Broomrape as a Major Constraint for Grass Pea (Lathyrus sativus) Production in Mediterranean Rain-Fed Environments
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Experimental Design
2.2. Statistical Analysis
3. Results
3.1. Analysis of Variance
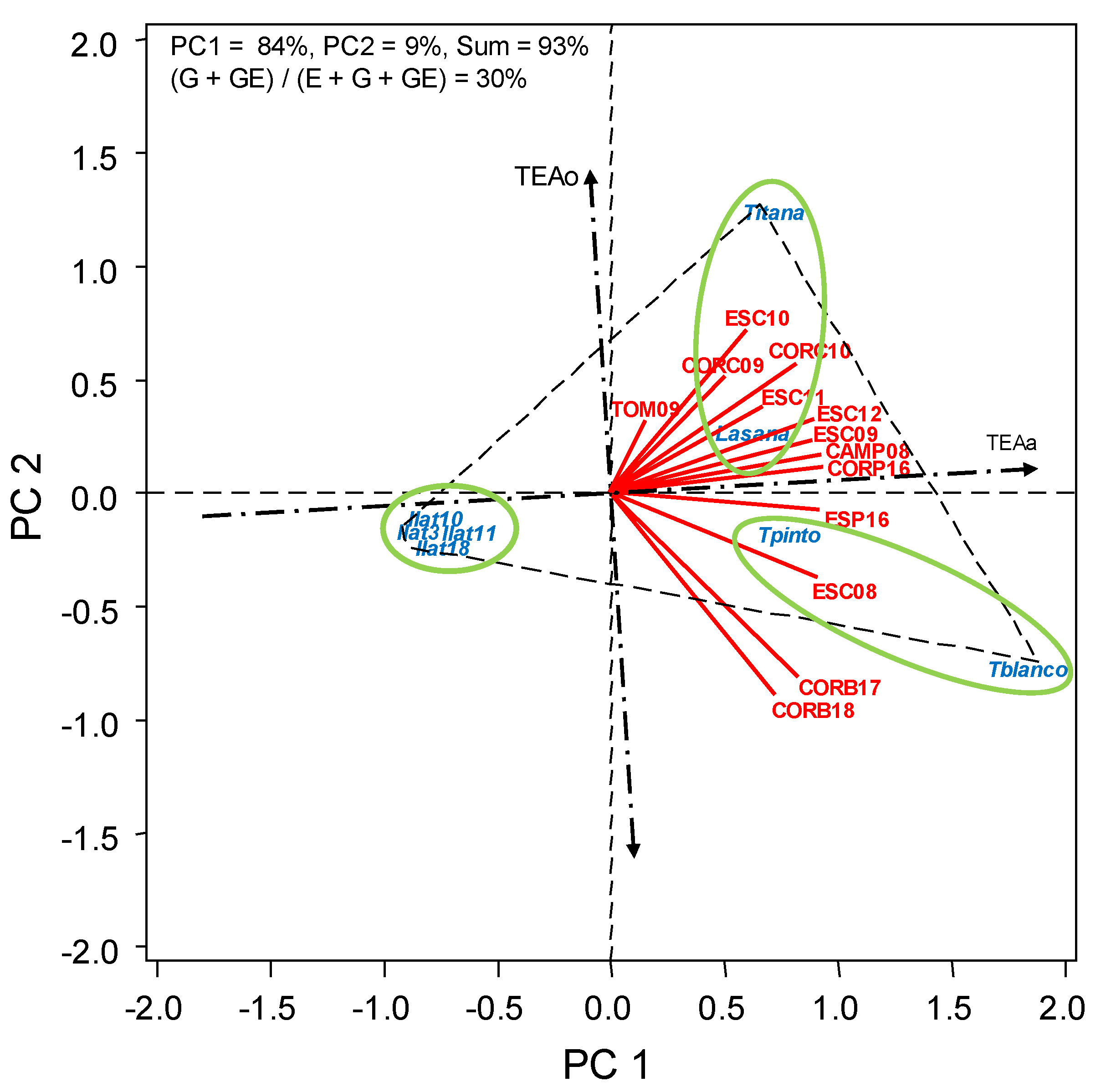
3.2. HA-GGE Analysis
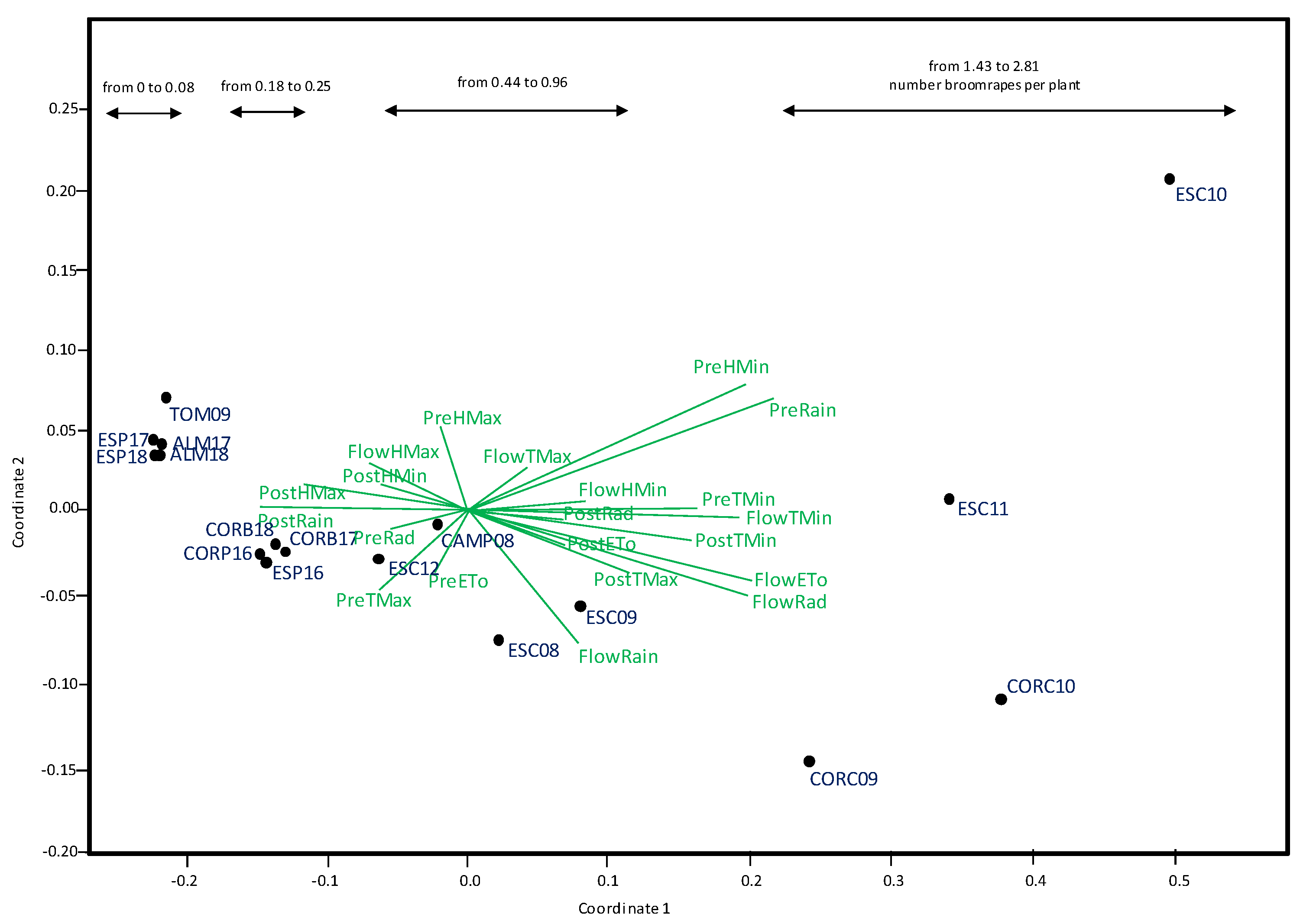
3.3. Effect of Climate Variables on Broomrape Inffection
3.4. Effect of Broomrape Infection, Flowering Date and All Climate Variables on Yield
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gonςalves, L.; Rubiales, D.; Vaz Patto, M.C. Grass pea prospective at the Mediterranean Basin. Legume Perspect. 2015, 10, 8–9. [Google Scholar]
- AESAN. Informe del Comité Científico de la Agencia Española de Seguridad Alimentaria y Nutrición (AESAN) Sobre el Consumo Humano Ocasional de Almortas (Lathyrus sativus). Revista Comité Científico nº 11. AESAN-2009-12. 2009. Available online: https://www.aesan.gob.es/AECOSAN/docs/documentos/seguridad_alimentaria/evaluacion_riesgos/informes_comite/ALMORTAS_CONSUMO.pdf (accessed on 2 November 2020).
- MAPAMA. 2019. Available online: https://www.mapa.gob.es/estadistica/pags/anuario/2019-Avance/CAPITULOSPDF/CAPITULO07/pdfc07_2.10.2.pdf (accessed on 2 November 2020).
- Lambein, F.; Travella, S.; Kuo, Y.; Van Montagu, M.; Heidje, M. Grass pea (Lathyrus sativus L.): Orphan crop, nutraceutical or just plain food? Planta 2019, 250, 821–838. [Google Scholar] [CrossRef] [PubMed]
- Dixit, G.P.; Parihar, A.K.; Bohra, A.; Singh, N.P. Achievements and prospects of grass pea (Lathyrus sativus L.) improvement for sustainable food production. Crop J. 2016, 4, 407–416. [Google Scholar] [CrossRef]
- Girma, D.; Korbu, L. Genetic improvement of grass pea (Lathyrus sativus) in Ethiopia: An unfulfilled promise. Plant Breed. 2012, 131, 231–236. [Google Scholar] [CrossRef]
- Fikre, A.; Negwo, T.; Kuo, Y.H.; Lambein, F.; Ahmed, S. Climatic, edaphic and altitudinal factors affecting yield and toxicity of Lathyrus sativus grown at five locations in Ethiopia. Food Chem. Toxicol. 2011, 49, 623–630. [Google Scholar] [CrossRef]
- Jiao, C.J.; Jiang, J.L.; Ke, L.M.; Cheng, W.; Li, F.M.; Li, Z.X.; Wang, C.Y. Factors affecting beta-ODAP content in Lathyrus sativus and their possible physiological mechanisms. Food Chem. Toxicol. 2011, 49, 543–549. [Google Scholar] [CrossRef]
- Getahun, H.; Lambein, F.; Vanhoorne, M.; Van der Stuyft, P. Food-aid cereals to reduce neurolathyrism related to grass-pea preparations during famine. Lancet 2003, 362, 1808–1810. [Google Scholar] [CrossRef]
- Kumar, S.; Gupta, P.; Barpete, S.; Sarker, A.; Amri, A.; Mathur, P.N.; Baum, M. Grass pea. In Genetic and Genomic Resources of Grain Legume Improvement; Singh, M., Upadhyaya, H.D., Bisht, I.S., Eds.; Elsevier Science: Oxford, UK, 2013; pp. 269–292. [Google Scholar]
- Hillocks, R.J.; Maruthi, M.N. Grass pea (Lathyrus sativus): Is there a case for further crop improvement? Euphytica 2012, 186, 647–654. [Google Scholar] [CrossRef]
- Rubiales, D.; Emeran, A.A.; Flores, F. Adaptation of grass pea (Lathyrus sativus) to Mediterranean environments. Agronomy 2020, 10, 1295. [Google Scholar] [CrossRef]
- Fernández-Aparicio, M.; Flores, F.; Rubiales, D. Escape and true resistance to crenate broomrape (Orobanche crenata Forsk.) in grass pea (Lathyrus sativus L.) germplasm. Field Crops Res. 2011, 125, 92–97. [Google Scholar] [CrossRef]
- Vaz Patto, M.C.; Skiba, B.; Pang, E.C.K.; Ochatt, S.J.; Lambein, F.; Rubiales, D. Lathyrus improvement for resistance against biotic and abiotic stresses: From classical breeding to marker assisted selection. Euphytica 2006, 147, 133–147. [Google Scholar] [CrossRef]
- Tavoletti, S.; Iommarini, L.; Crinò, P.; Granati, E. Collection and evaluation of grasspea (Lathyrus sativus L.) germplasm of central Italy. Plant Breed. 2005, 124, 388–391. [Google Scholar] [CrossRef]
- Piergiovanni, A.R.; Lupo, F.; Zaccardelli, M. Environmental effect on yield, composition and technological seed traits of some Italian ecotypes of grass pea (Lathyrus sativus L.). J. Sci. Food Agric. 2011, 91, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Grela, E.R.; Rybiński, W.; Matras, J.; Sobolewska, S. Variability of phenotypic and morphological characteristics of some Lathyrus sativus L. and Lathyrus cicera L. accessions and nutritional traits of their seeds. Genet. Resour. Crop Evol. 2012, 59, 1687–1703. [Google Scholar] [CrossRef]
- McIntosh, M.S. Analysis of combined experiments. Agron. J. 1983, 75, 153–155. [Google Scholar] [CrossRef]
- Yan, W.; Holland, J.B. A heritability-adjusted GGE biplot for test environment evaluation. Euphytica 2010, 171, 355–369. [Google Scholar] [CrossRef]
- Flores, F.; Hybl, M.; Knudsen, J.C.; Marget, P.; Muel, F.; Nadal, S.; Narits, L.; Raffiot, B.; Sass, O.; Solis, I.; et al. Adaptation of spring faba bean types across European climates. Field Crops Res. 2013, 145, 1–9. [Google Scholar] [CrossRef]
- Burgueño, J.; Crossa, J.; Vargas, M. SAS Programs for Graphing GE and GGE Biplots; Biometrics and Statistics Unit, CIMMYT, Int.: El Batan, Mexico, 2003. [Google Scholar]
- Yan, W.K.; Hunt, L.A.; Sheng, Q.L.; Szlavnics, Z. Cultivar evaluation and mega-environment investigation based on the GGE biplot. Crop Sci. 2000, 40, 597–605. [Google Scholar] [CrossRef]
- Anderson, M.J. A new method for non-parametric multivariate analysis of variance. Austral. Ecol. 2001, 26, 32–46. [Google Scholar]
- Yang, R.C.; Crossa, J.; Cornelius, P.L.; Burgueño, J. Biplot analysis of genotype × environment interaction: Proceed with caution. Crop Sci. 2009, 49, 1564–1576. [Google Scholar] [CrossRef]
- Kruskal, J.B. Multidimensional scaling by optimizing goodness of fit to a nonmetric hypothesis. Psychometrika 1964, 29, 1–27. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 4. Available online: http://palaeo-electronica.org/2001_1/past/issue1_01.htm (accessed on 2 November 2020).
- Yan, W.K. GGE biplot. A windows application for graphical analysis of multienvironment trial data and other types of two-way data. Agron. J. 2001, 93, 1111–1118. [Google Scholar] [CrossRef]
- Aznar-Fernández, T.; Carrillo-Perdomo, E.; Flores, F.; Rubiales, D. Identification and multi-environment validation of resistance to pea weevil (Bruchus pisorum) in Pisum germplasm. J. Pest. Sci. 2017, 91, 505–514. [Google Scholar] [CrossRef]
- Hanbury, C.D.; Siddique, K.H.M.; Galwey, N.W.; Cocks, P.S. Genotype-environment interaction for seed yield and ODAP concentration of Lathyrus sativus L. and L. cicera L. in Mediterranean type environments. Euphytica 1999, 110, 45–60. [Google Scholar] [CrossRef]
- Rubiales, D.; Fernández-Aparicio, M. Innovations in parasitic weeds management in legume crops. A review. Agron. Sustain. Dev. 2012, 32, 433–449. [Google Scholar] [CrossRef]
- Fernández-Aparicio, M.; Flores, F.; Rubiales, D. The Effect of Orobanche crenata Infection Severity in Faba Bean, Field Pea, and Grass Pea Productivity. Front. Plant Sci. 2016, 7, 1409. [Google Scholar] [CrossRef]
- Linke, K.H.; Abd El-Moneim, A.M.; Saxena, M.C. Variation in resistance of some forage legumes species to Orobanche crenata Forsk. Field Crops Res. 1993, 32, 277–285. [Google Scholar] [CrossRef]
- Rubiales, D. Parasitic plants, wild relatives and the nature of resistance. New Phytol. 2003, 160, 459–461. [Google Scholar] [CrossRef]
- Rubiales, D.; Alcántara, C.; Pérez-de-Luque, A.; Gil, J.; Sillero, J.C. Infection of chickpea (Cicer arietinum) by crenate broomrape (Orobanche crenata) as influenced by sowing date and weather conditions. Agronomie 2003, 23, 359–362. [Google Scholar] [CrossRef]
- Sauerborn, J.; Masri, H.; Saxena, M.C.; Erskine, W. A rapid test to screen lentil under laboratory conditions for susceptibility to Orobanche. Lens Newsl. 1987, 14, 15–16. [Google Scholar]
- Fondevilla, S.; Fernández-Aparicio, M.; Satovic, Z.; Emeran, A.A.; Torres, A.M.; Moreno, M.T.; Rubiales, D. Identification of quantitative trait loci for specific mechanisms of resistance to Orobanche crenata in pea. Mol. Breed. 2010, 25, 259–272. [Google Scholar] [CrossRef]
- Rubiales, D. Can we breed for durable resistance to broomrapes? Phytopathol. Mediterr. 2018, 57, 170–185. [Google Scholar] [CrossRef]
- Pérez-de-Luque, A.; Flores, F.; Rubiales, D. Differences in Crenate Broomrape Parasitism Dynamics on Three Legume Crops Using a Thermal Time Model. Front. Plant Sci. 2016, 7, 1910. [Google Scholar] [CrossRef] [PubMed]
- Pérez-de-Luque, A.; Sillero, J.C.; Moral, A.; Cubero, J.I.; Rubiales, D. Effect of sowing date and host resistance on the establishment of Orobanche crenata in faba bean and common vetch. Weed Res. 2004, 44, 282–288. [Google Scholar] [CrossRef]


| Environm. | Location | Growing Season | Soil Type | Soil pH | Latit. | Longit. | Altit. | AvTmax (°C) | AvTmin (°C) | Rain (mm) |
|---|---|---|---|---|---|---|---|---|---|---|
| CAMP08 | Campillo | 2007–2008 | Vertisol | 7.5–8 | 37°20′ N | 4°51′ W | 461 | 18.8 | 7.8 | 264 |
| TOM09 | Tomejil | 2008–2009 | Vertisol | 7–7.5 | 37°30′ N | 5°57′ W | 12 | 22.6 | 7.5 | 219 |
| CORC09 | Córdoba | 2008–2009 | Cambisol | 6.5–7 | 37°50′ N | 4°50′ W | 90 | 21.7 | 7.9 | 280 |
| CORC10 | Córdoba | 2009–2010 | Cambisol | 6.5–7 | 37°50′ N | 4°50′ W | 90 | 21.3 | 9.4 | 626 |
| ESC08 | Escacena | 2007–2008 | Fluvisol | 7–7.5 | 37°25′ N | 6°15′ W | 88 | 20.7 | 10.1 | 391 |
| ESC09 | Escacena | 2008–2009 | Fluvisol | 7–7.5 | 37°25′ N | 6°15′ W | 88 | 21.4 | 9.4 | 252 |
| ESC10 | Escacena | 2009–2010 | Fluvisol | 7–7.5 | 37°25′ N | 6°15′ W | 88 | 20.9 | 10.5 | 886 |
| ESC11 | Escacena | 2010–2011 | Fluvisol | 7–7.5 | 37°25′ N | 6°15′ W | 88 | 22.1 | 11.8 | 534 |
| ESC12 | Escacena | 2011–2012 | Fluvisol | 7–7.5 | 37°25′ N | 6°15´W | 88 | 21.9 | 10.1 | 164 |
| CORP16 | Córdoba | 2015–2016 | Cambisol | 7–7.2 | 37°86′ N | 4°79′ W | 94 | 25.9 | 11.3 | 519 |
| CORB17 | Córdoba | 2016–2017 | Vertisol | 7.2–7.8 | 37°87′ N | 4°78′ W | 97 | 27.6 | 12 | 467 |
| CORB18 | Córdoba | 2017–2018 | Vertisol | 7.2–7.8 | 37°87′ N | 4°78′ W | 97 | 25.5 | 10.7 | 488 |
| ESP16 | Espiel | 2015–2016 | Cambisol | 7.8–8 | 38°19′ N | 5°01′ W | 587 | 23.9 | 9.9 | 485 |
| ESP17 | Espiel | 2016–2017 | Cambisol | 7.8–8 | 38°19′ N | 5°01′ W | 587 | 23.5 | 9.5 | 464 |
| ESP18 | Espiel | 2017–2018 | Cambisol | 7.8–8 | 38°19′ N | 5°01′ W | 587 | 21.4 | 7.7 | 558 |
| ALM17 | Almodóvar | 2016–2017 | Fluvisol | 8–8.3 | 37°77′ N | 5°03´W | 84 | 26.1 | 10.9 | 424 |
| ALM18 | Almodóvar | 2017–2018 | Fluvisol | 8–8.3 | 37°77′ N | 5°03′ W | 84 | 24.5 | 10.2 | 589 |
| Trait | Source | df a | Sum Squares b | Explained Variation c | % of PC1 + PC2 d |
|---|---|---|---|---|---|
| E | 16 | 876430554 *** | 81 | ||
| Grain yield (kg ha−1) | G | 7 | 32860384 *** | 3 | 48 + 29 |
| GE | 112 | 165378470 *** | 16 | ||
| E | 11 | 26099 *** | 52 | ||
| Flowering date | G | 7 | 14534 *** | 29 | 72 + 10 |
| GE | 77 | 9004 *** | 18 | ||
| E | 12 | 208 *** | 70 | ||
| Broomrapes infection | G | 7 | 55 *** | 19 | 84 + 9 |
| GE | 84 | 34 *** | 11 |
| Environment | Grain Yield (kg ha−1) | Mean | SE | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Tblanco | Tpinto | Ilat11 | Ilat18 | Ilat10 | Ilat3 | Lasana | Titana | |||
| ESC10 | 84 | 301 | 287 | 255 | 322 | 476 | 55 | 60 | 209 | 34 |
| ESC11 | 17 | 17 | 145 | 592 | 146 | 864 | 55 | 74 | 239 | 62 |
| CAMP08 | 137 | 106 | 607 | 547 | 173 | 340 | 810 | 510 | 404 | 59 |
| CORC10 | 11 | 62 | 920 | 629 | 696 | 800 | 138 | 129 | 423 | 91 |
| ESC08 | 333 | 301 | 908 | 1227 | 1534 | 645 | 703 | 634 | 786 | 99 |
| CORC09 | 371 | 70 | 2011 | 1197 | 1556 | 1370 | 1065 | 100 | 967 | 156 |
| ESC09 | 437 | 768 | 1619 | 1464 | 2343 | 1614 | 1988 | 1487 | 1465 | 136 |
| ESC12 | 1711 | 1077 | 1422 | 1436 | 1227 | 1589 | 1827 | 2869 | 1645 | 120 |
| ESP18 | 1765 | 1872 | 2127 | 2314 | 2069 | 2348 | 2683 | 2975 | 2269 | 98 |
| CORB18 | 1882 | 1954 | 2224 | 2329 | 2168 | 2767 | 3515 | 2736 | 2447 | 169 |
| ESP16 | 2807 | 2047 | 2933 | 3123 | 1963 | 2490 | 3154 | 3930 | 2806 | 174 |
| ALM18 | 2432 | 2554 | 3417 | 2884 | 3245 | 2790 | 3858 | 3816 | 3125 | 133 |
| CORB17 | 2753 | 2895 | 2610 | 2582 | 3018 | 3177 | 4125 | 4467 | 3203 | 148 |
| CORP16 | 2460 | 2493 | 2270 | 2297 | 3090 | 3707 | 3802 | 6807 | 3366 | 326 |
| ESP17 | 3291 | 3351 | 3060 | 3517 | 3553 | 4033 | 4718 | 5246 | 3846 | 150 |
| ALM17 | 3799 | 3869 | 3810 | 4488 | 4201 | 4898 | 5311 | 6025 | 4550 | 182 |
| TOM09 | 5365 | 8131 | 4325 | 4083 | 3963 | 3631 | 4985 | 2915 | 4675 | 358 |
| Mean | 1744 | 1865 | 2041 | 2057 | 2075 | 2208 | 2517 | 2634 | 2143 | 83 |
| SE | 217 | 282 | 184 | 187 | 191 | 201 | 257 | 308 | 83 | |
| Environment | No. Broomrapes per Plant by Accession | Mean | SE | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ilat3 | Ilat18 | Ilat11 | Ilat10 | Lasana | Tpinto | Titana | Tblanco | |||
| ESP17 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| ALM17 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| ESP18 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| ALM18 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| TOM09 | 0 | 0 | 0 | 0 | 0.65 | 0 | 0 | 0 | 0.08 | 0.08 |
| CORB18 | 0 | 0 | 0 | 0 | 0.27 | 0.36 | 0.51 | 0.83 | 0.18 | 0.06 |
| ESP16 | 0 | 0 | 0 | 0 | 0.15 | 0.35 | 0.41 | 0.68 | 0.20 | 0.01 |
| CORP16 | 0 | 0 | 0 | 0 | 0.19 | 0.36 | 0.45 | 0.64 | 0.20 | 0.01 |
| CORB17 | 0 | 0 | 0 | 0 | 0.31 | 0.40 | 0.42 | 0.87 | 0.25 | 0.06 |
| ESC12 | 0.05 | 0.08 | 0.17 | 0.16 | 0.38 | 0.63 | 0.97 | 1.13 | 0.44 | 0.09 |
| CAMP08 | 0.10 | 0.03 | 0.1 | 0.10 | 0.50 | 1.00 | 1.10 | 1.50 | 0.55 | 0.11 |
| ESC08 | 0.30 | 0.57 | 0.40 | 0.50 | 0.83 | 1.24 | 0.90 | 1.83 | 0.82 | 0.10 |
| ESC09 | 0.47 | 0.46 | 0.39 | 0.53 | 1.40 | 1.26 | 1.38 | 1.80 | 0.96 | 0.12 |
| CORC09 | 0.40 | 0.28 | 0.83 | 1.63 | 0.98 | 2.60 | 2.46 | 2.28 | 1.43 | 0.34 |
| ESC11 | 1.62 | 1.19 | 1.50 | 1.53 | 2.36 | 2.43 | 2.22 | 2.24 | 1.89 | 0.13 |
| CORC10 | 1.08 | 1.38 | 0.83 | 1.08 | 2.16 | 3.16 | 2.91 | 2.62 | 1.90 | 0.22 |
| ESC10 | 2.01 | 2.15 | 2.12 | 2.44 | 4.11 | 2.09 | 3.83 | 3.78 | 2.81 | 0.22 |
| Mean | 0.35 | 0.36 | 0.37 | 0.47 | 0.84 | 0.93 | 1.03 | 1.19 | 0.69 | 0.06 |
| SE | 0.09 | 0.09 | 0.08 | 0.10 | 0.16 | 0.15 | 0.16 | 0.16 | 0.06 | |
| Environment | Days to 50% Flowering | Mean | SE | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ilat11 | Ilat10 | Ilat18 | Ilat3 | Titana | Tblanco | Lasana | Tpinto | |||
| CORP16 | 78 | 78 | 78 | 78 | 84 | 84 | 94 | 84 | 82 | 1.1 |
| ESP16 | 78 | 78 | 78 | 78 | 84 | 84 | 94 | 84 | 82 | 1.1 |
| ALM18 | 86 | 86 | 86 | 93 | 86 | 98 | 98 | 101 | 92 | 1.3 |
| CORB18 | 88 | 87 | 87 | 88 | 92 | 99 | 93 | 101 | 92 | 1.1 |
| CORC09 | 96 | 96 | 96 | 100 | 107 | 109 | 107 | 109 | 103 | 1.3 |
| ESC11 | 91 | 98 | 101 | 98 | 120 | 104 | 105 | 112 | 104 | 2.2 |
| ESP18 | 77 | 80 | 111 | 112 | 109 | 109 | 121 | 113 | 104 | 3.2 |
| ESP17 | 91 | 98 | 92 | 113 | 115 | 113 | 109 | 115 | 106 | 2.0 |
| ESC10 | 87 | 106 | 98 | 91 | 104 | 118 | 116 | 128 | 106 | 3.3 |
| ALM17 | 91 | 98 | 91 | 114 | 115 | 113 | 113 | 116 | 106 | 2.3 |
| CORB17 | 90 | 106 | 92 | 112 | 114 | 114 | 114 | 118 | 107 | 2.1 |
| CORC10 | 105 | 105 | 104 | 105 | 114 | 119 | 118 | 118 | 111 | 1.5 |
| Mean | 88 | 93 | 93 | 98 | 104 | 105 | 107 | 108 | 100 | 0.8 |
| SE | 1.4 | 1.9 | 1.6 | 2.1 | 2.4 | 2.1 | 1.7 | 2.3 | 0.8 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rubiales, D.; Barilli, E.; Flores, F. Broomrape as a Major Constraint for Grass Pea (Lathyrus sativus) Production in Mediterranean Rain-Fed Environments. Agronomy 2020, 10, 1931. https://doi.org/10.3390/agronomy10121931
Rubiales D, Barilli E, Flores F. Broomrape as a Major Constraint for Grass Pea (Lathyrus sativus) Production in Mediterranean Rain-Fed Environments. Agronomy. 2020; 10(12):1931. https://doi.org/10.3390/agronomy10121931
Chicago/Turabian StyleRubiales, Diego, Eleonora Barilli, and Fernando Flores. 2020. "Broomrape as a Major Constraint for Grass Pea (Lathyrus sativus) Production in Mediterranean Rain-Fed Environments" Agronomy 10, no. 12: 1931. https://doi.org/10.3390/agronomy10121931
APA StyleRubiales, D., Barilli, E., & Flores, F. (2020). Broomrape as a Major Constraint for Grass Pea (Lathyrus sativus) Production in Mediterranean Rain-Fed Environments. Agronomy, 10(12), 1931. https://doi.org/10.3390/agronomy10121931







