Growth and Ginsenosides Content of Ginseng Sprouts According to LED-Based Light Quality Changes
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Light Treatments
2.3. Growth Characteristics
2.4. Photosynthetic Parameters
2.5. Total Saponin Content
2.6. Ginsenosides Content
2.7. Statistical Analysis
3. Results
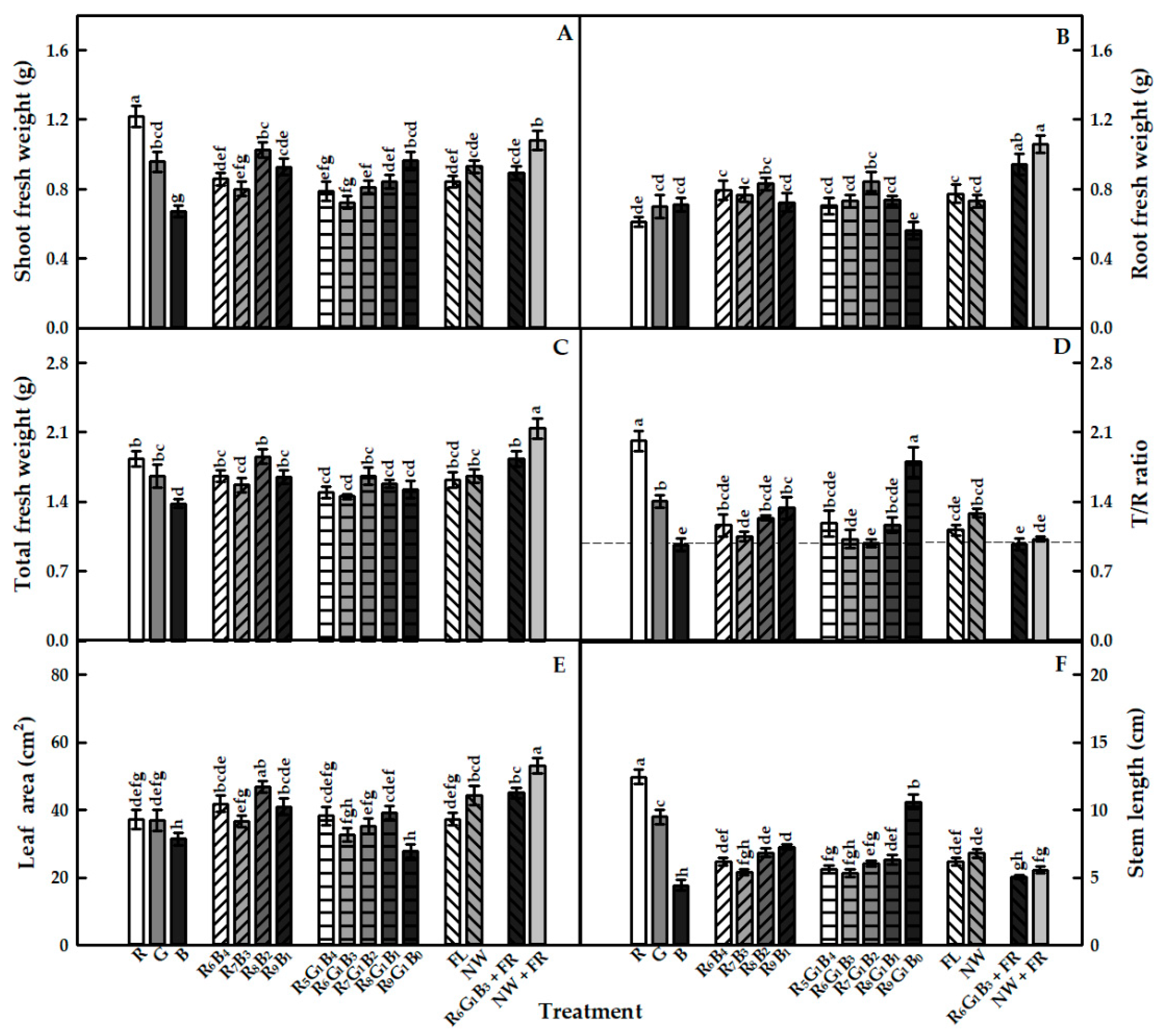
3.1. Growth Characteristics
3.2. Photosynthetic Parameters
3.3. Total Saponin Content
3.4. Ginsenosides Content
4. Discussion
4.1. Growth Characteristics
4.2. Photosynthetic Parameters
4.3. Total Saponin and Ginsenosides Contents
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kim, D.H.; Moon, Y.S.; Lee, T.H.; Jung, J.S.; Suh, H.W.; Song, D.K. The inhibitory effect of ginseng saponins on the stress-induced plasma interleukin-6 level in mice. J. Neurosci. Lett. 2003, 353, 13–16. [Google Scholar] [CrossRef] [PubMed]
- López, M.V.N.; Cuadrado, M.P.G.-S.; Ruiz-Poveda, O.M.P.; Del Fresno, A.M.V.; Accame, M.E.C. Neuroprotective effect of individual ginsenosides on astrocytes primary culture. Biochim. Biophys. Acta Gen. Subj. 2007, 1770, 1308–1316. [Google Scholar] [CrossRef] [PubMed]
- Vuksan, V.; Sung, M.-K.; Sievenpiper, J.L.; Stavro, P.M.; Jenkins, A.L.; Di Buono, M.; Lee, K.-S.; Leiter, L.A.; Nam, K.Y.; Arnason, J.T.; et al. Korean red ginseng (Panax ginseng) improves glucose and insulin regulation in well-controlled, type 2 diabetes: Results of a randomized, double-blind, placebo-controlled study of efficacy and safety. Nutr. Metab. Cardiovasc. Dis. 2008, 18, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Luo, J.-G.; Kong, L. Determination of 10 ginsenosides in Panax ginseng of different harvest times based on HPLC fingerprints and principal component analysis. Nat. Prod. Res. 2013, 27, 851–854. [Google Scholar] [CrossRef] [PubMed]
- Li, X.G.; Yan, Y.Z.; Jin, X.; Kim, Y.K.; Uddin, M.R.; Kim, Y.B.; Bae, H.; Kim, Y.C.; Lee, S.W.; Park, S.U. Ginsenoside content in the leaves and roots of Panax ginseng at different ages. Life Sci. 2012, 9, 670–683. [Google Scholar]
- Kim, G.S.; Hyun, D.Y.; Kim, Y.O.; Lee, S.E.; Kwon, H.; Cha, S.W.; Park, C.B.; Kim, Y.B. Investigation of ginsenosides in different parts of Panax ginseng cultured by hydroponics. Korean J. Hortic. Sci. 2010, 28, 216–226. [Google Scholar]
- Choi, S.Y.; Cho, C.-W.; Lee, Y.-M.; Kim, S.-S.; Lee, S.-H.; Kim, K.-T. Comparison of Ginsenoside and Phenolic Ingredient Contents in Hydroponically-cultivated Ginseng Leaves, Fruits, and Roots. J. Ginseng. Res. 2012, 36, 425–429. [Google Scholar] [CrossRef]
- Kozai, T. Resource use efficiency of closed plant production system with artificial light: Concept, estimation and application to plant factory. Proc. Jpn. Acad. Ser. B 2013, 89, 447–461. [Google Scholar] [CrossRef]
- Bian, Z.H.; Yang, Q.C.; Liu, W.K. Effects of light quality on the accumulation of phytochemicals in vegetables produced in controlled environments: A review. J. Sci. Food Agric. 2015, 95, 869–877. [Google Scholar] [CrossRef]
- Schmitt, J.; Wulff, R.D. Light spectral quality, phytochrome and plant competition. Trends Ecol. Evol. 1993, 8, 47–51. [Google Scholar] [CrossRef]
- Smith, H. Light Quality, Photoperception, and Plant Strategy. Annu. Rev. Plant Physiol. 1982, 33, 481–518. [Google Scholar] [CrossRef]
- Son, K.-H.; Oh, M.M. Leaf Shape, Growth, and Antioxidant Phenolic Compounds of Two Lettuce Cultivars Grown under Various Combinations of Blue and Red Light-emitting Diodes. HortScience 2013, 48, 988–995. [Google Scholar] [CrossRef]
- Kozai, T. Smart Plant Factory: The Next Generation Indoor Vertical Farms, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2018; pp. 3–14. [Google Scholar]
- Jeon, Y.-M.; Son, K.-H.; Kim, S.-M.; Oh, M.M. Growth and bioactive compounds as affected by irradiation with various spectrum of light-emitting diode lights in dropwort. Hortic. Environ. Biotechnol. 2017, 58, 467–478. [Google Scholar] [CrossRef]
- Kim, E.-Y.; Park, S.-A.; Park, B.-J.; Lee, Y.; Oh, M.M. Growth and antioxidant phenolic compounds in cherry tomato seedlings grown under monochromatic light-emitting diodes. Hortic. Environ. Biotechnol. 2014, 55, 506–513. [Google Scholar] [CrossRef]
- Lee, J.W.; Son, K.H.; Lee, J.H.; Kim, Y.J.; Oh, M.M. Growth and Biochemical Responses of Ice Plant Irradiated by Various Visible Light Spectra in Plant Factories. Hortic. Sci. Technol. 2019, 37, 598–608. [Google Scholar]
- Carvalho, R.F.; Takaki, M.; Azevedo, R.A. Plant pigments: The many faces of light perception. Acta Physiol. Plant. 2010, 33, 241–248. [Google Scholar] [CrossRef]
- Sullivan, J.A.; Deng, X.-W. From seed to seed: The role of photoreceptors in Arabidopsis development. Dev. Biol. 2003, 260, 289–297. [Google Scholar] [CrossRef]
- Li, J.; Hikosaka, S.; Goto, E. Effects of light quality and photosynthetic photon flux on growth and carotenoid pigments in spinach (Spinacia oleracea L.). Acta Hortic. 2011, 105–110. [Google Scholar] [CrossRef]
- Pennisi, G.; Blasioli, S.; Cellini, A.; Maia, L.; Crepaldi, A.; Braschi, I.; Spinelli, F.; Nicola, S.; Fernandez, J.A.; Stanghellini, C.; et al. Unraveling the Role of Red:Blue LED Lights on Resource Use Efficiency and Nutritional Properties of Indoor Grown Sweet Basil. Front. Plant Sci. 2019, 10, 305. [Google Scholar] [CrossRef]
- Zhang, X.; He, D.; Niu, G.; Yan, Z.; Song, J. Effects of environment lighting on the growth, photosynthesis, and quality of hydroponic lettuce in a plant factory. Int. J. Agric. Biol. Eng. 2018, 11, 33–40. [Google Scholar] [CrossRef]
- Hiai, S.; Oura, H.; Nakajima, T. Color reaction of some sapogenins and saponins with vanillin and sulfur1c acid. Planta Med. 1976, 29, 116–122. [Google Scholar] [CrossRef]
- Wang, J.; Lu, W.; Tong, Y.; Yang, Q. Leaf Morphology, Photosynthetic Performance, Chlorophyll Fluorescence, Stomatal Development of Lettuce (Lactuca sativa L.) Exposed to Different Ratios of Red Light to Blue Light. Front. Plant Sci. 2016, 7, 250. [Google Scholar] [CrossRef] [PubMed]
- Nanya, K.; Ishigami, Y.; Hikosaka, S.; Goto, E. Effects of blue and red light on stem elongation and flowering of tomato seedlings. Acta Hortic. 2012, 956, 261–266. [Google Scholar] [CrossRef]
- Bae, J.-H.; Park, S.-Y.; Oh, M.M. Supplemental irradiation with far-red light-emitting diodes improves growth and phenolic contents in Crepidiastrum denticulatum in a plant factory with artificial lighting. Hortic. Environ. Biotechnol. 2017, 58, 357–366. [Google Scholar] [CrossRef]
- Folta, K.M.; Maruhnich, S.A. Green light: A signal to slow down or stop. J. Exp. Bot. 2007, 58, 3099–3111. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-J.; Son, K.-H.; Oh, M.M. Increase in biomass and bioactive compounds in lettuce under various ratios of red to far-red LED light supplemented with blue LED light. Hortic. Environ. Biotechnol. 2016, 57, 139–147. [Google Scholar] [CrossRef]
- Morgan, D.C.; Smith, H. Linear relationship between phytochrome photoequilibrium and growth in plants under simulated natural radiation. Nat. Cell Biol. 1976, 262, 210–212. [Google Scholar] [CrossRef]
- Morgan, D.C.; Smith, H. The relationship between phytochrome-photoequilibrium and Development in light grown Chenopodium album L. Planta 1978, 142, 187–193. [Google Scholar] [CrossRef]
- Child, R.; Morgan, D.C.; Smith, H. Control of development in Chenopodium album l. by shadelight: The effect of light quality (red:far-red ratio) on morphogenesis. New Phytol. 1981, 89, 545–555. [Google Scholar] [CrossRef]
- Park, Y.; Runkle, E.S. Far-red radiation promotes growth of seedlings by increasing leaf expansion and whole-plant net assimilation. Environ. Exp. Bot. 2017, 136, 41–49. [Google Scholar] [CrossRef]
- Van Gelderen, K.; Kang, C.; Pierik, R. Light Signaling, Root Development, and Plasticity. Plant Physiol. 2018, 176, 1049–1060. [Google Scholar] [CrossRef] [PubMed]
- McCree, K. Test of current definitions of photosynthetically active radiation against leaf photosynthesis data. Agric. Meteorol. 1972, 10, 443–453. [Google Scholar] [CrossRef]
- Sood, S.; Gupta, V.; Tripathy, B.C. Photoregulation of the Greening Process of Wheat Seedlings Grown in Red Light. Plant Mol. Biol. 2005, 59, 269–287. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Ito, H.; Tanaka, R.; Tanaka, N.K.; Yoshida, K.; Okada, K. Chlorophyll a oxygenase (CAO) is involved in chlorophyll b formation from chlorophyll a. Proc. Natl. Acad. Sci. USA 1998, 95, 12719–12723. [Google Scholar] [CrossRef]
- Park, Y.; Runkle, E.S. Far-red radiation and photosynthetic photon flux density independently regulate seedling growth but interactively regulate flowering. Environ. Exp. Bot. 2018, 155, 206–216. [Google Scholar] [CrossRef]
- Shi, W.; Wang, Y.; Li, J.; Zhang, H.; Ding, L. Investigation of ginsenosides in different parts and ages of Panax ginseng. Food Chem. 2007, 102, 664–668. [Google Scholar] [CrossRef]
- Lim, W.; Mudge, K.W.; Vermeylen, F. Effects of Population, Age, and Cultivation Methods on Ginsenoside Content of Wild American Ginseng (Panax quinquefolium). J. Agric. Food Chem. 2005, 53, 8498–8505. [Google Scholar] [CrossRef]
- Lee, J.G.; Oh, S.S.; Cha, S.H.; Jang, Y.A.; Kim, S.Y.; Um, Y.C.; Cheong, S.R. Effects of red/blue light ratio and short-term light quality conversion on growth and anthocyanin contents of baby leaf lettuce. Prot. Hortic. Plant Fact. 2010, 19, 351–359. [Google Scholar]
- Fournier, A.R.; Proctor, J.T.; Gauthier, L.; Khanizadeh, S.; Bélanger, A.; Gosselin, A.; Dorais, M. Understory light and root ginsenosides in forest-grown Panax quinquefolius. Phytochemistry 2003, 63, 777–782. [Google Scholar] [CrossRef]



| Shoot Ginsenosides Content (mg g−1) | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group 1 | Treatment | Panaxadiol (PD) | Panaxatriol (PT) | Total Value | PD/PT | ||||||||||||||||||
| Rb1 | Rb2 | Rc | Rd | Rg1 | Rg2 | Rg3 | Re | Rf | |||||||||||||||
| Mono | R | 1.36 | j 2 | 0.68 | fgh | 0.25 | gh | 3.34 | fg | 10.40 | f | 1.02 | h | 0.10 | h | 3.65 | l | 0.14 | f | 20.93 | j | 0.37 | |
| G | 1.98 | hi | 1.18 | d | 0.28 | bcde | 5.81 | bc | 13.93 | cd | 1.67 | def | 0.12 | g | 4.35 | k | 0.11 | g | 29.44 | fgh | 0.46 | ||
| B | 3.25 | bc | 1.11 | de | 0.15 | i | 4.86 | d | 10.43 | f | 1.58 | ef | 0.15 | ef | 10.78 | a | 0.24 | a | 32.55 | defg | 0.40 | ||
| RB | R6B4 | 2.59 | fg | 1.49 | c | 0.27 | cdefg | 5.77 | bc | 13.71 | cde | 2.15 | c | 0.17 | de | 5.08 | ij | 0.11 | g | 31.33 | efgh | 0.48 | |
| R7B3 | 3.11 | cd | 1.20 | d | 0.24 | gh | 4.84 | d | 14.24 | bcd | 1.88 | cde | 0.14 | fg | 9.23 | b | 0.21 | bcd | 35.10 | bcde | 0.37 | ||
| R8B2 | 2.39 | g | 0.97 | de | 0.29 | bc | 3.79 | ef | 12.98 | de | 1.57 | ef | 0.15 | ef | 5.40 | i | 0.15 | f | 27.69 | hi | 0.37 | ||
| R9B1 | 2.52 | g | 0.89 | ef | 0.29 | bc | 4.02 | e | 14.10 | bcd | 1.45 | fg | 0.16 | de | 6.49 | fg | 0.21 | bcd | 30.13 | fgh | 0.34 | ||
| RGB | R5G1B4 | 2.87 | de | 0.44 | hi | 0.25 | gh | 2.40 | h | 14.89 | bc | 1.00 | h | 0.18 | cd | 6.24 | g | 0.16 | ef | 28.42 | gh | 0.26 | |
| R6G1B3 | 3.05 | cd | 0.54 | ghi | 0.30 | b | 2.83 | gh | 17.17 | a | 1.06 | h | 0.17 | cd | 7.77 | d | 0.19 | cde | 33.08 | cdef | 0.26 | ||
| R7G1B2 | 2.78 | ef | 0.50 | ghi | 0.25 | fgh | 2.59 | h | 14.30 | bcd | 1.10 | h | 0.21 | ab | 8.30 | c | 0.21 | abc | 30.24 | fgh | 0.25 | ||
| R8G1B1 | 2.10 | h | 0.31 | i | 0.26 | defgh | 1.81 | i | 13.24 | cde | 0.64 | i | 0.18 | cd | 6.01 | gh | 0.21 | abc | 24.77 | j | 0.22 | ||
| R9G1B0 | 1.75 | i | 0.71 | fg | 0.26 | efgh | 3.49 | ef | 12.02 | ef | 1.15 | gh | 0.14 | fg | 4.63 | jk | 0.15 | f | 24.30 | ij | 0.34 | ||
| W | FL | 3.12 | de | 1.14 | c | 0.24 | a | 5.34 | b | 15.90 | a | 1.69 | cd | 0.17 | a | 9.07 | hi | 0.23 | ef | 36.89 | bcd | 0.36 | |
| NW | 2.93 | cd | 1.44 | d | 0.33 | h | 6.07 | cd | 17.32 | ab | 1.94 | def | 0.21 | cde | 5.61 | b | 0.16 | ab | 36.02 | bc | 0.43 | ||
| FR | R6G1B3 + FR | 3.50 | a | 2.52 | a | 0.27 | cdef | 7.88 | a | 15.90 | ab | 3.50 | a | 0.19 | bc | 7.08 | e | 0.19 | cde | 41.03 | a | 0.53 | |
| NW + FR | 3.40 | ab | 1.77 | b | 0.29 | bcd | 6.17 | b | 17.44 | a | 2.54 | b | 0.18 | cd | 6.90 | ef | 0.18 | de | 38.87 | ab | 0.43 | ||
| Root Ginsenosides Content (mg g−1) | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group 1 | Treatment | Panaxadiol (PD) | Panaxatriol (PT) | Total Value | PD/PT | ||||||||||||||||
| Rb1 | Rb2 | Rc | Rd | Rg1 | Rg2 | Re | Rf | ||||||||||||||
| Mono | R | 1.15 | abc 2 | 0.60 | cdef | 0.12 | bcdef | 0.33 | cdefg | 3.86 | abc | 0.72 | a | 0.33 | c | 0.34 | bc | 7.45 | abc | 0.42 | |
| G | 0.98 | bcd | 0.45 | ghi | 0.10 | cdef | 0.28 | fg | 3.59 | abcd | 0.53 | c | 0.27 | c | 0.34 | bc | 6.54 | bcd | 0.39 | ||
| B | 1.14 | abc | 0.83 | a | 0.10 | def | 0.31 | defg | 3.00 | def | 0.77 | a | 1.39 | a | 0.52 | a | 8.06 | a | 0.42 | ||
| RB | R6B4 | 0.65 | f | 0.39 | i | 0.13 | bcdef | 0.29 | efg | 2.26 | fg | 0.36 | d | 0.27 | c | 0.34 | bc | 4.69 | e | 0.45 | |
| R7B3 | 0.91 | de | 0.50 | fgh | 0.07 | ef | 0.32 | cdefg | 2.79 | efg | 0.53 | e | 0.98 | b | 0.42 | ab | 6.52 | bcd | 0.38 | ||
| R8B2 | 0.91 | de | 0.54 | efg | 0.13 | bcde | 0.36 | bcde | 3.21 | bcde | 0.57 | bc | 0.28 | c | 0.29 | cd | 6.29 | cd | 0.45 | ||
| R9B1 | 1.29 | a | 0.72 | b | 0.17 | abc | 0.42 | a | 3.44 | abcde | 0.78 | a | 0.35 | c | 0.34 | bc | 7.51 | abc | 0.53 | ||
| RGB | R5G1B4 | 1.11 | abcd | 0.71 | bc | 0.21 | a | 0.40 | ab | 3.31 | bcde | 0.74 | a | 0.27 | c | 0.27 | cd | 7.02 | abcd | 0.53 | |
| R6G1B3 | 1.16 | ab | 0.67 | bcd | 0.17 | abcd | 0.33 | cdefg | 3.43 | abcde | 0.68 | ab | 0.34 | c | 0.30 | bcd | 7.06 | abcd | 0.49 | ||
| R7G1B2 | 0.91 | de | 0.54 | ef | 0.12 | bcdef | 0.27 | g | 2.84 | defg | 0.56 | bc | 0.47 | c | 0.25 | cd | 5.96 | d | 0.45 | ||
| R8G1B1 | 1.24 | a | 0.72 | b | 0.17 | ab | 0.36 | abcd | 4.14 | a | 0.76 | a | 0.32 | c | 0.33 | bc | 8.04 | a | 0.45 | ||
| R9G1B0 | 1.25 | a | 0.73 | b | 0.12 | bcdef | 0.34 | bcdef | 3.92 | ab | 0.75 | a | 0.33 | c | 0.31 | bcd | 7.77 | ab | 0.46 | ||
| W | FL | 0.99 | bcd | 0.67 | bcde | 0.10 | bcd | 0.31 | abc | 2.85 | cde | 0.74 | ab | 0.42 | c | 0.28 | cd | 6.37 | bcd | 0.48 | |
| NW | 1.00 | bcd | 0.64 | bcd | 0.14 | cdef | 0.38 | defg | 3.11 | defg | 0.69 | a | 0.25 | c | 0.23 | cd | 6.44 | bcd | 0.50 | ||
| FR | R6G1B3 + FR | 0.72 | ef | 0.40 | hi | 0.06 | f | 0.27 | g | 2.17 | g | 0.40 | d | 0.43 | c | 0.19 | d | 4.65 | e | 0.45 | |
| NW + FR | 0.93 | cd | 0.57 | def | 0.15 | abcd | 0.33 | cdefg | 2.89 | defg | 0.57 | bc | 0.28 | c | 0.28 | cd | 6.01 | d | 0.49 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.-J.; Nguyen, T.K.L.; Oh, M.-M. Growth and Ginsenosides Content of Ginseng Sprouts According to LED-Based Light Quality Changes. Agronomy 2020, 10, 1979. https://doi.org/10.3390/agronomy10121979
Kim Y-J, Nguyen TKL, Oh M-M. Growth and Ginsenosides Content of Ginseng Sprouts According to LED-Based Light Quality Changes. Agronomy. 2020; 10(12):1979. https://doi.org/10.3390/agronomy10121979
Chicago/Turabian StyleKim, Yoon-Jeong, Thi Kim Loan Nguyen, and Myung-Min Oh. 2020. "Growth and Ginsenosides Content of Ginseng Sprouts According to LED-Based Light Quality Changes" Agronomy 10, no. 12: 1979. https://doi.org/10.3390/agronomy10121979
APA StyleKim, Y.-J., Nguyen, T. K. L., & Oh, M.-M. (2020). Growth and Ginsenosides Content of Ginseng Sprouts According to LED-Based Light Quality Changes. Agronomy, 10(12), 1979. https://doi.org/10.3390/agronomy10121979




