1. Introduction
Iron (Fe) is one of the micronutrients which is responsible for the quality and quantity of crop yields and, therefore, its deficiency significantly impacts agricultural production at the global level [
1,
2]. In fact, being crucial for the proper functioning of metabolic processes related to electron transport such as respiration and photosynthesis as well as those connected with the biosynthesis of fundamental molecules, e.g., chlorophyll [
3,
4], Fe plays a crucial role in the whole metabolism (anabolic and catabolic) of plants. When Fe availability in the growth medium is not adequate, crops display typical visual symptoms such as interveinal leaf chlorosis of the younger leaves and stunted development of the whole plant, making the diagnosis of the nutritional disorder rather easy to make [
5]. At the cellular level, instead, the Fe shortage induces serious imbalances in the ultrastructure and functionality of chloroplasts, as 90% of leaf-Fe is present in chloroplasts [
6,
7], with relevant implications for all the metabolic pathways carried out in these organelles.
The Fe content of soils is high, being the fourth element in Earth’s crust in percentage. Therefore, the widespread limited availability of Fe for plant nutrition is not related to its low absolute soil content, but rather to its extremely low solubility. In particular, Fe deficiency is a typical feature of alkaline soils [
5]. Considering that these soils represent about 25% of Earth’s surface [
8], from an agronomical point of view, the consistency of this problem is evident. To worsen the context, there is then the buffer capacity of these soils which, by influencing the pH at the root surface, can depress the functionality of the mechanisms underlying nutrient acquisition by the crops [
9,
10]. Therefore, it is clear why the management of plant Fe nutrition has been the focus of great attention in recent decades. In this context, it should be mentioned that specific targets such as (i) the enhanced plant availability of the soil endogenous Fe forms, (ii) the higher use efficiency of the exogenous Fe sources (fertilizers) supplied foliarly or to the soil and/or (iii) the more efficient metabolic use of the metal acquired by crops have been addressed by applying different approaches. Considering the prediction of a constant increase in population density as well as in the food demand in the near future, concurrently with the decrease in the arable land surface, it is clear how urgent it is to identify comprehensive answers (i.e., novel agronomic practices) to the problem of plant nutrition management. In this regard, it should be necessary to privilege strategies that also meet the great current challenge of agriculture to protect the environment. In this respect and specifically in a context of more sustainable agriculture, plant biostimulants can be surely considered one of the valid tools. In fact, they are defined novel “environmental-friendly” natural products capable of contributing considerably to the achievement of high yields and good-quality products. This group of natural products includes both substances/molecules and microorganisms that, even if applied to soil or directly to the plants in a very small quantity, allow crops to enhance the efficiency of the whole nutrition acquisition process, the tolerance levels to abiotic stresses and/or the expression of the quality traits of crop products [
11] and references therein. These beneficial effects of biostimulants are generally ascribed to an indirect effect on soil properties and microbial activities at the rhizosphere (which, in turn, promote plant growth and development) or to a direct action on the plant traits contributing to its growth [
12]. With respect to the latter, it has been reported that some categories of biostimulants could be able to improve plant nutritional status by increasing the efficiency of the nutrient acquisition process, regardless of the nutrients’ levels directly supplied with the biostimulant [reviewed in 11].
This action could be mainly ascribed to three major groups of substances, including humic substances [
13], hormone-containing products [
14] and protein hydrolysates [
15]. With respect to the latter, they are nitrogen(N)-containing compounds obtained by thermal, chemical and/or enzymatic hydrolysis of various raw materials of plant or animal origin. Vegetal-derived protein hydrolysates have been proven to be very effective in enhancing crop performances through the increase in plant nutrient availability, nutrient uptake and metabolic use, abiotic stress resistance and the expression of crop quality traits [
16,
17].
The aim of this work is to evaluate the effect of a legume-derived protein hydrolysate on plant growth and development and to verify the biostimulant’s ability to mitigate the effects of Fe deficiency. To this purpose, two different crops (tomato (
Solanum lycopersicum L.—cv. AKRAI F1) and cucumber (
Cucumis sativus L.—cv. EKRON F1)) were chosen as model plants because of their economic and social importance. Tomato is one of the most popular vegetables in the world and the world’s third largest vegetable crop after potato and onion. The leading tomato-producing countries in the world are China, India, USA, Turkey, Egypt, Iran, Italy, Spain and Brazil, with a global cultivated area of about 4.73 million hectares and a production of 163.96 million tons (FAO, 2016). Both tomatoes and tomato-based products (tomato sauce and juice) have great nutritional value, being rich in several nutrients, including vitamins [
18].
Moreover, cucumber is an important vegetable crop and represents an important food for the human diet, with a wide cultivation area and a market area mostly including countries such as Greece, Holland, Finland and Germany [
19].
The increased consumption of both products has triggered a significant acreage extension for many countries worldwide. However, both tomato and cucumber cultivation urgently need major transformation, including overall improvement of the production system through varietal renewal, but also the development of new agronomic practices, including fertilizer management.
During the last 40 years, agricultural production has nearly doubled thanks to the application of large doses of fertilizers, the use of increasingly high-yielding varieties and the intensive use of water and pesticides [
20]. Such intensive cropping systems have, in some cases, severely compromised the soil quality and fertility, resulting in a series of critical issues such as limited nutrients’ content, soil acidification, soil salinization, drop in soil biological activity and a significant depletion of the organic matter content. In this context, the current challenges of food safety and security, concurrently with the progressive decrease in the agricultural land surface, urge identifying novel agronomic practices allowing for quali-quantitatively improving primary productions in a context of increasing agricultural sustainability. In this respect, in recent years, the research interest toward the application of biostimulants in agriculture has been clearly intensified. Among biostimulants, protein hydrolysates are particularly interesting for their demonstrated ability to modulate the molecular and physiological processes that promote plant growth, favor increased yield and alleviate the impact of abiotic stress on crops [
21].
The purpose of this study is thus to verify whether foliar application of the protein hydrolysate could improve growth parameters (expressed as shoot and root fresh weight and relative chlorophyll content) as well as nutrient accumulation in both shoot and root tissues of tomato and cucumber plants. Ultimately, the combination of limited Fe supply and biostimulant application will allow further evaluating the effect of the protein hydrolysate on the plant’s ability to cope with Fe deficiency, by analyzing changes in the activity of root Fe
3+-chelate reductase, since both crops rely on an Fe
3+ reduction-based mechanism for micronutrient acquisition [
22].
2. Materials and Methods
2.1. Plant Growth Conditions
Tomato (
Solanum lycopersicum L.—cv. AKRAI F1) and cucumber (
Cucumis sativus L.—cv. EKRON F1) seeds were germinated on perlite soaked with distilled water in the dark at 24 °C for 4 days. Half of the homogeneous seedlings were transferred in 2 L plastic pots (6 seedlings/pot), filled with a continuously aerated full nutrient solution (NS) [
23] (control, C), having the following composition: (mM) 2 Ca(NO
3)
2, 0.7 K
2SO
4, 0.1 KH
2PO
4, 0.1 KCl and 0.5 MgSO
4; and (µM) 10 H
3BO
3, 0.5 MnSO
4, 0.2 CuSO
4, 0.5 ZnSO
4, 0.01 (NH
4)
6Mo
7O
24 and 40 Fe
3+-EDTA. The other half of the homogeneous seedlings were transferred to the same 2 L pots (6 seedlings/pot), containing the same full NS, but with a lower Fe concentration (4 µM Fe
3+-EDTA, F).
Plants were grown hydroponically in a climate chamber with a day/night cycle of 16/8 h, temperature regime of 27/20 °C, light intensity of about 200 µmol m−2 s−1 at the plant level and relative humidity of 70%. The NS was continuously aerated and changed every three days.
After 8 and 15 days, half of the plants of each condition (C, control, and F, Fe-deficient condition) were foliarly treated with a protein hydrolysate solution by means of a spray bottle applying 300 mg per plant of hydrolysate at the concentration of 3 mL L
−1. The commercial legume seed-based hydrolysate Trainer
® (Italpollina S.p.A. Rivoli Veronese, Italy) had a total N concentration of 50 g kg
−1 containing free amino acids and soluble peptides [
21]. The hydrolysate contained the following amino acids: (g kg
−1) Ala (12), Arg (18), Asp (34), Cys (3), Glu (54), Gly (12), His (8), Ile (13), Leu (22), Lys (18), Met (4), Phe (15), Pro (15), Thr (11), Trp (3), Tyr (11) and Val (14).
Cucumber and tomato plants were harvested after 17 and 22 days from sowing, respectively. Shoots and roots were separated, and their fresh biomass was determined by weighing.
2.2. Determination of Relative Chlorophyll Content
At harvest (17 and 22 days after sowing for cucumber and tomato, respectively), the relative chlorophyll content per unit area was measured on the youngest fully expanded leaves of each plant by using a portable chlorophyll meter SPAD-502 (Konica Minolta, Osaka, Japan), and recorded values were expressed as SPAD units.
2.3. Determination of Root Fe3+-Chelate Reductase Activity
The Fe
3+-chelate reductase activity of tomato and cucumber roots was assayed colorimetrically, using bathophenantrolinedisulfonate (BPDS) reagent [
24,
25]. Briefly, roots of intact plants were carefully rinsed in deionized water and incubated in darkness at room temperature in a continuously aerated assay solution, with the following composition: 0.5 mM CaSO
4, 10 mM MES-KOH (pH 5.5), 0.25 mM Fe
3+-EDTA and 0.6 mM BPDS. After 20 min, the absorbance of the assay solution was determined at 535 nm with a spectrophotometer [
26]. The reduced Fe was calculated on the basis of the concentration of the Fe
2+-BPDS
3 complex, using the molar extinction coefficient of 22.1 mM
−1 cm
−1, and was expressed in μmol Fe reduced h
−1 g
−1 root FW.
2.4. Determination of Macro- and Micronutrients
Shoots and roots of both plant species were dried at 60 °C until constant weight was reached and ball-milled (Mixer Mill, MM 400, Retsch, Italy) to obtain a homogeneous powder. Samples were then acid-digested with ultra-pure HNO3 (650 mL L−1; Carlo Erba, Milano, Italy) using a Single Reaction Chamber Microwave (SRC, UltraWAVE, Milestone Inc, Shelton, CT, USA). Subsequently, mineralized samples were filtered and element concentrations were determined by ICP-OES (Arcos Ametek, Spectro, Germany), using tomato leaves (SRM 1573a) and spinach leaves (SRM 1570a) as external certified reference materials.
2.5. Statistical Analysis
Each reported value represents the mean ± standard error (SE) of three independent experiments (biological replicates) run in triplicate (technical replicates). Statistical analysis was carried out using SigmaPlot Version 12.2 on Windows 10 64 bit. Statistical significance was tested by one-way ANOVA analysis with the Student–Newman–Keuls post hoc test at * p < 0.05, ** p < 0.01 and *** p < 0.001. Principle component analysis (PCA) was performed with PAST Version 4.03 for Windows.
4. Discussion
The quali-quantitative improvement of primary productions is an important challenge considering the need to increase agricultural sustainability. In this respect, in recent years, the research interest toward the application of biostimulants has been clearly intensified. Among biostimulants, protein hydrolysates are particularly interesting for their demonstrated ability to modulate the molecular and physiological processes that promote plant growth, favor increased yield and alleviate the impact of abiotic stress on crops [
21].
In this work, we investigated and characterized the effect of a legume-derived protein hydrolysate on the growth and development of tomato and cucumber plants. These two plant species were selected for their wide diffusion and economic relevance in the horticultural sector of the Mediterranean area. Furthermore, we evaluated the ability of this product to mitigate the effects of Fe shortage. Iron deficiency represents a critical issue in the life cycle of many crops, particularly in calcareous soils, which are the majority in the Mediterranean area.
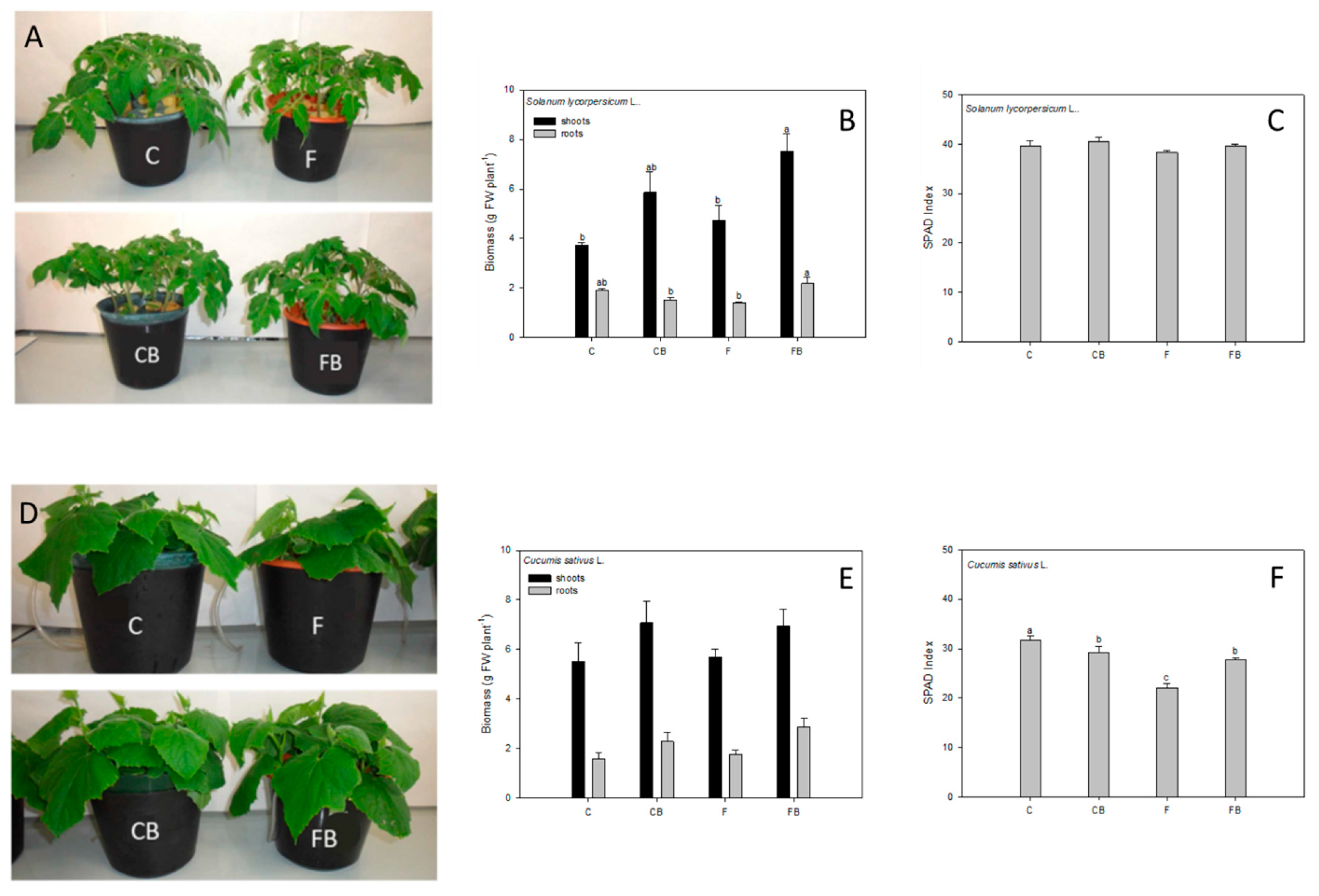
The results here presented show that the application of the protein hydrolysates did not affect the growth rate of both tomato and cucumber plants adequately fed with all the nutrients (
Figure 1B,E). Furthermore, no significant difference in relative chlorophyll content was found between tomato plants grown in the nutrient solution adequately supplied with all the nutrients (C) and those supplied with the protein hydrolysates (CB). On the contrary, cucumber plants supplied with biostimulants exhibited lower relative chlorophyll contents than control plants (
Figure 1C,F). However, the PCA analysis enabled the identification of significant changes induced by the supply of biostimulants. Indeed, the nutritional composition of both roots and shoots led to a clear separation of C and CB samples in both species (
Figure 3). In particular, data shown in both
Tables S1 and S2 indicate that K accumulation was limited in plants exposed to the protein hydrolysates. In this respect, it is worth mentioning that K is a nutrient playing a crucial role in plant metabolism, being involved in the stomatal opening and closure, directly affecting plant photosynthetic capacity as well as the synthesis and, therefore, the source/sink allocation of carbohydrates [
27]. Therefore, the drop in K content here recorded is very likely to cause an irregular ripening of tomato as well as a lower consistency of cucumber fruits, thus resulting in a poorer crop quality [
28]. However, it is important to highlight that in both crops, the accumulation of Ca, which could be important in the post-harvest phase [
29], was not affected by the biostimulant supply (
Tables S1 and S2).
On the other hand, in cucumber plants, the accumulation as well as the allocation at the shoot level of some essential nutrients resulted in being increased as a consequence of the root exposure to the protein hydrolysate. In particular, the Cu and Zn accumulation increased in CB plants, but only at the root level, whereas S and Mo increased only at the shoot level, likely suggesting the occurrence of changes in the metabolic activity of plants. Indeed, it has been suggested that Mo can induce the synthesis of abscisic acid and nitric oxide, which are involved in the regulation of root system growth [
30]. Thus, the different levels of Mo accumulated in cucumber plants (
Table S2) could be functional to the improvement of the plants’ ability to acquire water and nutrients, obtained through greater development of the root system, as suggested by the slight increase in root biomass accumulation in cucumber plants treated with the biostimulant (CB,
Figure 1E).
The second working hypothesis of this work was that the protein hydrolysate could be able to improve plant capability to cope with Fe deficiency stress. The most common symptoms of Fe deficiency are leaf chlorosis and an increase in root biomass accumulation due to the development of numerous secondary roots [
31,
32,
33]. In particular, Fe-deficient plants trigger the number and length of absorbent hairs [
34] and the formation of “transfer” cells [
35]. Measurements of plant growth parameters showed divergence in plant response to the application of biostimulants between the two selected species (
Figure 1). For tomato, the root biomass of plants exposed to solely Fe deficiency stress appeared significantly reduced in comparison with control plants, whereas plants exposed to both Fe deficiency and biostimulant treatment (FB condition) reached values not significantly different from those exposed to the C and CB conditions (
Figure 1B). On the contrary, in cucumber plants, no significant effects on plant growth were observed regardless of the nutrient supply or biostimulant application (
Figure 1E).
The higher expansion of the root apparatus observed in tomato plants can be considered a general response of seedlings to Fe shortage, as previously described [
36,
37], and suggests a positive effect of the biostimulant. In addition, biostimulant treatment not only promoted the growth of plant roots, thus improving the stress tolerance of plants, but also stimulated the growth of the aerial part of the FB tomato plants which reached values two times greater than the control plants C (
Figure 1B). Considering leaf chlorosis caused by Fe shortage, it has been observed that the drop in the relative chlorophyll content (−30% compared to control C) only occurred in cucumber plants (
Figure 1F), but this response was minimized in FB plants (Fe-deficient but treated with the biostimulant) compared to F ones. It is interesting to note that FB plants showed a relative chlorophyll content that was not significantly different from that measured in the CB plants, grown in the presence of the optimal Fe concentration, and was only 5% lower than that determined in control plants C (
Figure 1F). Therefore, this effect could be ascribed to the biostimulant’s ability to improve the plant’s efficiency to face Fe deficiency stress.
It is well known that dicots such as tomato and cucumber plants are characterized by a Fe
3+ reduction-based mechanism for nutrient acquisition and rely on the enhancement of this activity to cope with Fe shortage [
22]. As expected, the data presented here (
Figure 2A) show exactly this behavior in plants exposed to the nutritional stress. However, when the Fe deficiency was associated with the supply of the biostimulant (FB condition), the extent of this enhancement was lower than that observed in the F condition (
Figure 2A). This result could be explained by hypothesizing that in FB plants, the biostimulant application may have improved both uptake and accumulation of Fe and therefore enzyme activity was modulated by plant nutritional status (Fe concentration in shoot and root tissues), rather than by the availability of the nutrient in the growth medium. Indeed, the levels of Fe accumulated in shoots of both FB tomato and cucumber plants treated with the biostimulants seem to confirm this hypothesis (
Figure 2B,C). Furthermore, it is interesting to note that Fe accumulation in FB tomato shoots was comparable to that recorded in the shoots of plants adequately fed with nutrients and supplied with the biostimulants (CB,
Figure 2B).
Finally, the PCA analysis computed with the macro- and micronutrient concentrations in shoot and root tissues of the plants evidenced a clear separation of the four different nutritional conditions, suggesting that the biostimulant not only improves Fe accumulation by Fe-deficient plants, but also triggers an adjustment of the whole plant nutritional status (
Figure 3). In particular, it is important to note that Fe shortage induced specific nutrient interactions in the plants regardless of the biostimulant supply. For instance, a synergistic Cu/Fe and Zn/Fe interaction was observed only in the shoots of tomato plants. On the other hand, cucumber plants revealed an enhanced Fe deficiency-induced Cu accumulation only in the roots and a synergistic Zn/Fe effect on both shoots and roots (
Tables S1 and S2). These results further corroborate the positive effect of biostimulants on plant performance (i.e., alleviating Fe stress) by increasing Fe concentration in plant tissues yet maintaining the Fe deficiency-induced synergistic effect of other essential micronutrients.
In conclusion, the results clearly show a limited effectiveness of this vegetal-derived protein hydrolysate when supplied to crops fed with an adequate availability of all the nutrients. On the other hand, its contribution to enhance plant performance in conditions of nutritional stress is of paramount importance, indicating a compensation effect. In fact, in an increasingly sustainable agriculture context, the possibility of having products/molecules capable of stimulating plants to grow and produce in non-optimal edaphic conditions and/or better exploiting the endogenous soil resources of nutrients is of particular value, also considering the environmental and economic benefits related to the limited need and, thus, application of fertilizers.