Natural Compounds as Elicitors of Plant Resistance Against Diseases and New Biocontrol Strategies
Abstract
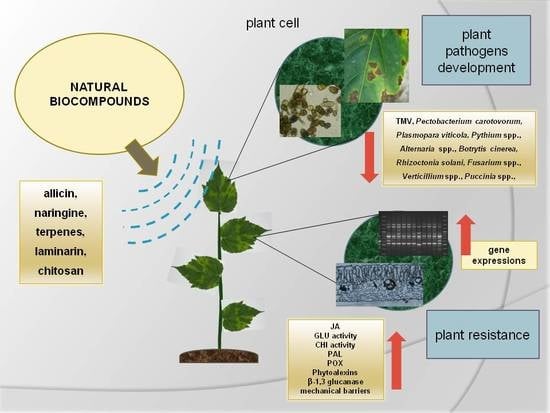
:1. Introduction
2. Natural Bioactive Compounds as Elicitors
3. Natural Compounds Against Plant Diseases
Mode of Action of Natural Elicitors
4. Commercial Uses of Natural Elicitors in Organic Plant Production
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Arunkumar, K.; Sivakumar, S.R.; Rengasamy, R. Review on Bioactive Potential in Seaweeds Marine Macroalgae: A Special Emphasis on Bioactivity of Seaweeds Against Plant Pathogens. Asian J. Plant Sci. 2010, 9, 227–240. [Google Scholar] [CrossRef] [Green Version]
- Pięta, D.; Patkowska, E.; Pastucha, A. The protective effect of biopreparations applied as the dressing for common bean (Phaseolus vulgaris L.) and pea (Pisum sativum L.). Acta Sci. Pol-Hortoru. 2005, 4, 59–69. [Google Scholar]
- Pastucha, A. Chitosan as a compound inhibiting the occurrence of soybean diseases. Acta Sci. Pol-Hortoru. 2008, 7, 41–55. [Google Scholar]
- Lucas, G.C.; Alves, E.; Pereira, R.B.; Perina, F.J.; Magela de Souza, R. Antibacterial activity of essential oils on Xanthomonas vesicatoria and control of bacteria spot in tomato. Pesq. Agropec. Bras. 2012, 47, 3. [Google Scholar] [CrossRef]
- Raja, N.; Masresha, G. Plant Based Biopesticides: Safer Alternative for Organic Food Production. J. Fertil. Pestic. 2015, 6, 2. [Google Scholar] [CrossRef]
- Abdulrahman, A.; Alkhail, A. Antifungal activity of some extract against some plant pathogenic fungi. Pak. J. Biol. Sci. 2005, 8, 413–417. [Google Scholar]
- Sadowski, C.; Lenc, L.; Łukanowski, A. Pytopathological aspect of onion seed production in organic farm. J. Res. Appl. Agric. Eng. 2009, 54, 80–84. [Google Scholar]
- Burgieł, Z.J.; Smagłowski, M. Fungistatyczne właściwości olejku z drzewa herbacianego. Prog. Plant Prot. 2008, 529, 13–18. [Google Scholar]
- Sultana, V.; Baloch, G.N.; Ara, J.; Esteshamul-Haque, S.; Tariq, R.M.; Athar, M. Seaweeds as alternative to chemical pesticides for the management of root diseases of sunflower and tomato. J. Appl. Bot. Food Quality. 2011, 84, 162–168. [Google Scholar]
- Stoleru, V.; Sellitto, V.M. Pest Control in Organic System. In Integrated Pest Management (IPM): Environmentally Sound Pest Management, 1st ed.; Gill, H., Ed.; IntechOpen: London, UK, 2016; pp. 1239–1560. [Google Scholar] [CrossRef] [Green Version]
- Freeman, B.C.; Battie, G.A. An overview of plant defenses against pathogens and herbivores. In The Plant Health Instructor; Iowa State University: Ames, IA, USA, 2008; Volume 94, pp. 1–12. [Google Scholar] [CrossRef] [Green Version]
- Babosha, A.V. Changes in lecitin activity in plants treated with resistance inducers. Biol. Bull. Russian Acad. Sci. 2004, 31, 51–55. [Google Scholar] [CrossRef]
- Aziz, A.; Poinssot, B.; Daire, X.; Adrian, M.; Bezier, A.; Lambert, B.; Joubert, J.M.; Pugin, A. Laminarin elicits defense responses in grapevine and induces protection against Botrytis cinerea and Plasmopara viticola. Mol. Plant Microbe. Interact. 2003, 16, 1118–1128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thakur, M.; Sohal, B.S. Role of elicitors in inducing resistance in plant pathogen infection: A review. ISRN Biochem. 2013, 2013, 762412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, J.D.G.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boller, T.; Felix, G. A renaissance of elicitors: Perception of micro-be-associated molecular patterns and danger signals by pattern-recognition receptors. Annu. Rev. Plant Biol. 2009, 60, 379–406. [Google Scholar] [CrossRef] [PubMed]
- Henry, G.; Thonart, P.; Ongena, M. PAMPs, MAMPs, DAMPs and others: An update on the diversity of plant immunity elicitors. Biotechnol. Agron. Soc. Environ. 2012, 16, 257–268. [Google Scholar]
- Schwessinger, B.; Ronald, P.C. Plant innate immunity: Perception of conserved microbial signatures. Annu. Rev. Plant. Biol. 2012, 63, 451–482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ranf, S. Sensing of molecular patterns through cell surface immune receptors. Curr. Opin. Plant. Biol. 2017, 38, 68–77. [Google Scholar] [CrossRef]
- Bigeard, J.; Colcombet, J.; Hirt, H. Signaling mechanisms in pattern—Triggered immunity (PTI). Mol. Plant 2015, 8, 521–539. [Google Scholar] [CrossRef] [Green Version]
- Conrath, U.; Beckers, G.J.; Flors, V.; García-Agustín, P.; Jakab, G.; Mauch, F.; Newman, M.A.; Pieterse, C.M.; Poinssot, B.; Pozo, M.J.; et al. Priming: Getting ready for battle. Mol. Plant Microbe. Interact. 2006, 19, 1062–1071. [Google Scholar] [CrossRef] [Green Version]
- Pastor, V.; Balmer, A.; Gamir, J.; Flors, V.; Mauch-Mani, B. Preparing to fight back: Generation and storage of priming compounds. Front. Plant Sci. 2014, 5, 295. [Google Scholar] [CrossRef] [Green Version]
- Wiesel, L.; Newton, A.C.; Elliott, I.; Booty, D.; Gilroy, E.M.; Birch, P.R.J.; Hein, I. Molecular effects of resistance elicitors from biological origin and their potential for crop protection. Front. Plant Sci. 2014, 5, 655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curtis, H.; Noll, U.; Störmann, J.; Slusarenko, A.J. Broad spectrum activity of the volatile phytoanticipin allicin in extracts of garlic (Allium sativum L.) against plant pathogenic bacteria, fungi and Oomycetes. Physiol. Mol. Plant Pathol. 2004, 65, 79–89. [Google Scholar] [CrossRef]
- Slusarenko, A.J.; Patel, A.; Portz, D. Control of plant diseases by natural products: Allicin from garlic as a case study. Eur. J. Plant Pathol. 2008, 121, 313–322. [Google Scholar] [CrossRef]
- Jamiołkowska, A.; Wagner, A. Effect of Garlic Pulp (Bioczos Plynny) on Some Fungi Pathogenic to Vegetables. In Proceedings of the Fourth International Conference on Non-Chemical Crop Protection Methods, Lille, France, 8–11 March 2011; AFPP: Harrogate, UK, 2011; pp. 213–220. [Google Scholar]
- Jamiołkowska, A. Preparaty Biotechniczne i Biologiczne w Ochronie Papryki Słodkiej (Capsicum Annuum L.) Przed Grzybami Chorobotwórczymi i Indukowaniu Reakcji Obronnych Roślin, 1st ed.; Rozprawy Naukowe UP w Lublinie, University of Life Sciences in Lublin: Lublin, Poland, 2013; Volume 379, p. 117. Available online: https://www.up.lublin.pl/5490/ (accessed on 13 November 2019).
- Göellner, K.; Conrath, U. Priming: it’s all the world to induced disease resistance. Eur. J. Plant Pathol. 2008, 121, 233–242. [Google Scholar] [CrossRef]
- Pohl, A.; Kalisz, A.; Sękara, A. Seaweed extracts’ multifactorial action: Influence on physiological and biochemical status of Solanaceae plants. Acta Agrobot. 2019, 72, 1–11. [Google Scholar] [CrossRef]
- Koziara, W.; Sulewska, H.; Panasiewicz, K. Effect of resistance stymulator application to some agricultural crops. J. Res. Appl. Agric. Eng. 2006, 51, 82–87. [Google Scholar]
- Céliz, G.; Daz, M.; Audisio, M.C. Antibacterial activity of naringin derivatives against pathogenic strains. J. App. Microbiol. 2011, 111, 731–738. [Google Scholar] [CrossRef] [Green Version]
- Saniewska, A.; Jarecka, A. Influence of endogenous grapefruit flavonoids (Citrus paradisi Macf.) on the growth and of development of two special forms of Fusarium oxysporum Schlecht. Prog. Plant Prot. 2006, 46, 517–520. [Google Scholar]
- Jamiołkowska, A. Laboratory effect of azoxystrobin (Amistar 250 SC) and grapefruit extract (Biosept 33SL) on growth of fungi colonizing zucchini plants. Acta Sci. Pol-Hortoru. 2011, 10, 245–257. [Google Scholar]
- Sadowski, C.; Lenc, L.; Koala, W. Investigations on the possibility of protection of organically grown red beet against fungal diseases. J. Res. Appl. Agric. Eng. 2007, 52, 38–44. [Google Scholar]
- Angelini, P.; Pagiotti, R.; Meghini, A.; Vianello, B. Antimicrobial activities of various essential oils against foodborne pathogenic or spoilage moulds. Ann. Microbiol. 2006, 56, 65–69. [Google Scholar] [CrossRef]
- Terzi, V.; Morcia, C.; Faccioli, P.; Vale, G.; Tacconi, G.; Malnati, M. In vitro antifungal activity of tea tree (Malaleuca alternifolia) essential oil and its major components against pathogens. Lett. Appl. Microbiol. 2007, 44, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Wang, J.; Shao, X.; Xu, F.; Wang, H. Antifungal modes of action of tea tree oil and its two characteristic components against Botrytis cinerea. J. App. Microbiol. 2015, 119, 1253–1262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Włodarek, A.; Robak, J. Możliwości stosowania środków pochodzenia naturalnego w ochronie sałaty w uprawie polowej i pod osłonami przed chorobami. Zesz. Nauk. Inst. Ogrod. 2013, 21, 43–47. [Google Scholar]
- Jimenez, E.; Dorta, F.; Medina, C.; Ramirez, A.; Ramirez, I.; Pena-Cortes, H. Anti-pathogenic activities of macro-algae extracts. Mar. Drugs 2011, 9, 739–756. [Google Scholar] [CrossRef] [Green Version]
- Sultana, V.; Esteshamul-Haque, S.; Ara, J. Management of root diseases of soybeen and tomato with seaweed application. Phytopathoiogy 2007, 97, 112. [Google Scholar]
- Jolivet, E.; Landlais-Jeannin, I.; Morot-Gaudry, J.F. Extracts of marine algae: Phyto-active properties and effects on cholesterol metabolism. Nutri. Res. 1991, 20, 585–598. [Google Scholar]
- Kurita, K. Chitin and Chitosan: Functional Biopolymers from Marine Crustaceans. Mar. Biotechnol. 2006, 8, 204–225. [Google Scholar] [CrossRef]
- El Hadrami, A.; Adam, L.R.; El Hadrami, I.; Daayf, F. Chitosan in plant protection. Mar. Drugs. 2010, 8, 968–987. [Google Scholar] [CrossRef]
- Cao, Y.; Liang, Y.; Tanaka, K.; Nguyen, C.T.; Jedrzejczak, R.P.; Joachimiak, A.; Stacey, G. The kinaze LYK5 is major chitin receptor in Arabidopsis and forms a chitin-induced complex with related kinase CERK1. Elife 2014, 3, e03766. [Google Scholar] [CrossRef]
- Hayafune, M.; Berisio, R.; Marchetti, R.; Silipo, A.; Kayama, M.; Desaki, Y.; Arima, S.; Squeglia, F.; Ruggiero, A.; Tokuyasu, K.; et al. Chitin-induced activation of immune signalling by the rice receptor CEBiP relies on a unique sandwich-type dimerization. Proc. Nat. Acad. Sci. USA 2014, 111, E404–E413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gai, O.Y.; Jiao, J.; Wang, X.; Liu, J.; Wang, Z.Y.; Fu, Y.J. Chitosan promoting formononetin and calycoisn accumulation in Astragalus membranaceus hairy root cultures via mitogen-activated protein kinase signaling cascades. Sci. Rep. 2019, 9, 10367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sealy, R.; Evans, M.R.; Rothrock, C. The effect of garlic extract and root substrate on soilborne fungal pathogens. HortTechnology 2007, 17, 169–173. [Google Scholar] [CrossRef] [Green Version]
- Patkowska, E. The use of biopreparations in the control of soybean endangered by pathogenic soil-borne fungi. Electron. J. Pol. Agric. Univ. 2006, 9. Available online: http://www.ejpau.media.pl/volume9/issue1/art-19.html (accessed on 13 November 2019).
- Salas, M.P.; Céliz, G.; Geronazzo, H.; Daz, M.; Resnik, S.L. Antifungal activity of natural and enzymatically-modified flavonoids isolated from citrus species. Food Chem. 2011, 124, 1411–1415. [Google Scholar] [CrossRef]
- Riccioni, L.; Orzali, L. Activity of tea tree (Malaleuca alternifolia, Chell) and thyme (Thymus vulgaris L.) essential oils against some pathogenic seed borne fungi. J. Essent. Oil Res. 2012, 23, 43–47. [Google Scholar] [CrossRef]
- Horoszkiewicz-Janka, J.; Michalski, T. Wpływ biopreparatów Bion 50 WG i Bio-algeen S 90 Plus 2 na zdrowotność i plonowanie jęczmienia jarego uprawianego w siewie czystym i w mieszankach z owsem. Biuletyn Instytutu Hodowli i Aklimatyzacji Roślin 2004, 234, 347–356. [Google Scholar]
- Vera, J.; Castro, J.; Contreras, R.A.; Gonzalez, A.; Moenne, A. Oligo-carrageenans induce a long-term and broad-range protection against pathogens in tobacco plants (var. Xanthi). Physiol. Mol. Plant Pathol. 2012, 79, 31–39. [Google Scholar] [CrossRef]
- Shukla, P.S.; Borza, T.; Critchley, A.T.; Prithiviray, B. Carrageeans from Red Seaweeds as Promoters of Growth and Elicitors of Defense response in Plants. Front. Mar. Sci. 2016, 3, 81. [Google Scholar] [CrossRef]
- Arunkumar, K.; Rengasamy, R. Evaluation of Antibacterial Potential of Seaweeds Occurring along the Coast of Tamil Nadu, India against the Plant Pathogenic Bacterium Xanthomonas oryzae pv. oryzae (Ishiyama) Dye. Bot. Mar. 2005, 435, 409–415. [Google Scholar] [CrossRef]
- Mazur, S.; Waksmundzka, A. Effect of some compounds on the decay of strawberry fruits caused by Botrytis cinerea Pers. Meded. Rijksuniv. Gent. Fak. Landbouwkd. Toegep. Biol. Wet. 2001, 66, 227–231. [Google Scholar] [PubMed]
- Szczeponek, A.; Mazur, S.; Nawrocki, J. The Usage of Chitosan in Protection of Some Peppermint and Lemon Balm Pathogens; Polish Chitin Society: Łódź, Poland, 2006; pp. 193–200. [Google Scholar]
- Chirkov, A.; Ina, V.I.; Surgucheva, W.A.; Letunova, E.V.; Yuvaritsev, A.A.; Tatarino, N.Y.; Varlamov, V.P. Effect of chitosan on systemic Viral Infection and some defence responses in potato plants. Russian J. Plant Physiol. 2001, 48, 774–779. [Google Scholar] [CrossRef]
- Walters, D.; Newton, A.; Lyon, G. Induced Resistance for Plant Defense: A Sustainable Approach to Crop Protection; Blackwell Publishing Ltd.: Oxford, UK, 2007; p. 258. [Google Scholar]
- Gayoso, C.; Pomar, F.; Merino, F.; Bernal, M.A. Oxidative metabolism and phenolic compounds in Capsicum annuum L. var. annuum infected by Phytophthora capsici Leon. Sci. Hortic. 2004, 102, 1–13. [Google Scholar] [CrossRef]
- Panina, Y.; Fravel, D.R.; Baker, C.J.; Shcherbakova, A. Biocontrol and plant pathogenic Fusarium oxysporum—Induced changes in phenolic compounds in tomato leaves and roots. J. Phytopathol. 2007, 155, 475–481. [Google Scholar] [CrossRef]
- Hassan, S.; Mathesius, U. The role of flavonoids in root-rhizosphere signaling: Opportunities and challenges for improving plant-microbe interaction. J. Exp. Bot. 2012, 63, 3429–3444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dakora, F.D. Plant flavonoids: Biological molecules for useful exploitation. Aust. J. Plant. Physiol. 1995, 22, 87–99. [Google Scholar] [CrossRef]
- Garcion, C.; Lamotte, O.; Métraux, J.P. Mechanism of defence to pathogens: Biochemistry and physiology. In Induced Resistance for Plant Defense: A Sustainable Approach to Crop Protection, 1st ed.; Walters, D., Newton, A., Lyon, G., Eds.; Blackwell Publishing Ltd.: Oxford, UK, 2007; pp. 109–132. [Google Scholar]
- Klarzynski, O.; Plesse, B.; Joubert, J.M.; Yvin, J.C.; Kopp, M.; Kloareg, B.; Fritig, B. Linear beta-1,3-glucans are elicitors of defense responses in tobacco. Plant Physiol. 2000, 124, 1027–1038. [Google Scholar] [CrossRef] [Green Version]
- Angioni, A.; Cabras, P.; Hallewing, G.; Pirisi, F.M.; Schirra, M. Synthesis and inhibitory activity of 7-geranoxycumarin agains Penicillium species in citrus fruit. Phytochemistry 1998, 47, 1521. [Google Scholar] [CrossRef]
- De Lorenzo, G.; Ferrari, S.; Giovannoi, M.; Mattei, B.; Cervone, F. Cell wall traits that influence development, immunity, and bioconversion. Plant J. 2019, 97, 134–147. [Google Scholar] [CrossRef]
- Hou, S.; Liu, Z.; Shen, X.; Wu, D. Damage-Associated Molecular Pattern-Triggered Immunity in Plants. Front. Plant Sci. 2019, 10, 646. [Google Scholar] [CrossRef]
- Buxdorf, K.; Rubinsky, G.; Barda, O.; Burdman, S.; Aharoni, A.; Levy, M. The transcription factor SISHINE3 modulates defense responses in tomato plants. Plant Mol. Biol. 2014, 84, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Plich, J. Resistance of the potato to Phytophthora infestans and its relations to earliness of cultivars—A review. Biuletyn Instytutu Hodowli i Aklimatyzacji Roślin 2007, 246, 61–71. [Google Scholar]
- Boudet, A.M. Lignins and lignification: Selected issues. Plant Physiol. Biochem. 2000, 38, 81–96. [Google Scholar] [CrossRef]
- Egea, C.; Ahmed, A.S.; Candela, M.; Candela, M.F. Elicitation of peroxidase activity and lignin biosynthesis in pepper suspension cells by Phytophthora capsici. J. Plant Physiol. 2001, 158, 151–158. [Google Scholar] [CrossRef]
- Humphreys, J.M.; Chapple, C. Rewriting the lignin roadmap. Curr. Opin. Plant Biol. 2002, 5, 224–229. [Google Scholar] [CrossRef]
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in Plant Science: A Global Perspective. Front. Plant Sci. 2017, 7, 2049. [Google Scholar] [CrossRef] [Green Version]
- Craigie, J.S. Seaweed extract stimuli in plant science and agriculture. J. Appl. Phycol. 2011, 23, 371–393. [Google Scholar] [CrossRef]
- Lötze, E.; Hoffman, E.W. Nutrient composition and content of various biological active compounds of three South African-based commercial seaweed biostimulants. J. Appl. Phycol. 2016, 28, 1379–1386. [Google Scholar] [CrossRef]
- Torre, L.A.; Battaglia, V.; Caradonia, F. An overview of the current plant biostimulant legislations in different European Member States. J. Sci. Food Agric. 2016, 96, 727–734. [Google Scholar] [CrossRef]
- Traon, D.; Amat, L.; Zotz, F.; du Jardin, P. A Legal Framework for Plant Biostimulants and Agronomic Fertiliser Additives in the EU–Report to the European Commission. DG Enterp. Ind. 2014, 115. [Google Scholar]
- Villaverde, J.J.; Sandin-Espấna, P.; Sevilla-Morañ, B.; López-Goti, C.; Alonso-Prados, J.L. Biopesticides from natural products: Current development, legislative framework and future trends. BioResources 2016, 11, 5618–5640. [Google Scholar] [CrossRef] [Green Version]

| Origin | Species Name | Form | Bioactive Natural Compound | Effective Against | Effects Shown | References |
|---|---|---|---|---|---|---|
| Vascular Plants | Allium spp. | garlic pulp | allicin | Agrobacterium tumefaciens, Pseudomonas syringae pv. maculicola, P. syringae pv. phaseolicola, Ervinia carotovora, Escherichia coli | in vitro | [24,25] |
| Alternaria alternata, Botrytis cinerea, Colletotrichum coccodes, Rhizoctonia solani | sweet pepper, strawberry | [6,26,27] | ||||
| Pythium aphanidermatum, Phytophthora infestans | in vitro, potato tubers | [24,25,47] | ||||
| Citrus spp. | grapefruit extract | naringin | Gram-positive pathogenic bacteria | in vitro | [31] | |
| Cercospora arachidicola | onion | [7] | ||||
| Pythium oligandrum, soil-borne fungi | bean, pea soybean | [48] | ||||
| Fusarium culmorum, F. oxysporum, F. solani, Rhizoctonia solani, Sclerotinia sclerotiorum | common bean, pea | [2] | ||||
| Fusarium semitectum, Aspergillus flavus, Aspergillus parasiticus, Penicillium expansum | in vitro | [49] | ||||
| Malaleuca alternifolia L. | tea tree oil | terpenes | Xanthomonas vesicatoria | in vitro | [4] | |
| terpenes (α-terpineol, terpinolene, 1,8-cineole) | Botrytis cinerea, Aspergillus fumigatus, Chaetomium globosum, Penicillium chrysogenum | in vitro | [35,37] | |||
| terpinen-4-ol, γ-terpinen, 1,8-cineole | Blumeria graminis f. sp. hordei, Fusarium graminearum, F. culmorum, Pyrenophora graminea | in vitro | [36] | |||
| terpenes | Ascochyta rabiei, Colletotrichum lindemuthianum, Drechslera avenae, Alternaria radicina, A. dauci | in vitro | [50] | |||
| Protists | brown algae (Ascophyllum nodosum) | algae extract | β-1,3-glucan laminarin | Pyrenophora teres, Rhynchosporium secalis, Puccinia hordei | spring barley, oat | [51] |
| brown algae (Laminaria digitata) | Botrytis cinerea, Plasmopara viticola | grapevine | [13] | |||
| several species of marine algae | Staphylococcus aureus, Escherichia coli, Bacillus subtilis, Streptococcus aureus, Proteus subtilis | several plants | [1] | |||
| pathogenic fungi, Rhizoctonia solani, Botrytis cinerea, Phytophthora cinnamomi | several strawberry | [1,39] | ||||
| marine algae (Spatoglossum variabile, Melanothamnus afaqhusainii, Halimeda tuna) | Macrophomina phaseolina, Fusarium solani, Rhizoctonia solani, Verticillium spp. | sunflower | [9] | |||
| red algae (Chondrus crispus, Gigartina stellata) | carrageenans | Tobacco mosaic virus (TMV), Pectobacterium carotovorum, Botrytis cinerea | tobacco | [52,53] | ||
| seaweeds | Xanthomonas oryzae pv. oryzae | in vitro | [54] | |||
| Animals | marine crustaceans (Crustacea) | chitosan, chitin (MAMPs) | Botrytis cinerea | strawberry | [55] | |
| Alternaria alternata, Botrytis cinerea, Fusarium spp., Pythium spp., Rhizoctonia solani | peppermint, soybean | [3,56] | ||||
| Sclerotinia sclerotiorum, Cladosporium cladosporioides | lemon balm, peppermint | [56] | ||||
| pathogenic bacteria and fungi | several plants | [43] |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jamiołkowska, A. Natural Compounds as Elicitors of Plant Resistance Against Diseases and New Biocontrol Strategies. Agronomy 2020, 10, 173. https://doi.org/10.3390/agronomy10020173
Jamiołkowska A. Natural Compounds as Elicitors of Plant Resistance Against Diseases and New Biocontrol Strategies. Agronomy. 2020; 10(2):173. https://doi.org/10.3390/agronomy10020173
Chicago/Turabian StyleJamiołkowska, Agnieszka. 2020. "Natural Compounds as Elicitors of Plant Resistance Against Diseases and New Biocontrol Strategies" Agronomy 10, no. 2: 173. https://doi.org/10.3390/agronomy10020173
APA StyleJamiołkowska, A. (2020). Natural Compounds as Elicitors of Plant Resistance Against Diseases and New Biocontrol Strategies. Agronomy, 10(2), 173. https://doi.org/10.3390/agronomy10020173