Biostimulant Seed Coating Treatments to Improve Cover Crop Germination and Seedling Growth
Abstract
:1. Introduction
2. Materials and Methods
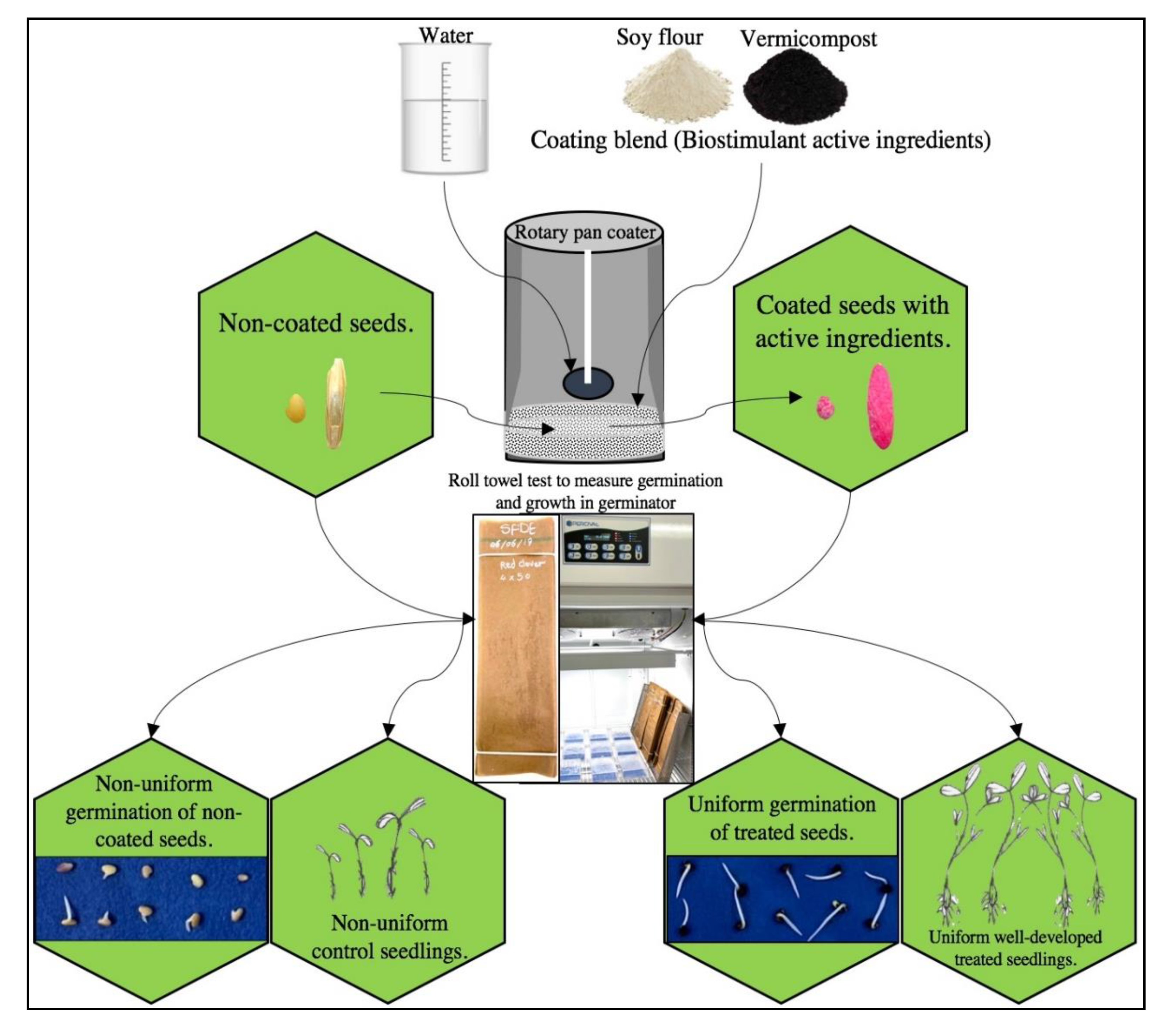
2.1. Seed and Coating Materials
2.2. Seed Coating
2.3. Seed Coat Physical Properties
2.3.1. Seed Coating Integrity Test
2.3.2. Mechanical Property Test
2.3.3. Seed Coating Hydration Test
2.4. Seed Germination and Seedling Growth Measurements
2.5. Statistical Analysis
3. Results and Discussion
3.1. Seed Coating Physical Properties
3.2. Germination and Seedling Growth of Soy Flour and Diatomaceous Earth Seed Coating
3.3. Germination and Seedling Growth of Soy Flour and Vermicompost Seed Coating
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Roser, M.; Ritchie, H.; Ortiz-Ospina, E. World Population Growth; Our World in Data; Oxford University-Global Change Data Lab (GCDL), 2019; Available online: https://ourworldindata.org/world-population-growth (accessed on 20 January 2020).
- Mu, S.; Zhou, S.; Chen, Y.; Li, J.; Ju, W.; Odeh, I.O.A. Assessing the impact of restoration-induced land conversion and management alternatives on net primary productivity in Inner Mongolian grassland, China. Glob. Planet. Chang. 2013, 108, 29–41. [Google Scholar] [CrossRef]
- Ni, J. Carbon storage in grasslands of China. J. Arid Environ. 2002, 50, 205–218. [Google Scholar] [CrossRef]
- Qiu, Y.; Chen, Z.; Hou, Z.F.; Song, T.; Mi, L.K.; Shao, Z.W. Numerical simulation and experiment on improving pelleted coating of forage grass seeds by vibration force field. Trans. CSAE 2017, 33, 86–93. [Google Scholar]
- Sheoran, V.; Sheoran, A.S.; Poonia, P. Soil reclamation of abandoned mine land by revegetation: A review. Int. J. Soil Sediment Water 2010, 3, 13. [Google Scholar]
- Skousen, J.; Zipper, C.E. Reclamation guidelines for surface mined land in South-Western Virginia. Chapter: Revegetation species and practices. Va. Coop. Ext. Publ. 1996, 460, 120. [Google Scholar]
- Balfourier, F.; Imbert, C.; Charmet, G. Evidence for phylogeographic structure in lolium species related to the spread of agriculture in Europe. A cpDNA study. Theor. Appl. Genet. 2000, 101, 131–138. [Google Scholar] [CrossRef]
- McKenna, P.; Cannon, N.; Conway, J.; Dooley, J.; Davies, W.P. Red clover (Trifolium pratense) in conservation agriculture: A compelling case for increased adoption. Int. J. Agric. Sustain. 2018, 16, 342–366. [Google Scholar] [CrossRef]
- Daryanto, S.; Fu, B.; Wang, L.; Jacinthe, P.A.; Zhao, W. Quantitative synthesis on the ecosystem services of cover crops. Earth-Sci. Rev. 2018, 185, 357–373. [Google Scholar] [CrossRef]
- Taylor, A.G.; Allen, P.S.; Bennett, M.A.; Bradford, K.J.; Burris, J.S.; Misra, M.K. Seed enhancements. Seed Sci. Res. 1998, 8, 245–256. [Google Scholar] [CrossRef]
- Snapp, S.; Price, R.; Morton, M. Seed priming of winter annual cover crops improves germination and emergence. Agron. J. 2008, 100, 1506–1510. [Google Scholar] [CrossRef]
- Harris, D.; Raghuwanshi, B.S.; Gangwar, J.S.; Singh, S.C.; Joshi, K.; Rashid, A.; Hollington, P.A. Evaluation by farmers of on-farm seed priming in wheat in India, Nepal and Pakistan. Exp. Agric. 2001, 37, 403–415. [Google Scholar] [CrossRef]
- Vartha, E.W.; Clifford, P.T.P. Effects of seed coating on establishment and survival of grasses, surface-sown on tussock grasslands. N. Z. J. Exp. Agric. 1973, 1, 39–43. [Google Scholar] [CrossRef]
- Falloon, R.E.; Fletcher, R.H. Increased herbage production from perennial ryegrass following fungicide seed treatment. N. Z. J. Agric. Res. 1983, 26, 1–5. [Google Scholar] [CrossRef]
- Amirkhani, M.; Netravali, A.N.; Huang, W.; Taylor, A.G. Investigation of soy protein-based biostimulant seed coating for broccoli seedling and plant growth enhancement. HortScience 2016, 51, 1121–1126. [Google Scholar] [CrossRef] [Green Version]
- Ou, C.M.; Mao, P.S. Progress in research and application of coating technology for grasses. Seed 2019, 11, 63–67. (In Chinese) [Google Scholar]
- Li, C.Y.; Zhang, F.; Liu, C.H.; Yu, F.S.; Li, Y.C. Screening of coating materials of the forage seeds. J. Northeast Agric. Univ. 2013, 4, 94–100, (In Chinese with English abstract). [Google Scholar]
- He, Z.X.; Mao, P.S.; Sun, Y.; Li, M. Review of grass seed coating technology. Acta Agrestia Sin. 2016, 2, 270–277, (In Chinese with English abstract). [Google Scholar]
- Rouphael, Y.; Colla, G. Synergistic biostimulatory action: Designing the next generation of plant biostimulants for sustainable agriculture. Front. Plant Sci. 2018, 9, 1655. [Google Scholar] [CrossRef] [Green Version]
- Colla, G.; Hoagland, L.; Ruzzi, M.; Cardarelli, M.; Bonini, P.; Canaguier, R.; Rouphael, Y. Biostimulant action of protein hydrolysates: Unraveling their effects on plant physiology and microbiome. Front. Plant Sci. 2017, 8, 2202. [Google Scholar] [CrossRef] [Green Version]
- Wilson, H.T.; Amirkhani, M.; Taylor, A.G. Evaluation of gelatin as a biostimulant seed treatment to improve plant performance. Front. Plant Sci. 2018, 9, 1006. [Google Scholar] [CrossRef] [Green Version]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef] [Green Version]
- Schmitt, A.; Koch, E.; Stephan, D.; Kromphardt, C.; Jahn, M.; Krauthausen, H.J. Evaluation of non-chemical seed treatment methods for the control of Phoma valerianellae on lamb’s lettuce seeds. J. Plant Dis. Prot. 2009, 116, 200–207. [Google Scholar] [CrossRef]
- Taylor, A.G. Seed treatments. In Encyclopedia of Applied Plant Sciences; Thomas, B., Murphy, D.J., Murray, B.G., Eds.; Elsevier Acad. Press: Oxford, UK, 2003; pp. 1291–1298. [Google Scholar]
- Amirkhani, M.; Netravali, A.N.; Taylor, A.G. Improving seedling growth uniformity and seed vigor index by using plant-based protein seed coating in tomato and broccoli NYSAES research symposium. In Proceedings of the NYSAES Research Symposium 2017, Geneva, NY, USA, 23 June 2017; p. 6. [Google Scholar]
- Amirkhani, M.; Mayton, H.S.; Loos, M.T.; Netravali, A.N.; Taylor, A.G. Biostimulant effect of a plant-based protein applied as a seed coating on selected crops. In Proceedings of the 3rd Annual Cornell AgriTech Research Symposium, Geneva, NY, USA, 29 June 2018; p. 4. [Google Scholar]
- Amirkhani, M.; Mayton, H.S.; Netravali, A.N.; Taylor, A.G. A seed coating delivery system for bio-based biostimulants to enhance plant growth. Sustainability 2019, 11, 5304. [Google Scholar] [CrossRef] [Green Version]
- Talley, B. Status and New Developments in Seed Coatings; Summit Seed Coating: Caldwell, ID, USA, 2011; Available online: https://uknowledge.uky.edu/ky_alfalfa/2012/Session/6/ (accessed on 28 August 2019).
- Cespi, M.; Bonacucina, G.; Misici-Falzi, M.; Golzi, R.; Boltri, L.; Palmieri, G.F. Stress relaxation test for the characterization of the viscoelasticity of pellets. Eur. J. Pharm. Biopharm. 2007, 67, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Texture Analysis. Texture: Measure and Analyze Properties. Disintegration. Available online: https://www.stablemicrosystems.com/MeasureDisintegration.html (accessed on 15 August 2019).
- AOSA. Rules for testing seeds. Principles and procedures. Assoc. Off. Seed Anal. 2014, 1, 6–25. [Google Scholar]
- Egli, D.B.; Hamman, B.; Rucker, M. Seed vigor and uniformity of seedling emergence in soybean. Seed Technol. 2010, 32, 87–95. [Google Scholar]
- Coolbear, P.; Francis, A.; Grierson, D. The effect of low temperature pre-sowing treatment on the germination performance and membrane integrity of artificially aged tomato seeds. J. Exp. Bot. 1984, 35, 1609–1617. [Google Scholar] [CrossRef]
- Abdul-Baki, A.A.; Anderson, J.D. Vigor determination in soybean seed by multiple criteria. Crop Sci. 1973, 13, 630–633. [Google Scholar] [CrossRef]
- Minitab (Version Express for PC). 2019. Available online: http://www.minitab.com/en-us/products/express (accessed on 28 August 2019).
- American Seed Trade Association (ASTA). The Guide to Seed Treatment Stewardship. For Applicators. Available online: www.seed-treatment-guide.com (accessed on 5 December 2019).
- Accinelli, C.; Abbas, H.K.; Little, N.S.; Kotowicz, J.K.; Mencarelli, M.; Shier, W.T. A liquid bioplastic formulation for film coating of agronomic seeds. Crop Prot. 2016, 89, 123–128. [Google Scholar] [CrossRef]
- Foqué, D.; Devarrewaere, W.; Verboven, P.; Nuyttens, D. Physical and chemical characteristics of abraded seed coating particles. Asp. Appl. Biol. 2014, 122, 85–94. [Google Scholar]
- Karlsons, A.; Osvalde, A.; Una Andersone-Ozola, U.; Ievinsh, G. Vermicompost from municipal sewage sludge affects growth and mineral nutrition of winter rye (Secale cereale) plants. J. Plant Nutr. 2015, 39, 765–780. [Google Scholar] [CrossRef]
- Tognetti, C.; Laos, F.; Mazzarino, M.J.; Hernández, M.T. Composting vs. vermicomposting: A comparison of end product quality. Compost Sci. Util. 2005, 13, 6–13. [Google Scholar] [CrossRef]
- Alwaneen, W.S. Effect of cow manure vermicompost on some growth parameters of alfalfa and Vinca rosa plants. Asian J. Plant Sci. 2016, 15, 81–85. [Google Scholar] [CrossRef]
- Fleming, T.R.; Fleming, C.C.; Levy, C.C.B.; Carlos, R.; Franck, H.; Nolasco, J.B.; Liu, F.Q. Biostimulants enhance growth and drought tolerance in Arabidopsis Thaliana and exhibit chemical priming action. Ann. Appl. Biol. 2019, 174, 153–165. [Google Scholar] [CrossRef]
- Rouphael, Y.; Cardarelli, M.; Bonini, P.; Colla, G. Synergistic action of a microbial-based biostimulant and a plant derived-protein hydrolysate enhances lettuce tolerance to alkalinity and salinity. Front. Plant Sci. 2017, 8, 131. [Google Scholar] [CrossRef] [Green Version]
- Van Oosten, M.J.; Pepe, O.; De Pascale, S.; Silletti, S.; Maggio, A. The role of biostimulants and bioeffectors as alleviators of abiotic stress in crop plants. Chem. Biol. Technol. Agric. 2017, 4, 5. [Google Scholar] [CrossRef] [Green Version]
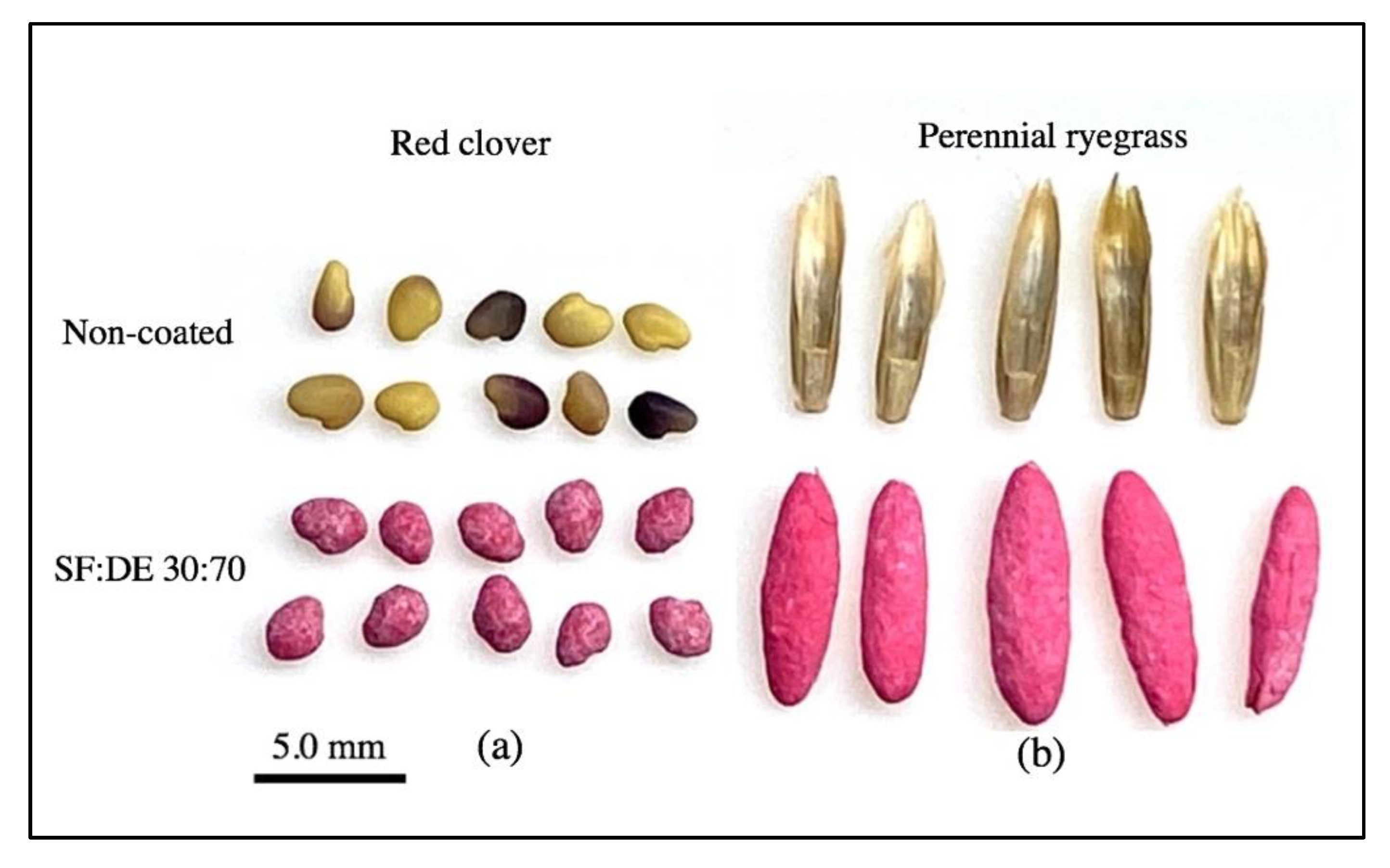


| Coating Materials | Abbreviation | Source |
|---|---|---|
| Soy Flour | SF | Archer Daniels Midland Co., Decatur, IL, USA |
| Diatomaceous Earth | DE | Perma-Guard, Inc., Albuquerque, NM, USA |
| Concentrated Vermicompost Extract (liquid) | CVE | Worm Power, Avon, NY, USA (concentrated by Caloris Engineering, Easton, MD, USA) |
| Micronized Vermicompost | MVC-2 | Worm Power, Avon, NY, USA |
| Micronized Vermicompost | MVC-3 | TerraVesco, Sonoma Valley Worm Farm, Sonoma, CA, USA |
| Crop | Treatment | WL (%) | DT (min) | Force (N) | TB (s) | RT (s) |
|---|---|---|---|---|---|---|
| Red clover | SF:DE 30:70 | 1.5 a * | 58 a | 16.2 a | 4.4 a | 0.3 a |
| SF:DE 40:60 | 1.2 ab | 75 b | 19.2 b | 5.1 b | 0.36 b | |
| SF:DE 50:50 | 0.6 bc | 100 c | 20.6 b | 5.6 c | 0.48 c | |
| SF:DE 60:40 | 0.4 c | 103 c | 23.9 c | 5.5 c | 0.5 c | |
| SF:MVC-2 | 1.2 ab | 78 b | 16.8 a | 4.4 a | 0.38 b | |
| SF:MVC-3 | 1.1 ab | 83 b | 16.5 a | 4.7 a | 0.32 a | |
| SF:DE:CVE | 1.0 ab | 80 b | 17.5 b | 4.9 a | 0.31 a | |
| Perennial ryegrass | SF:DE 30:70 | 1.4 A * | 40 A | 15.3 A | 5.7 A | 0.2 A |
| SF:DE 40:60 | 1.2 AB | 58 B | 17.9 B | 6.0 B | 0.38 B | |
| SF:DE 50:50 | 0.5 B | 90 C | 20.9 C | 6.4 C | 0.51 C | |
| SF:MVC-2 | 1.3 B | 60 B | 15.6 A | 5.4 A | 0.24 A | |
| SF:MVC-3 | 0.9 BC | 55 B | 16.1 A | 5.5 A | 0.22 A | |
| SF:DE:CVE | 0.9 BC | 60 B | 15.8 A | 5.8 A | 0.31 B |
| Crop | Treatment | Gmax (%) | T50 (h) | GU (h) | Shoot (cm) | Root (cm) | SVI |
|---|---|---|---|---|---|---|---|
| Red clover | Control | 95 b * | 35 a | 37 a | 3.6 b | 2.4 b | 5.7 b |
| SF:DE 30:70 | 98 a | 27 b | 27 b | 4.1 a | 3.0 a | 7.0 a | |
| SF:DE 40:60 | 99 a | 29 b | 25 b | 4.3 a | 2.9 a | 7.1 a | |
| SF:DE 50:50 | 96 b | 30 b | 27 b | 4.1 a | 2.9 a | 6.7 a | |
| SF:DE 60:40 | 96 b | 34 a | 28 b | 3.9 a | 2.7 a | 6.3 b | |
| Perennial ryegrass | Control | 85 A * | 75 B | 39 B | 6.5 B | 5.5 B | 10.2 B |
| SF:DE 30:70 | 83 A | 79 A | 40 B | 7.6 A | 6.2 A | 11.5 A | |
| SF:DE 40:60 | 83 A | 80 A | 42 B | 7.4 A | 6.3 A | 11.4 A | |
| SF:DE 50:50 | 80 B | 83 A | 47 A | 7.2 A | 5.9 A | 10.5 A |
| Crop | Treatment | Gmax (%) | T50 (h) | GU (h) | Shoot (cm) | Root (cm) | DW (g) | SVI |
|---|---|---|---|---|---|---|---|---|
| Red clover | Control | 94 b * | 36 a | 35 a | 3.7 b | 2.5 b | 0.05 b | 6.0 b |
| SF:DE | 98 a | 26 b | 27 b | 4.2 a | 2.8 a | 0.07 a | 6.9 a | |
| SF:MVC-2 | 99 a | 26 b | 24 b | 4.4 a | 2.9 a | 0.07 a | 7.3 a | |
| SF:MVC-3 | 99 a | 25 b | 25 b | 4.7 a | 3.0 a | 0.08 a | 7.6 a | |
| SF:DE:CVE | 98 a | 27 b | 25 b | 4.5 a | 3.2 a | 0.07 a | 7.5 a | |
| Perennial ryegrass | Control | 87 A * | 76 A | 41 B | 6.5 B | 5.9 B | 0.10 B | 10.8 B |
| SF:DE | 85 A | 78 A | 43 B | 7.9 A | 6.5 A | 0.13 A | 12.3 A | |
| SF:MVC-2 | 86 A | 77 A | 40 B | 8.1 A | 6.8 A | 0.14 A | 12.8 A | |
| SF:MVC-3 | 82 B | 81 A | 48 A | 8.3 A | 6.6 A | 0.13 A | 12.2 A | |
| SF:DE:CVE | 85 A | 77 A | 43 B | 8.4 A | 6.6 A | 0.15 A | 12.8 A |
| Crop | WL (%) | Force (N) | |
|---|---|---|---|
| Red clover | DT (min) | −0.99 *** | +0.92 ** |
| WL (%) | - | −0.94 ** | |
| Perennial ryegrass | DT (min) | −0.99 *** | +0.99 *** |
| WL (%) | - | −0.96 *** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, Y.; Amirkhani, M.; Mayton, H.; Chen, Z.; Taylor, A.G. Biostimulant Seed Coating Treatments to Improve Cover Crop Germination and Seedling Growth. Agronomy 2020, 10, 154. https://doi.org/10.3390/agronomy10020154
Qiu Y, Amirkhani M, Mayton H, Chen Z, Taylor AG. Biostimulant Seed Coating Treatments to Improve Cover Crop Germination and Seedling Growth. Agronomy. 2020; 10(2):154. https://doi.org/10.3390/agronomy10020154
Chicago/Turabian StyleQiu, Yi, Masoume Amirkhani, Hilary Mayton, Zhi Chen, and Alan G. Taylor. 2020. "Biostimulant Seed Coating Treatments to Improve Cover Crop Germination and Seedling Growth" Agronomy 10, no. 2: 154. https://doi.org/10.3390/agronomy10020154
APA StyleQiu, Y., Amirkhani, M., Mayton, H., Chen, Z., & Taylor, A. G. (2020). Biostimulant Seed Coating Treatments to Improve Cover Crop Germination and Seedling Growth. Agronomy, 10(2), 154. https://doi.org/10.3390/agronomy10020154






