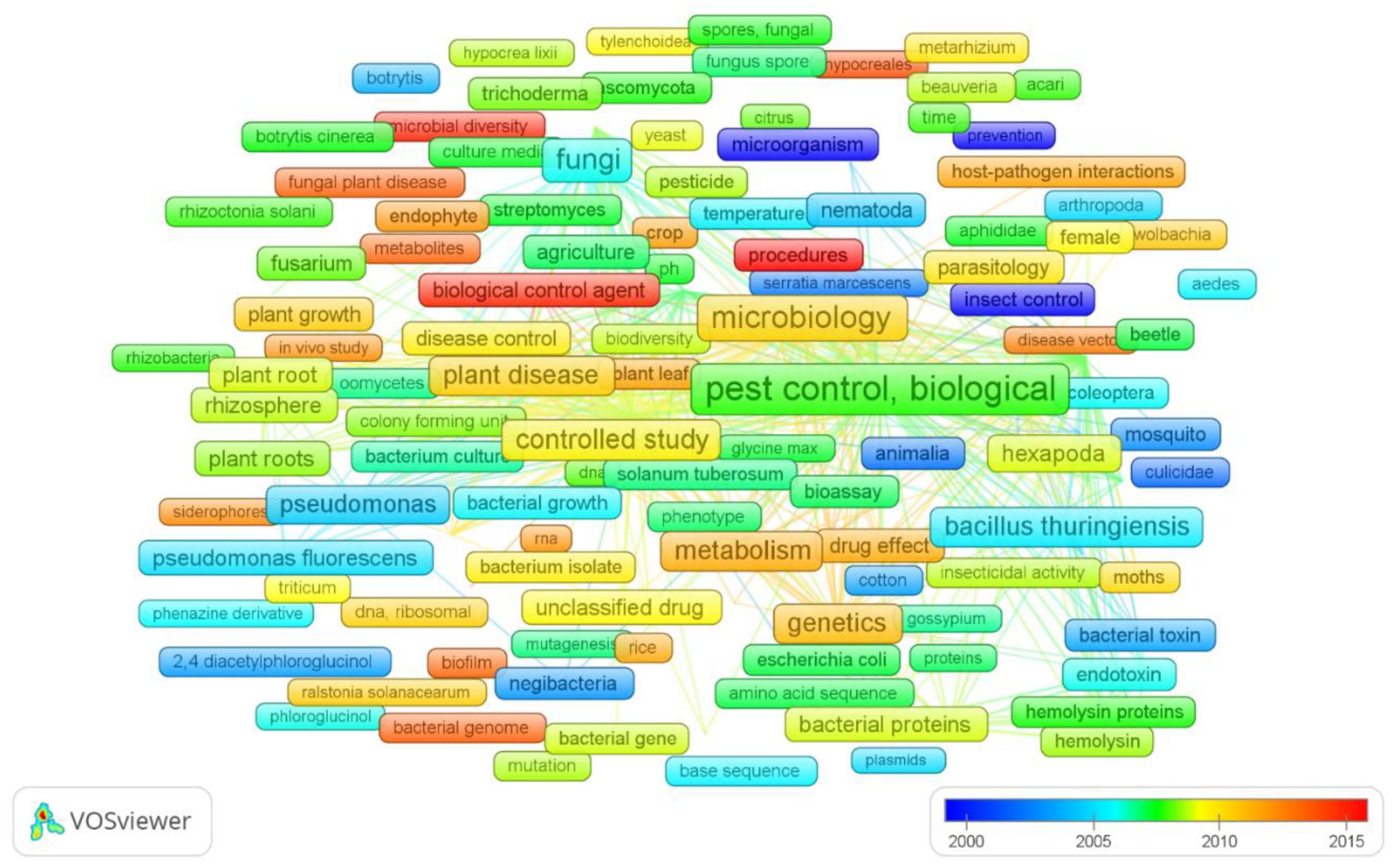


| Rank | Journal | Pub. | Country | Pub. | Institute | Pub. |
|---|---|---|---|---|---|---|
| 1 | Applied and Environmental Microbiology | 97 | United States | 351 | USDA Agricultural Research Service, Washington DC | 65 |
| 2 | Journal of Invertebrate Pathology | 81 | China | 167 | United States Department of Agriculture | 45 |
| 3 | Journal of Applied Microbiology | 47 | India | 111 | ETH Zurich | 32 |
| 4 | Applied Microbiology and Biotechnology | 39 | France | 82 | University of Florida | 22 |
| 5 | Pest Management Science | 36 | United Kingdom | 82 | Wageningen University and Research Centre | 20 |
| 6 | Canadian Journal of Microbiology | 33 | Germany | 76 | Ministry of Education China | 19 |
| 7 | Journal of Economic Entomology | 29 | Spain | 76 | Chinese Academy of Agricultural Sciences | 19 |
| 8 | Biological Control | 28 | Brazil | 69 | Université de Lausanne UNIL | 18 |
| 9 | Frontiers in Microbiology | 25 | Canada | 57 | USDA ARS Beltsville Agricultural Research Center | 18 |
| 10 | Letters in Applied Microbiology | 23 | Switzerland | 53 | Zhejiang University | 18 |
| Rank | Author (year) | Title | Journal | Citations |
|---|---|---|---|---|
| 1 | Harman, et al. [55] | Trichoderma species—Opportunistic, avirulent plant symbionts | Nature Reviews Microbiology | 1806 |
| 2 | Lugtenberg and Kamilova [56] | Plant-growth-promoting rhizobacteria | Annual Review of Microbiology | 1626 |
| 3 | Haas and Défago [57] | Biological control of soil-borne pathogens by fluorescent pseudomonads | Nature Reviews Microbiology | 1337 |
| 4 | Compant, Duffy, Nowak, Clément and Barka [1] | Use of plant growth-promoting bacteria for biocontrol of plant diseases: Principles, mechanisms of action, and future prospects | Applied and Environmental Microbiology | 1258 |
| 5 | Verschuere, et al. [58] | Probiotic bacteria as biological control agents in aquaculture | Microbiology and Molecular Biology Reviews | 1206 |
| 6 | Whipps [59] | Microbial interactions and biocontrol in the rhizosphere | Journal of Experimental Botany | 1068 |
| 7 | Hallmann, et al. [60] | Bacterial endophytes in agricultural crops | Canadian Journal of Microbiology | 1039 |
| 8 | Stouthamer, et al. [61] | Wolbachia pipientis: Microbial manipulator of arthropod reproduction | Annual Review of Microbiology | 906 |
| 9 | Dillon and Dillon [18] | The gut bacteria of insects: Nonpathogenic interactions | Annual Review of Entomology | 804 |
| 10 | Gatesoupe [62]. | The use of probiotics in aquaculture | Aquaculture | 791 |
| 11 | Berg [63] | Plant-microbe interactions promoting plant growth and health: Perspectives for controlled use of microorganisms in agriculture | Applied Microbiology and Biotechnology | 705 |
| 12 | Ferré and Van Rie [22] | Biochemistry and genetics of insect resistance to Bacillus thuringiensis | Annual Review of Entomology | 683 |
| 13 | Bravo, et al. [64] | Mode of action of Bacillus thuringiensis Cry and Cyt toxins and their potential for insect control | Toxicon | 679 |
| 14 | Hoitink and Boehm [65] | Biocontrol within the context of soil microbial communities: A substrate-dependent phenomenon | Annual Review of Phytopathology | 607 |
| 15 | Vorholt [66] | Microbial life in the phyllosphere | Nature Reviews Microbiology | 600 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Rosas, F.; Figueroa-Rodríguez, K.A.; García-Pacheco, L.A.; Velasco-Velasco, J.; Sangerman-Jarquín, D.M. Microorganisms and Biological Pest Control: An Analysis Based on a Bibliometric Review. Agronomy 2020, 10, 1808. https://doi.org/10.3390/agronomy10111808
Hernández-Rosas F, Figueroa-Rodríguez KA, García-Pacheco LA, Velasco-Velasco J, Sangerman-Jarquín DM. Microorganisms and Biological Pest Control: An Analysis Based on a Bibliometric Review. Agronomy. 2020; 10(11):1808. https://doi.org/10.3390/agronomy10111808
Chicago/Turabian StyleHernández-Rosas, Francisco, Katia A. Figueroa-Rodríguez, Luis A. García-Pacheco, Joel Velasco-Velasco, and Dora M. Sangerman-Jarquín. 2020. "Microorganisms and Biological Pest Control: An Analysis Based on a Bibliometric Review" Agronomy 10, no. 11: 1808. https://doi.org/10.3390/agronomy10111808
APA StyleHernández-Rosas, F., Figueroa-Rodríguez, K. A., García-Pacheco, L. A., Velasco-Velasco, J., & Sangerman-Jarquín, D. M. (2020). Microorganisms and Biological Pest Control: An Analysis Based on a Bibliometric Review. Agronomy, 10(11), 1808. https://doi.org/10.3390/agronomy10111808





