Effects of Plant-Soil Feedback on Switchgrass Productivity Related to Microbial Origin
Abstract
1. Introduction
2. Materials and Methods
3. Results
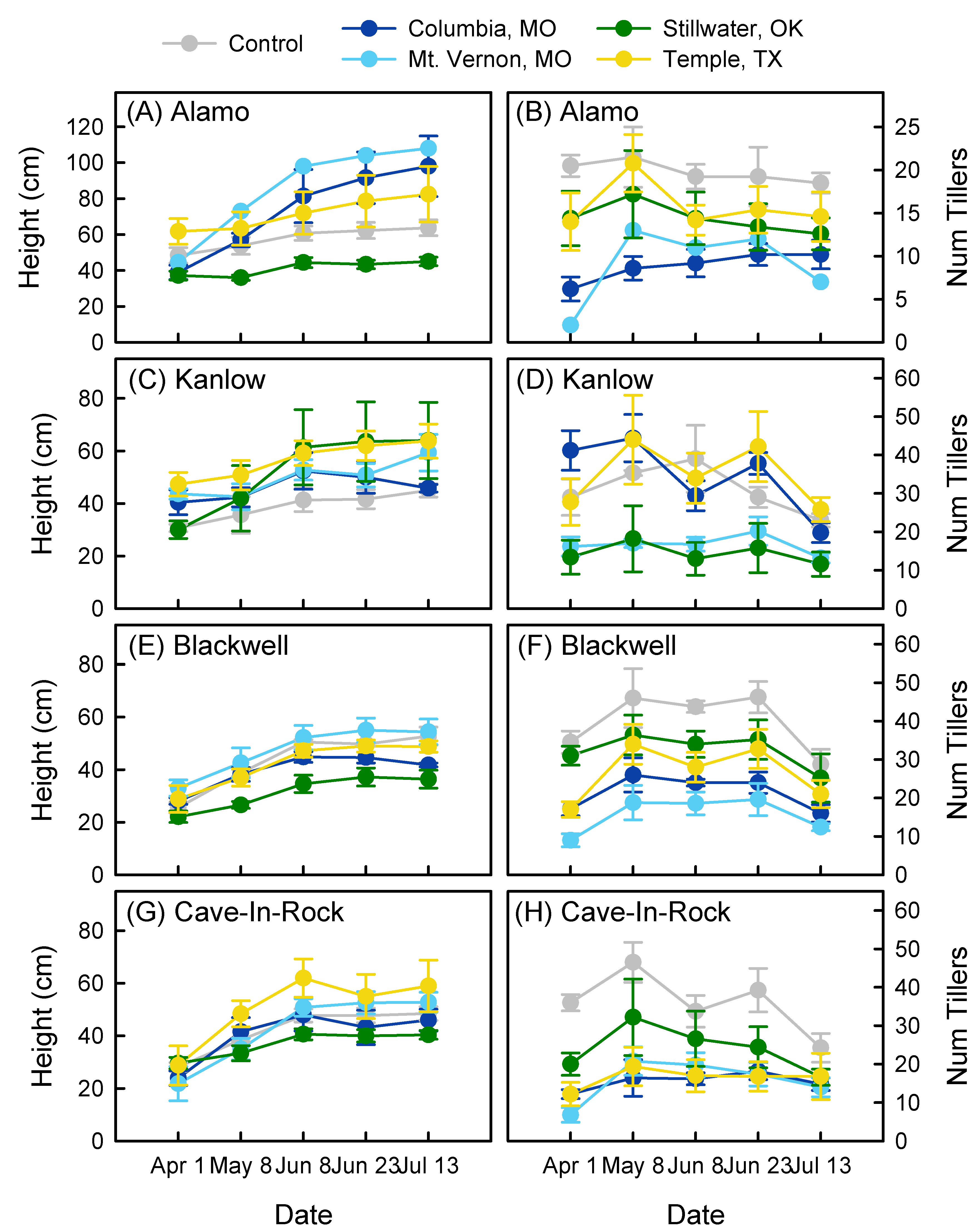
3.1. Growth over Time
3.2. Biomass and Feedback at Harvest
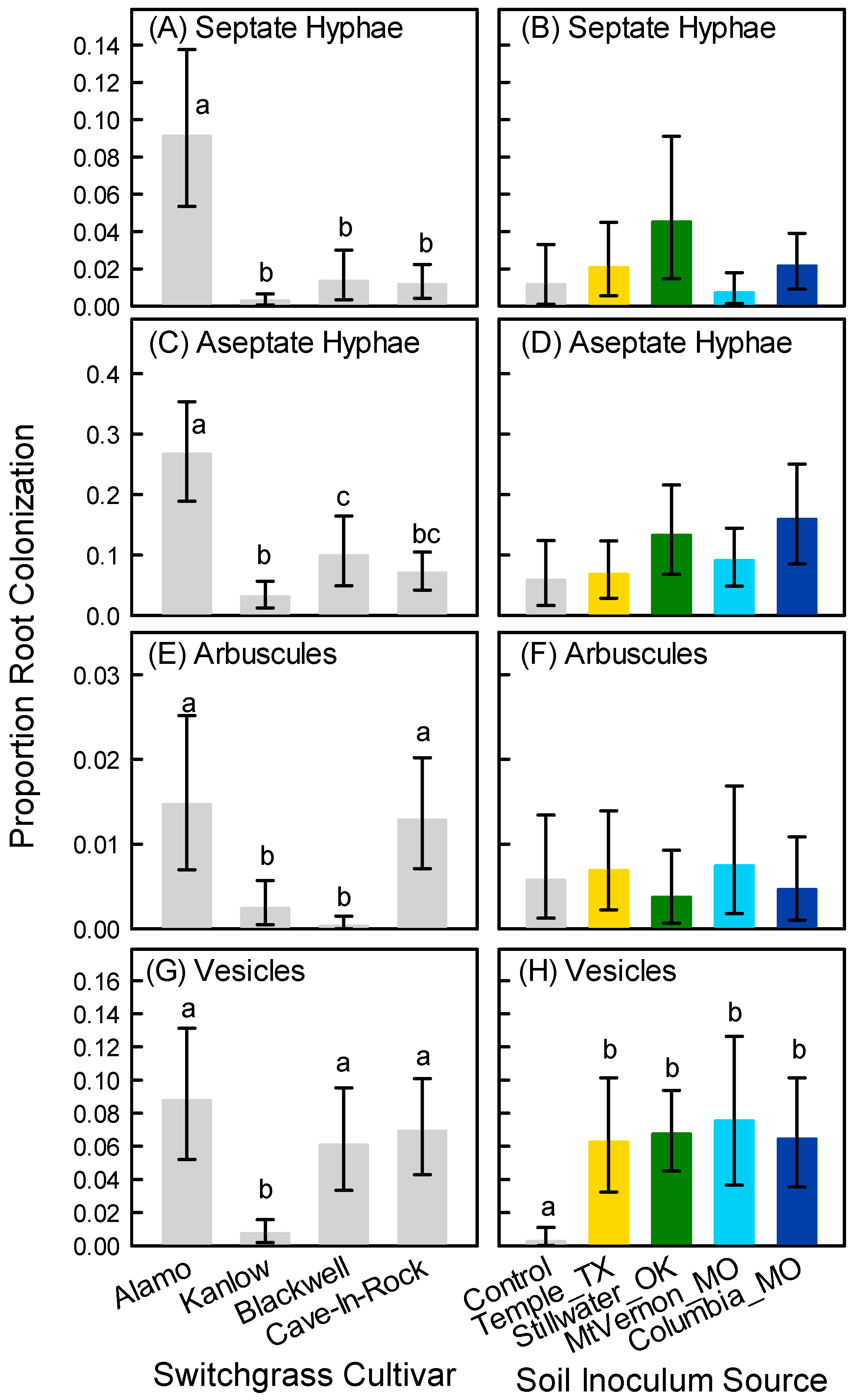
3.3. Root Colonization at Harvest
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aspinwall, M.J.; Lowry, D.B.; Taylor, S.H.; Juenger, T.E.; Hawkes, C.V.; Johnson, M.-V.V.; Kiniry, J.R.; Fay, P.A. Genotypic variation in traits linked to climate and aboveground productivity in a widespread C4grass: Evidence for a functional trait syndrome. New Phytol. 2013, 199, 966–980. [Google Scholar] [CrossRef] [PubMed]
- Casler, M.D.; Vogel, K.P.; Taliaferro, C.M.; Ehlke, N.J.; Berdahl, J.D.; Brummer, E.C.; Kallenbach, R.L.; West, C.P.; Mitchell, R.B. Latitudinal and Longitudinal Adaptation of Switchgrass Populations. Crop. Sci. 2007, 47, 2249–2260. [Google Scholar] [CrossRef]
- Casler, M.D.; Vogel, K.P.; Taliaferro, C.M.; Wynia, R.L. Latitudinal Adaptation of Switchgrass Populations. Crop. Sci. 2004, 44, 293–303. [Google Scholar] [CrossRef]
- Kiniry, J.R.; Anderson, L.C.; Johnson, M.-V.V.; Behrman, K.D.; Brakie, M.; Burner, D.; Cordsiemon, R.L.; Fay, P.A.; Fritschi, F.B.; Houx, J.H., III; et al. Perennial Biomass Grasses and the Mason–Dixon Line: Comparative Productivity across Latitudes in the Southern Great Plains. BioEnergy Res. 2012, 6, 276–291. [Google Scholar] [CrossRef]
- Kiniry, J.R.; Johnson, M.-V.V.; Bruckerhoff, S.B.; Kaiser, J.U.; Cordsiemon, R.L.; Harmel, R.D. Clash of the Titans: Comparing Productivity Via Radiation Use Efficiency for Two Grass Giants of the Biofuel Field. BioEnergy Res. 2011, 5, 41–48. [Google Scholar] [CrossRef]
- Lowry, D.B.; Behrman, K.D.; Grabowski, P.; Morris, G.P.; Kiniry, J.R.; Juenger, T.E. Adaptations between Ecotypes and along Environmental Gradients in Panicum virgatum. Am. Nat. 2014, 183, 682–692. [Google Scholar] [CrossRef]
- Kiniry, J.R.; Sanchez, H.; Greenwade, J.; Seidensticker, E.; Bell, J.R.; Pringle, F.; Peacock, G.; Rives, J. Simulating grass productivity on diverse range sites in Texas. J. Soil Water Conserv. 2002, 57, 144. [Google Scholar]
- Kulmatiski, A.; Beard, K.H.; Stevens, J.R.; Cobbold, S.M. Plant-soil feedbacks: A meta-analytical review. Ecol. Lett. 2008, 11, 980–992. [Google Scholar] [CrossRef]
- Cortois, R.; Schröder-Georgi, T.; Weigelt, A.; Van Der Putten, W.H.; De Deyn, G.B. Plant–soil feedbacks: Role of plant functional group and plant traits. J. Ecol. 2016, 104, 1608–1617. [Google Scholar] [CrossRef]
- Mariotte, P.; Mehrabi, Z.; Bezemer, T.M.; De Deyn, G.B.; Kulmatiski, A.; Drigo, B.; Veen, G.; Van Der Heijden, M.G.; Kardol, P. Plant–Soil Feedback: Bridging Natural and Agricultural Sciences. Trends Ecol. Evol. 2018, 33, 129–142. [Google Scholar] [CrossRef]
- Dias, T.; Dukes, A.; Antunes, P.M. Accounting for soil biotic effects on soil health and crop productivity in the design of crop rotations. J. Sci. Food Agric. 2015, 95, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A.R.; Inouye, B.D.; Johnson, M.T.J.; Underwood, N.; Vellend, M. Ecological consequences of genetic diversity. Ecol. Lett. 2008, 11, 609–623. [Google Scholar] [CrossRef] [PubMed]
- Crawford, K.M.; Hawkes, C.V. Soil precipitation legacies influence intraspecific plant–soil feedback. Ecology 2020, 101, e03142. [Google Scholar] [CrossRef]
- Ronsheim, M.L. Plant Genotype Influences Mycorrhiza Benefits and Susceptibility to a Soil Pathogen. Am. Midl. Nat. 2016, 175, 103–112. [Google Scholar] [CrossRef]
- Pérez-Izquierdo, L.; Zabal-Aguirre, M.; González-Martínez, S.C.; Buée, M.; Verdú, M.; Rincón, A.; Goberna, M. Plant intraspecific variation modulates nutrient cycling through its below ground rhizospheric microbiome. J. Ecol. 2019, 107, 1594–1605. [Google Scholar] [CrossRef]
- Revillini, D.; Gehring, C.A.; Johnson, N.C. The role of locally adapted mycorrhizas and rhizobacteria in plant–soil feedback systems. Funct. Ecol. 2016, 30, 1086–1098. [Google Scholar] [CrossRef]
- Van Grunsven, R.H.A.; Van Der Putten, W.H.; Bezemer, T.M.; Tamis, W.L.M.; Berendse, F.; Veenendaal, E.M. Reduced plant? Soil feedback of plant species expanding their range as compared to natives. J. Ecol. 2007, 95, 1050–1057. [Google Scholar] [CrossRef]
- Mills, K.E.; Bever, J.D. Maintenance of diversity within plant communtiies: Soil pathogens as agents of negative feedback. Ecology 1998, 79, 1595–1601. [Google Scholar] [CrossRef]
- Petermann, J.S.; Fergus, A.J.F.; Turnbull, L.A.; Schmid, B. Janzen-Connell Effects Are Widespread And Strong Enough To Maintain Diversity In Grasslands. Ecology 2008, 89, 2399–2406. [Google Scholar] [CrossRef]
- Maron, J.L.; Klironomos, J.; Waller, L.; Callaway, R.M. Invasive plants escape from suppressive soil biota at regional scales. J. Ecol. 2013, 102, 19–27. [Google Scholar] [CrossRef]
- Johnson, N.C.; Wilson, G.W.T.; Bowker, M.A.; Wilson, J.A.; Miller, R.M. Resource limitation is a driver of local adaptation in mycorrhizal symbioses. Proc. Natl. Acad. Sci. USA 2010, 107, 2093. [Google Scholar] [CrossRef] [PubMed]
- Meisner, A.; Gera Hol, W.H.; de Boer, W.; Krumins, J.A.; Wardle, D.A.; van der Putten, W.H. Plant–soil feedbacks of exotic plant species across life forms: A meta-analysis. Biol. Invasions 2014, 16, 2551–2561. [Google Scholar] [CrossRef]
- Porter, C.L., Jr. An analysis of variation between upland and lowland switchgrass, Panicum virgatum L., in central Oklahoma. Ecology 1966, 47, 980–992. [Google Scholar] [CrossRef]
- Brunken, J.N.; Estes, J.R. Cytological and morphological variation in Panicum virgatum L. Southwest. Nat. 1975, 19, 379–385. [Google Scholar] [CrossRef]
- Stroup, J.A.; Sanderson, M.A.; Muir, J.P.; McFarland, M.J.; Reed, R.L. Comparison of growth and performance in upland and lowland switchgrass types to water and nitrogen stress. Bioresour. Technol. 2003, 86, 65–72. [Google Scholar] [CrossRef]
- Aurangzaib, M.; Moore, K.J.; Archontoulis, S.V.; Heaton, E.A.; Lenssen, A.W.; Fei, S. Compositional differences among upland and lowland switchgrass ecotypes grown as a bioenergy feedstock crop. Biomass Bioenergy 2016, 87, 169–177. [Google Scholar] [CrossRef]
- Hawkes, C.V.; Kivlin, S.N.; Du, J.; Eviner, V.T. The temporal development and additivity of plant-soil feedback in perennial grasses. Plant Soil 2013, 369, 141–150. [Google Scholar] [CrossRef]
- Merryweather, J.W.; Fitter, A.H. A modified method for elucidating the structure of the fungal partner in a vesicular-arbuscular mycorrhiza. Mycol. Res. 1991, 95, 1435–1437. [Google Scholar] [CrossRef]
- McGonigle, T.P.; Miller, M.H.; Evans, D.G.; Fairchild, G.L.; Swan, J.A. A new method which gives an objective measure of colonization of roots by vesicular—Arbuscular mycorrhizal fungi. New Phytol. 1990, 115, 495–501. [Google Scholar] [CrossRef]
- Pernilla Brinkman, E.; Van der Putten, W.H.; Bakker, E.-J.; Verhoeven, K.J.F. Plant–soil feedback: Experimental approaches, statistical analyses and ecological interpretations. J. Ecol. 2010, 98, 1063–1073. [Google Scholar] [CrossRef]
- Hopkins, A.A.; Vogel, K.P.; Moore, K.J.; Johnson, K.D.; Carlson, I.T. Genotypic Variability and Genotype × Environment Interactions among Switchgrass Accessions from the Midwestern USA. Crop. Sci. 1995, 35, 565–571. [Google Scholar] [CrossRef]
- Cassida, K.A.; Muir, J.P.; Hussey, M.; Read, J.; Venuto, B.; Ocumpaugh, W. Biomass Yield and Stand Characteristics of Switchgrass in South Central U.S. Environments. Crop. Sci. 2005, 45, 673–681. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Tran, H.; Shan, L.; Kim, J.; Childs, K.L.; Ervin, E.H.; Frazier, T.P.; Zhao, B. Assessment of drought tolerance of 49 switchgrass (Panicum virgatum) genotypes using physiological and morphological parameters. Biotechnol. Biofuels 2015, 8, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Aspinwall, M.J.; Fay, P.A.; Hawkes, C.V.; Lowry, D.B.; Khasanova, A.; Bonnette, J.; Whitaker, B.K.; Johnson, N.; Juenger, T.E. Intraspecific variation in precipitation responses of a widespread C4grass depends on site water limitation. J. Plant Ecol. 2016, 10, 310–321. [Google Scholar] [CrossRef]
- Klironomos, J.N. Feedback with soil biota contributes to plant rarity and invasiveness in communities. Nat. Cell Biol. 2002, 417, 67–70. [Google Scholar] [CrossRef]
- Semchenko, M.; Leff, J.W.; Lozano, Y.M.; Saar, S.; Davison, J.; Wilkinson, A.; Jackson, B.G.; Pritchard, W.J.; De Long, J.R.; Oakley, S.; et al. Fungal diversity regulates plant-soil feedbacks in temperate grassland. Sci. Adv. 2018, 4, eaau4578. [Google Scholar] [CrossRef]
- Emery, S.M.; Kinnetz, E.R.; Bell-Dereske, L.; Stahlheber, K.A.; Gross, K.L.; Pennington, D. Low variation in arbuscular mycorrhizal fungal associations and effects on biomass among switchgrass cultivars. Biomass Bioenergy 2018, 119, 503–508. [Google Scholar] [CrossRef]
- Ulbrich, T.C.; Friesen, M.L.; Roley, S.S.; Tiemann, L.K.; E Evans, S. Intraspecific variability in root traits and edaphic conditions influence soil microbiomes across 12 switchgrass cultivars. Phytobiomes J. 2020. [Google Scholar] [CrossRef]

| Source Location | Soil Series | Soil Taxonomic Class | Soil Texture | Latitude | pH | Organic Matter % | Cation Exchange Capacity (mEq per 100 g Soil) |
|---|---|---|---|---|---|---|---|
| Temple, TX, USA | Houston | Udic Haplusterts | black clay | 31.04 | 7.9 | 2.38 | 45.9 |
| Stillwater, OK, USA | Kirkland | Udertic Paleustolls | silt loam | 36.12 | 5.9 | 1.83 | 49.2 |
| Mt. Vernon, MO, USA | Gerald | Aeric Fragiaqualfs | silt loam | 37.07 | 5.9 | 1.70 | 10.2 |
| Columbia, MO, USA | Mexico | Vertic Epiaqualfs | silt loam | 38.89 | 6.3 | 1.67 | 16.3 |
| Previous Field Yields (Mg ha−1 ± 1 SD) | |||||||
|---|---|---|---|---|---|---|---|
| Cultivar | State of Origin | Latitude of Origin | Ecotype | Temple, TX, USA | Stillwater, OK, USA | Mt. Vernon, MO, USA | Columbia, MO, USA |
| Alamo | Texas | 28 | Lowland | 30.6 ± 26.5 | 15.0 ± 7.0 | 15.1 ± 7.0 | 20.9 ± 8.9 |
| Kanlow | Oklahoma | 35 | Lowland | 13.8 ± 7.3 | 12.4 ± 0.9 | 21.2 ± 9.7 | 21.2 ± 7.0 |
| Blackwell | Oklahoma | 37 | Upland | 4.8 ± 2.8 | 8.8 ± 3.6 | 11.6 ± 1.9 | 6.7 ± 1.4 |
| Cave-In-Rock | Illinois | 38 | Upland | 4.8 ± 1.5 | 12.5 ± 3.6 | 14.8 ± 3.5 | 8.9 ± 1.3 |
| Height | Tillers | ||||||
|---|---|---|---|---|---|---|---|
| df | MS | F | p | MS | F | p | |
| Between-subjects | |||||||
| Cultivar | 3 | 10447.75 | 14.57 | <0.001 | 3173.17 | 11.78 | <0.001 |
| Soil | 4 | 3745.74 | 5.22 | 0.001 | 2596.87 | 9.64 | <0.001 |
| Cultivar × Soil | 12 | 1331.51 | 1.86 | 0.055 | 994.60 | 3.69 | <0.001 |
| Error | 71 | 717.01 | 269.27 | ||||
| Within-Subjects | |||||||
| Date | 4 | 7805.84 | 107.36 | <0.001 | 1205.94 | 30.20 | <0.001 |
| Date × Cultivar | 12 | 178.01 | 2.45 | 0.029 | 86.47 | 2.17 | 0.032 |
| Date × Soil | 16 | 94.63 | 1.30 | 0.195 | 53.69 | 1.34 | 0.205 |
| Date × Cultivar × Soil | 48 | 187.94 | 2.59 | <0.001 | 51.86 | 1.30 | 0.145 |
| Error | 71 | 72.71 | 39.93 | ||||
| Biomass | Feedback | |||||||
|---|---|---|---|---|---|---|---|---|
| Treatment | df | MS | F | p | df | MS | F | p |
| Cultivar | 3 | 0.244 | 1.594 | 0.198 | 3 | 1.760 | 11.417 | <0.001 |
| Soil | 4 | 2.910 | 19.023 | <0.001 | 3 | 1.271 | 8.246 | <0.001 |
| Cultivar × soil | 12 | 0.265 | 1.729 | 0.078 | 9 | 0.234 | 1.515 | 0.163 |
| Error | 74 | 0.153 | 61 | 0.154 | ||||
| Septate Hyphae | Aseptate Hyphae | Arbuscules | Vesicles | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| df | MS | F | p | MS | F | p | MS | F | p | MS | F | p | |
| Cultivar | 3 | 0.198 | 14.327 | <0.001 | 0.488 | 14.473 | <0.001 | 0.050 | 12.508 | <0.001 | 0.21 | 17.990 | <0.001 |
| Soil | 4 | 0.048 | 3.486 | 0.012 | 0.110 | 3.253 | 0.016 | 0.001 | 0.319 | 0.864 | 0.17 | 14.531 | <0.001 |
| Cultivar × Soil | 12 | 0.028 | 2.020 | 0.034 | 0.053 | 1.570 | 0.119 | 0.013 | 3.142 | 0.001 | 0.029 | 2.497 | 0.008 |
| Error | 74 | 0.014 | 0.034 | 0.004 | 0.012 | ||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiniry, J.R.; Arthur, C.E.; Banick, K.M.; Fritschi, F.B.; Wu, Y.; Hawkes, C.V. Effects of Plant-Soil Feedback on Switchgrass Productivity Related to Microbial Origin. Agronomy 2020, 10, 1860. https://doi.org/10.3390/agronomy10121860
Kiniry JR, Arthur CE, Banick KM, Fritschi FB, Wu Y, Hawkes CV. Effects of Plant-Soil Feedback on Switchgrass Productivity Related to Microbial Origin. Agronomy. 2020; 10(12):1860. https://doi.org/10.3390/agronomy10121860
Chicago/Turabian StyleKiniry, James R., Caroline E. Arthur, Katherine M. Banick, Felix B. Fritschi, Yanqi Wu, and Christine V. Hawkes. 2020. "Effects of Plant-Soil Feedback on Switchgrass Productivity Related to Microbial Origin" Agronomy 10, no. 12: 1860. https://doi.org/10.3390/agronomy10121860
APA StyleKiniry, J. R., Arthur, C. E., Banick, K. M., Fritschi, F. B., Wu, Y., & Hawkes, C. V. (2020). Effects of Plant-Soil Feedback on Switchgrass Productivity Related to Microbial Origin. Agronomy, 10(12), 1860. https://doi.org/10.3390/agronomy10121860






