Carbon Isotope Measurements to Determine the Turnover of Soil Organic Matter Fractions in a Temperate Forest Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling and Laboratory Measurements Before Incubation
2.2. Incubation Experiment
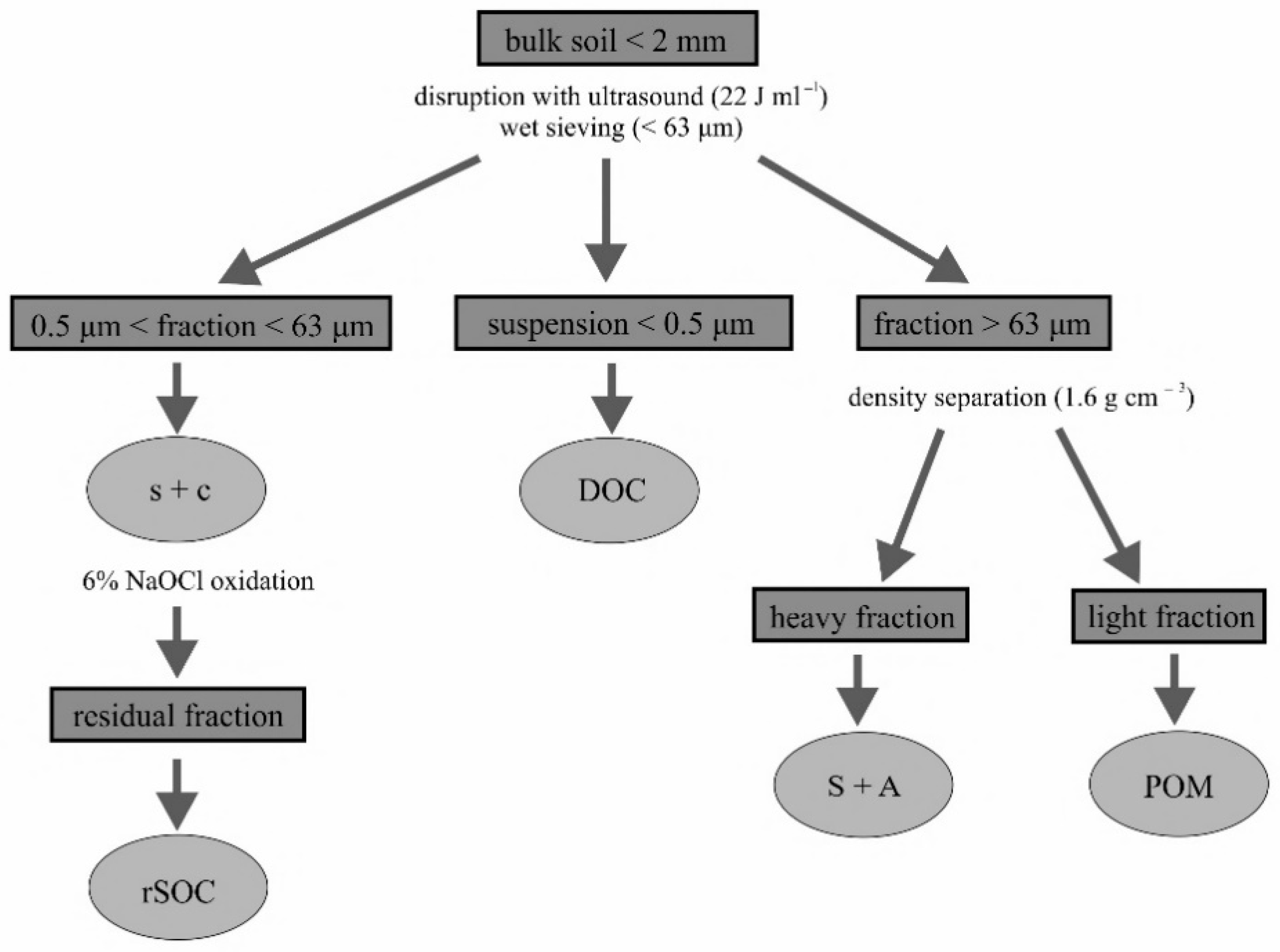
2.3. Soil Fractionation
2.4. Total C, N, and δ13C Measurements
2.5. 14C Measurements
2.6. FT-IR Measurements
2.7. Statistical Analysis
3. Results and Discussion
3.1. Total C and N Concentration in the Soil Fractions
3.2. Quality Assessment of SOM Fractions Using FT-IR Spectroscopy
3.3. Evaluation of C Isotope Changes in SOM Fractions and Turnover Time of the SOM Fractions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kögel-Knabner, I.; Amelung, W. Dynamics, Chemistry, and Preservation of Organic Matter in Soils. In Treatise on Geochemistry; Holland, H., Turekian, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 157–215. ISBN 9780080983004. [Google Scholar]
- von Lützow, M.; Kögel-Knabner, I.; Ludwig, B.; Matzner, E.; Flessa, H.; Ekschmitt, K.; Guggenberger, G.; Marschner, B.; Kalbitz, K. Stabilization mechanisms of organic matter in four temperate soils: Development and application of a conceptual model. J. Plant Nutr. Soil Sci. 2008, 171, 111–124. [Google Scholar] [CrossRef]
- Trumbore, S.E. Age of soil organic matter and soil respiration. Ecol. Appl. 2000, 10, 399–411. [Google Scholar] [CrossRef]
- Kuzyakov, Y. Sources of CO2 efflux from soil and review of partitioning methods. Soil Biol. Biochem. 2006, 38, 425–448. [Google Scholar] [CrossRef]
- Schmidt, M.W.I.; Torn, M.S.; Abiven, S.; Dittmar, T.; Guggenberger, G.; Janssens, I.A.; Kleber, M.; Kögel-Knabner, I.; Lehmann, J.; Manning, D.A.C.; et al. Persistence of soil organic matter as an ecosystem property. Nature 2011, 478, 49–56. [Google Scholar] [CrossRef] [Green Version]
- Creamer, C.A.; Filley, T.R.; Boutton, T.W. Long-term incubations of size and density separated soil fractions to inform soil organic carbon decay dynamics. Soil Biol. Biochem. 2013, 57, 496–503. [Google Scholar] [CrossRef]
- Trumbore, S.E.; Chadwick, O.A.; Amundson, R. Rapid exchange between soil carbon and atmospheric carbon dioxide driven by temperature change. Science 1996, 272, 393–396. [Google Scholar] [CrossRef] [Green Version]
- Balesdent, J.; Wagner, G.H.; Mariotti, A. Soil organic matter turnover in long-term field experiments as revealed by Carbon-13 natural bbundance. Soil Sci. Soc. Am. J. 1988, 52, 118–124. [Google Scholar] [CrossRef]
- Saviozzi, A.; Vanni, G.; Cardelli, R. Carbon mineralization kinetics in soils under urban environment. Appl. Soil Ecol. 2014, 73, 64–69. [Google Scholar] [CrossRef]
- Percival, H.J.; Parfitt, R.L.; Scott, N.A. Factors controlling soil carbon levels in New Zealand grasslands. Soil Sci. Soc. Am. J. 2000, 64, 1623–1630. [Google Scholar] [CrossRef]
- Kleber, M.; Mikutta, R.; Torn, M.S.; Jahn, R. Poorly crystalline mineral phases protect organic matter in acid subsoil horizons. Eur. J. Soil Sci. 2005, 56, 717–725. [Google Scholar] [CrossRef]
- Wei, H.; Guenet, B.; Vicca, S.; Nunan, N.; Asard, H.; AbdElgawad, H.; Shen, W.; Janssens, I.A. High clay content accelerates the decomposition of fresh organic matter in artificial soils. Soil Biol. Biochem. 2014, 77, 100–108. [Google Scholar] [CrossRef]
- Leifeld, J.; Fuhrer, J. Long-term management effects on soil organic matter in two cold, high-elevation grasslands: Clues from fractionation and radiocarbon dating. Eur. J. Soil Sci. 2009, 60, 230–239. [Google Scholar] [CrossRef]
- Dondini, M.; Hastings, A.; Saiz, G.; Jones, M.B.; Smith, P. The potential of Miscanthus to sequester carbon in soils: Comparing field measurements in Carlow, Ireland to model predictions. GCB Bioenergy 2009, 1, 413–425. [Google Scholar] [CrossRef]
- Carvalhais, N.; Forkel, M.; Khomik, M.; Bellarby, J.; Jung, M.; Migliavacca, M.; Μu, M.; Saatchi, S.; Santoro, M.; Thurner, M.; et al. Global covariation of carbon turnover times with climate in terrestrial ecosystems. Nature 2014, 514, 213–217. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Trumbore, S.E.; Torn, M.S.; Harden, J.W.; Vaughn, L.J.S.; Allison, S.D.; Randerson, J.T. Radiocarbon constraints imply reduced carbon uptake by soils during the 21st century. Science 2016, 353, 1419–1424. [Google Scholar] [CrossRef] [Green Version]
- Lehmann, J.; Kleber, M. The contentious nature of soil organic matter. Nature 2015, 528, 60–68. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014, Update 2015 International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Reports No. 106; FAO: Rome, Italy, 2015. [Google Scholar]
- Gee, G.W.; Bauder, J.W. Particle-size analysis. In Methods of Soil Analysis: Part 1—Physical and Mineralogical Methods; Klute, A., Ed.; Soil Science Society of America: Madison, WI, USA, 1986; pp. 383–411. [Google Scholar]
- Frøseth, R.B.; Bleken, M.A. Effect of low temperature and soil type on the decomposition rate of soil organic carbon and clover leaves, and related priming effect. Soil Biol. Biochem. 2015, 80, 156–166. [Google Scholar] [CrossRef]
- Zimmermann, M.; Leifeld, J.; Schmidt, M.W.I.; Smith, P.; Fuhrer, J. Measured soil organic matter fractions can be related to pools in the RothC model. Eur. J. Soil Sci. 2007, 58, 658–667. [Google Scholar] [CrossRef]
- Craig, H. The geochemistry of the stable carbon isotopes. Geochim. Cosmochim. Acta 1953, 3, 53–92. [Google Scholar] [CrossRef]
- Coplen, T.B.; Brand, W.A.; Gehre, M.; Gröning, M.; Meijer, H.A.J.; Toman, B.; Verkouteren, R.M. New Guidelines for δ 13 C Measurements. Anal. Chem. 2006, 78, 2439–2441. [Google Scholar] [CrossRef] [Green Version]
- Balesdent, J.; Mariotti, A. Measurement of soil organic matter turnover using 13C natural abundance. In Mass Spectrometry of Soils; Boutton, T.W., Yamasaki, S.I., Eds.; Marcel Dekker Inc.: New York, NY, USA, 1996; pp. 83–111. ISBN 0-8247-9699-3. [Google Scholar]
- Amelung, W.; Brodowski, S.; Sandhage-Hofmann, A.; Bol, R. Combining biomarker with stable isotope analyses for assessing the transformation and turnover of soil organic matter. Adv. Agron. 2008, 100, 155–250. [Google Scholar] [CrossRef]
- Jull, A.J.T.; Burr, G.S.; Beck, J.W.; Hodgins, G.W.L.; Biddulph, D.L.; Gann, J.; Hatheway, A.L.; Lange, T.E.; Lifton, N.A. Application of accelerator mass spectrometry to environmental and paleoclimate studies at the University of Arizona. Radioact. Environ. 2006, 8, 3–23. [Google Scholar]
- Molnár, M.; Janovics, R.; Major, I.; Orsovszki, J.; Gönczi, R.; Veres, M.; Leonard, A.G.; Castle, S.M.; Lange, T.E.; Wacker, L.; et al. Status Report of the New AMS 14C Sample Preparation Lab of the Hertelendi Laboratory of Environmental Studies (Debrecen, Hungary). Radiocarbon 2013, 55, 665–676. [Google Scholar] [CrossRef] [Green Version]
- Rinyu, L.; Molnár, M.; Major, I.; Nagy, T.; Veres, M.; Kimák, Á.; Wacker, L.; Synal, H.A. Optimization of sealed tube graphitization method for environmental C-14 studies using MICADAS. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2013, 294, 270–275. [Google Scholar] [CrossRef]
- Janovics, R.; Futó, I.; Molnár, M. Sealed tube combustion method with MnO2 for AMS 14C Measurement. Radiocarbon 2018, 60, 1347–1355. [Google Scholar] [CrossRef]
- Molnár, M.; Rinyu, L.; Veres, M.; Seiler, M.; Wacker, L.; Synal, H.-A. EnvironMICADAS: A Mini 14C AMS with Enhanced Gas Ion Source Interface in the Hertelendi Laboratory of Environmental Studies (HEKAL), Hungary. Radiocarbon 2013, 55, 338–344. [Google Scholar] [CrossRef] [Green Version]
- Wacker, L.; Christl, M.; Synal, H.A. Bats: A new tool for AMS data reduction. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2010, 268, 976–979. [Google Scholar] [CrossRef]
- Stenström, K.E.; Skog, G.; Georgiadou, E.; Genberg, J.; Johansson, A. A Guide to Radiocarbon Units and Calculations; Lund University: Lund, Sweden, 2011. [Google Scholar]
- Stuiver, M.; Polach, H.A. Discussion Reporting of 14C Data. Radiocarbon 1977, 19, 355–363. [Google Scholar] [CrossRef] [Green Version]
- Udvardi, B.; Kovács, I.J.; Kónya, P.; Földvári, M.; Füri, J.; Budai, F.; Falus, G.; Fancsik, T.; Szabó, C.; Szalai, Z.; et al. Application of attenuated total reflectance Fourier transform infrared spectroscopy in the mineralogical study of a landslide area, Hungary. Sediment. Geol. 2014, 313, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Inbar, Y.; Chen, Y.; Hadar, Y. Solid-state carbon-13 nuclear magnetic resonance and infrared spectroscopy of composted organic matter. Soil Sci. Soc. Am. J. 1989, 53, 1695–1701. [Google Scholar] [CrossRef]
- Haberhauer, G.; Rafferty, B.; Strebl, F.; Gerzabek, M.H. Comparison of the composition of forest soil litter derived from three different sites at various decompositional stages using FTIR spectroscopy. Geoderma 1998, 83, 331–342. [Google Scholar] [CrossRef]
- Haberhauer, G.; Gerzabek, M. Drift and transmission FT-IR spectroscopy of forest soils: An approach to determine decomposition processes of forest litter. Vib. Spectrosc. 1999, 19, 413–417. [Google Scholar] [CrossRef]
- Gerzabek, M.H.; Antil, R.S.; Kogel-Knabner, I.; Knicker, H.; Kirchmann, H.; Haberhauer, G. How are soil use and management reflected by soil organic matter characteristics: A spectroscopic approach. Eur. J. Soil Sci. 2006, 57, 485–494. [Google Scholar] [CrossRef] [Green Version]
- Martín, J.V.; Miralles De Imperial, R.; Calvo, R.; Garcia, M.C.; Leon-Cófreces, C.; Delgado, M.M. Carbon mineralisation kinetics of poultry manure in two soils. Soil Res. 2012, 50, 222–228. [Google Scholar] [CrossRef]
- Thiessen, S.; Gleixner, G.; Wutzler, T.; Reichstein, M. Both priming and temperature sensitivity of soil organic matter decomposition depend on microbial biomass-An incubation study. Soil Biol. Biochem. 2013, 57, 739–748. [Google Scholar] [CrossRef] [Green Version]
- Shahbaz, M.; Kuzyakov, Y.; Heitkamp, F. Decrease of soil organic matter stabilization with increasing inputs: Mechanisms and controls. Geoderma 2017, 304, 76–82. [Google Scholar] [CrossRef]
- Dou, X.; He, P.; Zhu, P.; Zhou, W. Soil organic carbon dynamics under long-term fertilization in a black soil of China: Evidence from stable C isotopes. Sci. Rep. 2016, 6, 21488. [Google Scholar] [CrossRef] [Green Version]
- Baisden, W.T.; Amundson, R.; Cook, A.C.; Brenner, D.L. Turnover and storage of C and N in five density fractions from California annual grassland surface soils. Glob. Biogeochem. Cycles 2002, 16, 64-1–64-16. [Google Scholar] [CrossRef]
- Sollins, P.; Swanston, C.; Kleber, M.; Filley, T.; Kramer, M.; Crow, S.; Caldwell, B.A.; Lajtha, K.; Bowden, R. Organic C and N stabilization in a forest soil: Evidence from sequential density fractionation. Soil Biol. Biochem. 2006, 38, 3313–3324. [Google Scholar] [CrossRef] [Green Version]
- Zimmermann, M.; Leifeld, J.; Abiven, S.; Schmidt, M.W.I.; Fuhrer, J. Sodium hypochlorite separates an older soil organic matter fraction than acid hydrolysis. Geoderma 2007, 139, 171–179. [Google Scholar] [CrossRef]
- Niemeyer, J.; Chen, Y.; Bollag, J.-M. Characterization of Humic Acids, Composts, and Peat by Diffuse Reflectance Fourier-Transform Infrared Spectroscopy. Soil Sci. Soc. Am. J. 1992, 56, 135–140. [Google Scholar] [CrossRef]
- Piccolo, A.; Zaccheo, P.; Genevini, P.G. Chemical characterization of humic substances extracted from organic-waste-amended soils. Bioresour. Technol. 1992, 40, 275–282. [Google Scholar] [CrossRef]
- Baldock, J.A.; Oades, J.M.; Waters, A.G.; Peng, X.; Vassallo, A.M.; Wilson, M.A. Aspects of the chemical structure of soil organic materials as revealed by solid-state 13C NMR spectroscopy. Biogeochemistry 1992, 16, 1–42. [Google Scholar] [CrossRef]
- Boutton, T.W. Stable carbon isotope ratios of soil organic matter and their use as indicators of vegetation and climate change. In Mass Spectrometry of Soils; Boutton, T.W., Yamasaki, S.I., Eds.; Marcel Dekker Inc.: New York, NY, USA, 1996; pp. 47–82. [Google Scholar]
- Keitel, C.; Matzarakis, A.; Rennenberg, H.; Gessler, A. Carbon isotopic composition and oxygen isotopic enrichment in phloem and total leaf organic matter of European beech (Fagus sylvatica L.) along a climate gradient. Plant Cell Environ. 2006, 29, 1492–1507. [Google Scholar] [CrossRef] [Green Version]
- Shang, C.; Tiessen, H. Carbon turnover and carbon-13 natural abundance in organo-mineral fractions of a tropical dry forest soil under cultivation. Soil Sci. Soc. Am. J. 2000, 64, 2149–2155. [Google Scholar] [CrossRef]
- Gerzabek, M.H.; Haberhauer, G.; Kirchmann, H. Soil organic matter pools and carbon-13 natural abundances in particle-size fractions of a long-term agricultural field experiment receiving organic amendments. Sci. Soc. Am. J. 2001, 65, 352–358. [Google Scholar] [CrossRef]
- Gunina, A.; Kuzyakov, Y. Pathways of litter C by formation of aggregates and SOM density fractions: Implications from 13C natural abundance. Soil Biol. Biochem. 2014, 71, 95–104. [Google Scholar] [CrossRef]
- Ågren, G.I.; Bosatta, E.; Balesdent, J. Isotope discrimination during decomposition of organic matter: A theoretical analysis. Soil Sci. Soc. Am. J. 1996, 60, 1121–1126. [Google Scholar] [CrossRef]
- Blair, N.; Leu, A.; Muñoz, E.; Olsen, J.; Kwong, E.; Des Marais, D. Carbon isotopic fractionation in heterotrophic microbial metabolism. Appl. Environ. Microbiol. 1985, 50, 996–1001. [Google Scholar] [CrossRef] [Green Version]
- Šantrůčková, H.; Bird, M.I.; Frouz, J.; Šustr, V.; Tajovský, K. Natural abundance of 13C in leaf litter as related to feeding activity of soil invertebrates and microbial mineralisation. Soil Biol. Biochem. 2000, 32, 1793–1797. [Google Scholar] [CrossRef]
- Trumbore, S.E.; Sierra, C.A.; Hicks Pries, C.E. Radiocarbon nomenclature, theory, models, and interpretation: Measuring age, determining cycling rates, and tracing source pools. In Radiocarbon and Climate Change; Schuur, E.A.G., Druffel, E.R.M., Trumbore, S.E., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 45–82. [Google Scholar]
- Leifeld, J.; Zimmermann, M.; Fuhrer, J.; Conen, F. Storage and turnover of carbon in grassland soils along an elevation gradient in the Swiss Alps. Glob. Chang. Biol. 2009, 15, 668–679. [Google Scholar] [CrossRef]
- Kögel-Knabner, I.; Guggenberger, G.; Kleber, M.; Kandeler, E.; Kalbitz, K.; Scheu, S.; Eusterhues, K.; Leinweber, P. Organo-mineral associations in temperate soils: Integrating biology, mineralogy, and organic matter chemistry. J. Plant Nutr. Soil Sci. 2008, 171, 61–82. [Google Scholar] [CrossRef]
- Gaudinski, J.B.; Trumbore, S.E.; Eric, A.; Zheng, S. Soil carbon cycling in a temperate forest: Radiocarbon-based estimates of residence times, sequestration rates and partitioning of fluxes. Biogeochemistry 2000, 51, 33–69. [Google Scholar] [CrossRef]
- Poeplau, C.; Don, A. Sensitivity of soil organic carbon stocks and fractions to different land-use changes across Europe. Geoderma 2013, 192, 189–201. [Google Scholar] [CrossRef]
- Yamashita, T.; Flessa, H.; John, B.; Helfrich, M.; Ludwig, B. Organic matter in density fractions of water-stable aggregates in silty soils: Effect of land use. Soil Biol. Biochem. 2006, 38, 3222–3234. [Google Scholar] [CrossRef]
- Mikutta, R.; Kleber, M.; Torn, M.S.; Jahn, R. Stabilization of soil organic matter: Association with minerals or chemical recalcitrance? Biogeochemistry 2006, 77, 25–56. [Google Scholar] [CrossRef]

| Fraction 1 | Treatment 2 | TOC (g kg−1) | TN (g kg−1) | C/N Ratio |
|---|---|---|---|---|
| POM | Native | 245 ± 25.4 3 a 4 | 9.84 ± 1.28 ab | 25.2 ± 1.31 a |
| Amended | 229 ± 16.4 a | 8.02 ± 0.552 a | 28.5 ± 0.43 b | |
| Control | 341 ± 3.39 b | 11.9 ± 0.099 b | 28.7 ± 0.21 b | |
| S + A | Native | 21.6 ± 0.13 b | 1.53 ± 0.009 b | 14.1 ± 0.03 a |
| Amended | 17.5 ± 0.36 a | 1.12 ± 0.023 a | 15.7 ± 0.05 c | |
| Control | 17.6 ± 0.11 a | 1.15 ± 0.006 a | 15.3 ± 0.11 b | |
| s + c | Native | 23.0 ± 0.18 c | 1.69 ± 0.002 c | 13.6 ± 0.10 a |
| Amended | 19.4 ± 0.16 a | 1.44 ± 0.011 a | 13.5 ± 0.05 a | |
| Control | 20.3 ± 0.03 b | 1.49 ± 0.008 b | 13.7 ± 0.06 a | |
| rSOC | Native | 7.72 ± 0.07 c | 0.21 ± 0.001 b | 36.5 ± 0.49 b |
| Amended | 5.05 ± 0.17 a | 0.17 ± 0.004 a | 30.2 ± 0.65 a | |
| Control | 6.56 ± 0.09 b | 0.20 ± 0.006 b | 33.2 ± 1.43 a | |
| Bulk recovery (mass %) | Native | 80.5 ± 0.39 a | 113.6 ± 0.33 b | |
| Amended | 134.3 ± 8.14 b | 101.6 ± 3.28 a | ||
| Control | 93.9 ± 0.20 a | 98.0 ± 0.31 a |
| Fraction 1 | Treatment | rA2920 | rA2850 | rA1730 | rA1640 | rA1515 | rA1420 | IAR |
|---|---|---|---|---|---|---|---|---|
| POM | Native | 12.7 ± 0.89 2 a 3 | 8.7 ± 0.62 a | 24.7 ± 0.87 ab | 23.1 ± 0.33 b | 11.2 ± 0.93 b | 10.9 ± 1.6 b | 1.9 ± 0.15 b |
| Amended | 13.6 ± 0.15 a | 8.8 ± 0.07 a | 24.1 ± 0.54 a | 23.5 ± 0.22 b | 7.0 ± 0.30 a | 6.8 ± 0.41 a | 1.7 ± 0.02 b | |
| Control | 17.8 ± 0.18 b | 12.6 ± 0.21 b | 26.4 ± 0.33 b | 20.7 ± 0.36 a | 8.5 ± 0.18 a | 5.4 ± 0.51 a | 1.2 ± 0.03 a | |
| S + A | Native | 10.8 ± 0.11 b | 6.7 ± 0.09 b | 7.1 ± 0.31 a | 21.6 ± 0.22 a | 5.7 ± 0.30 a | 12.7 ± 0.57 a | 2.0 ± 0.01 a |
| Amended | 8.9 ± 0.37 a | 5.5 ± 0.26 a | 6.5 ± 0.31 a | 22.2 ± 0.22 ab | 8.5 ± 0.70 ab | 19.8 ± 1.6 b | 2.5 ± 0.10 b | |
| Control | 8.1 ± 0.40 a | 4.9 ± 0.24 a | 6.2 ± 0.32 a | 23.5 ± 0.72 b | 9.0 ± 1.2 b | 20.7 ± 1.7 b | 2.9 ± 0.14 c | |
| s + c | Native | 7.9 ± 0.19 ab | 4.5 ± 0.13 ab | 7.0 ± 0.18 a | 24.7 ± 0.29 a | 7.5 ± 0.74 b | 20.7 ± 1.3 b | 3.1 ± 0.08 a |
| Amended | 8.4 ± 0.23 b | 5.0 ± 0.16 b | 6.4 ± 0.19 a | 24.4 ± 0.48 a | 5.1 ± 0.29 a | 13.0 ± 0.78 a | 2.9 ± 0.12 a | |
| Control | 7.4 ± 0.29 a | 4.4 ± 0.18 a | 6.9 ± 0.26 a | 24.5 ± 0.67 a | 5.4 ± 0.43 a | 15.5 ± 1.5 a | 3.3 ± 0.16 a | |
| rSOC | Native | 8.3 ± 0.21 b | 5.3 ± 0.13 b | 4.0 ± 0.40 c | 18.0 ± 0.28 a | 11.3 ± 0.37 a | 21.9 ± 1.0 a | 2.2 ± 0.03 a |
| Amended | 3.5 ± 0.14 a | 2.5 ± 0.09 a | 2.1 ± 0.13 a | 20.0 ± 0.74 a | 15.3 ± 0.37 b | 18.7 ± 2.1 a | 5.8 ± 0.30 b | |
| Control | 7.4 ± 0.52 b | 4.9 ± 0.36 b | 3.2 ± 0.16 b | 20.2 ± 0.75 a | 11.1 ± 0.49 a | 18.2 ± 1.9 a | 2.8 ± 0.24 a |
| Fraction 1 | Fmaize 2(%) | MRT 3 (Years) | 14C activity (pMC) | Δ14C (‰) |
|---|---|---|---|---|
| POM | 11.7 ± 2.5 4 | 3.6 | 108.4 ± 0.19 | 75.2 ± 1.9 |
| S + A | 3.8 ± 2.8 | 11.5 | 106.0 ± 0.21 | 51.0 ± 2.1 |
| s + c | 2.7 ± 3.0 | 16.1 | 105.8 ± 0.19 | 49.7 ± 1.9 |
| rSOC | 0.18 ± 2.5 | 249.7 | 93.3 ± 0.24 | −75.0 ± 2.4 |
| Bulk soil | 4.3 ± 2.9 | 10.2 | 108.7 ± 0.17 | 78.2 ± 1.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zacháry, D.; Filep, T.; Jakab, G.; Molnár, M.; Kertész, T.; Király, C.; Hegyi, I.; Gáspár, L.; Szalai, Z. Carbon Isotope Measurements to Determine the Turnover of Soil Organic Matter Fractions in a Temperate Forest Soil. Agronomy 2020, 10, 1944. https://doi.org/10.3390/agronomy10121944
Zacháry D, Filep T, Jakab G, Molnár M, Kertész T, Király C, Hegyi I, Gáspár L, Szalai Z. Carbon Isotope Measurements to Determine the Turnover of Soil Organic Matter Fractions in a Temperate Forest Soil. Agronomy. 2020; 10(12):1944. https://doi.org/10.3390/agronomy10121944
Chicago/Turabian StyleZacháry, Dóra, Tibor Filep, Gergely Jakab, Mihály Molnár, Titanilla Kertész, Csilla Király, István Hegyi, Lilla Gáspár, and Zoltán Szalai. 2020. "Carbon Isotope Measurements to Determine the Turnover of Soil Organic Matter Fractions in a Temperate Forest Soil" Agronomy 10, no. 12: 1944. https://doi.org/10.3390/agronomy10121944
APA StyleZacháry, D., Filep, T., Jakab, G., Molnár, M., Kertész, T., Király, C., Hegyi, I., Gáspár, L., & Szalai, Z. (2020). Carbon Isotope Measurements to Determine the Turnover of Soil Organic Matter Fractions in a Temperate Forest Soil. Agronomy, 10(12), 1944. https://doi.org/10.3390/agronomy10121944







