Paclobutrazol Application Favors Yield Improvement of Maize Under Semiarid Regions by Delaying Leaf Senescence and Regulating Photosynthetic Capacity and Antioxidant System During Grain-Filling Stage
Abstract
1. Introduction
2. Materials and Methods
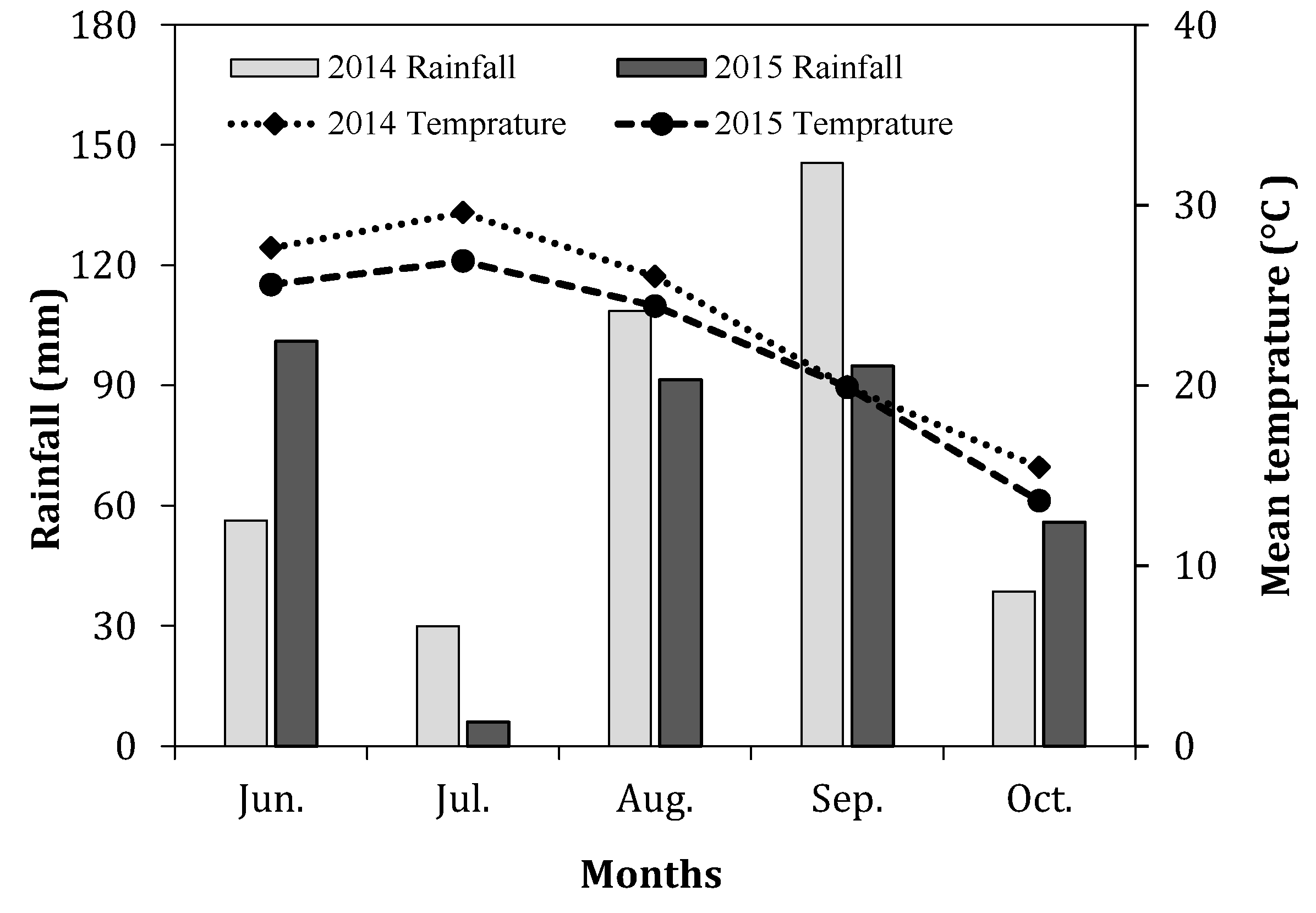
2.1. Site Description
2.2. Seed Treatment and Experimental Design
2.3. Plants Sampling and Measurements
2.3.1. Determination of Green Leaf Area and Senescence Rate
2.3.2. Assay of Chlorophyll and Carotenoid Content
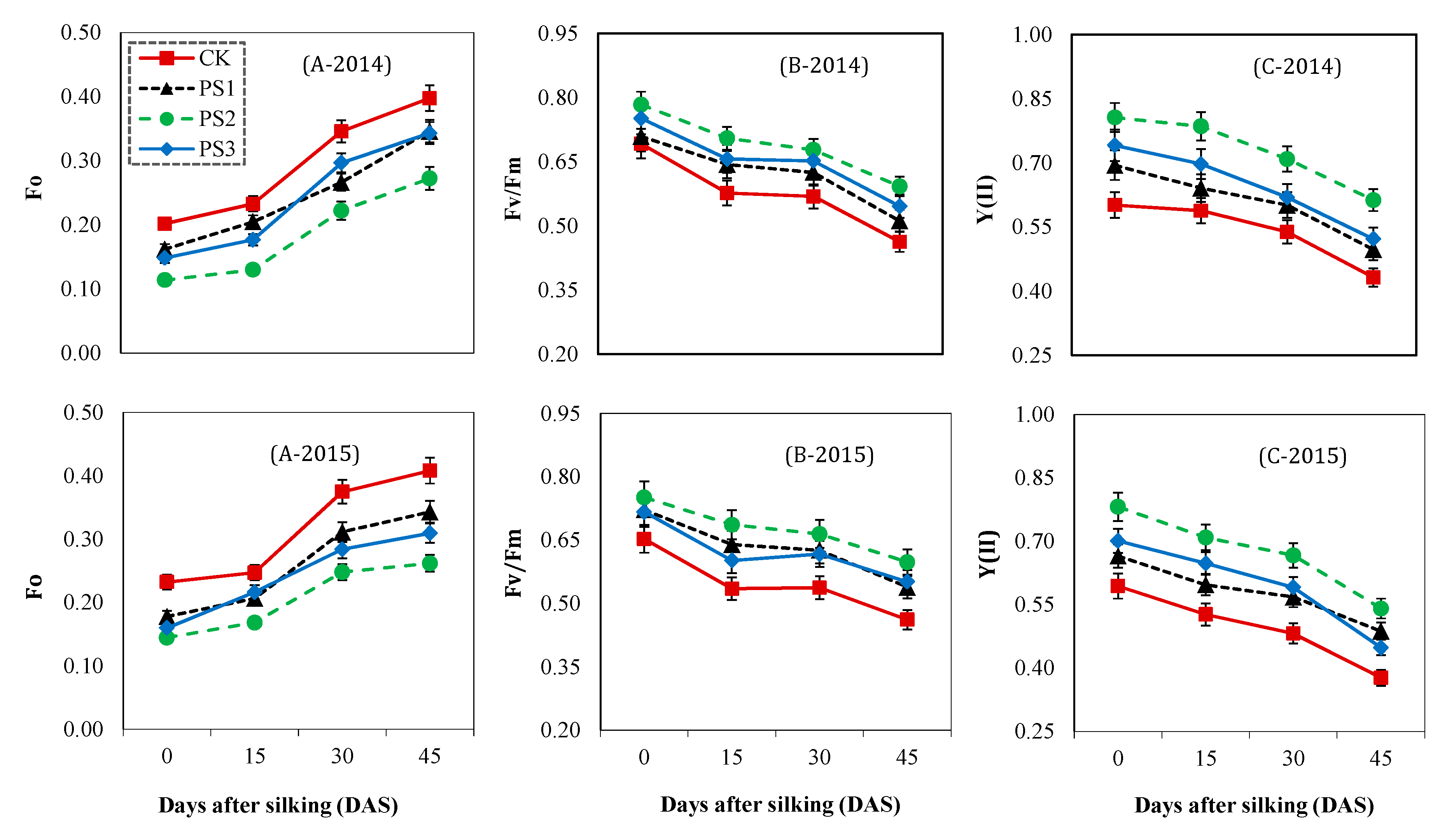
2.3.3. Net Photosynthetic Rate and Chlorophyll Fluorescence Analysis
2.3.4. Preparation of Enzyme Extracts and Assay of Antioxidant Enzymes
2.3.5. Estimation of Soluble Sugars and Protein
2.3.6. Determination of Malonaldehyde (MDA) and Reactive Oxygen Species (ROS)
2.3.7. Grain Yield and Yield Components
2.4. Statistical Analysis
3. Results
3.1. Ear Size
3.2. Grain Yield And Yield Components
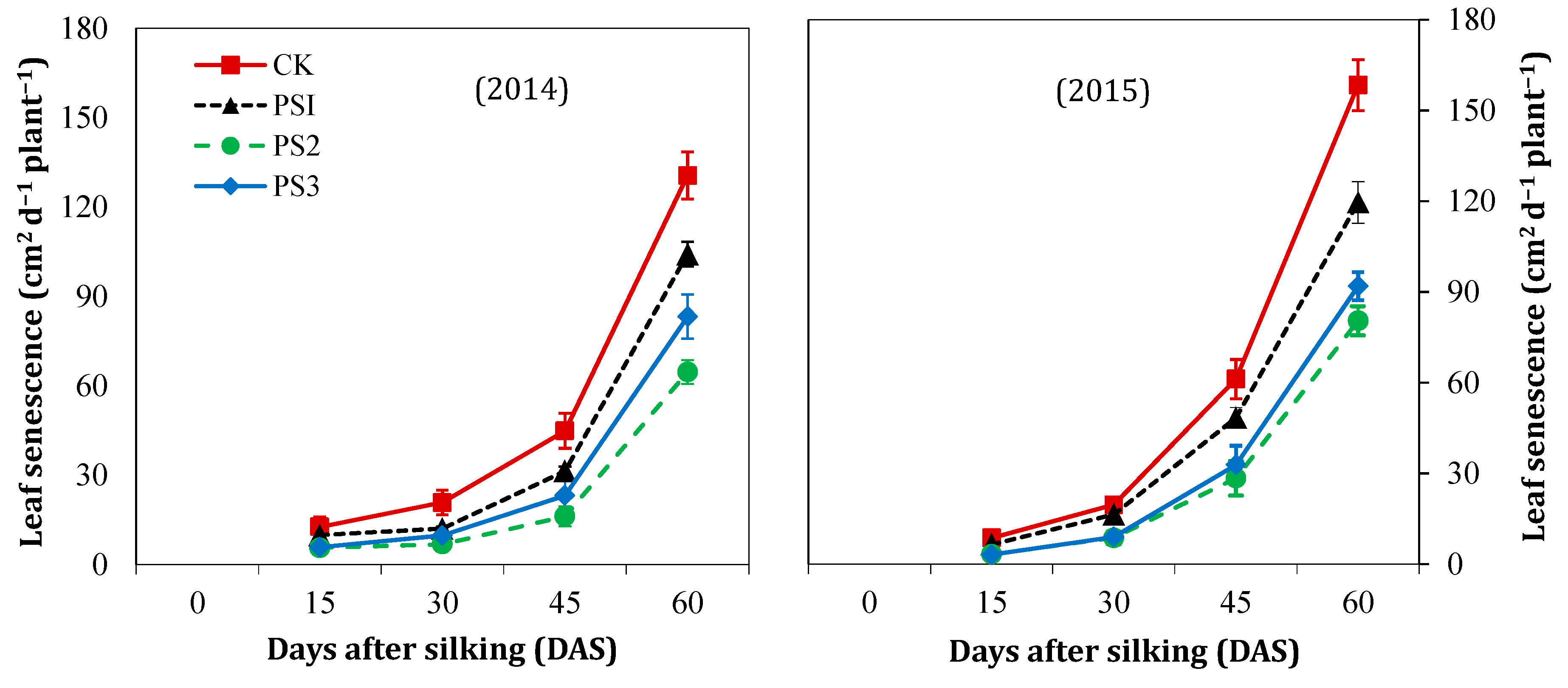
3.3. Leaf Senescence Rate
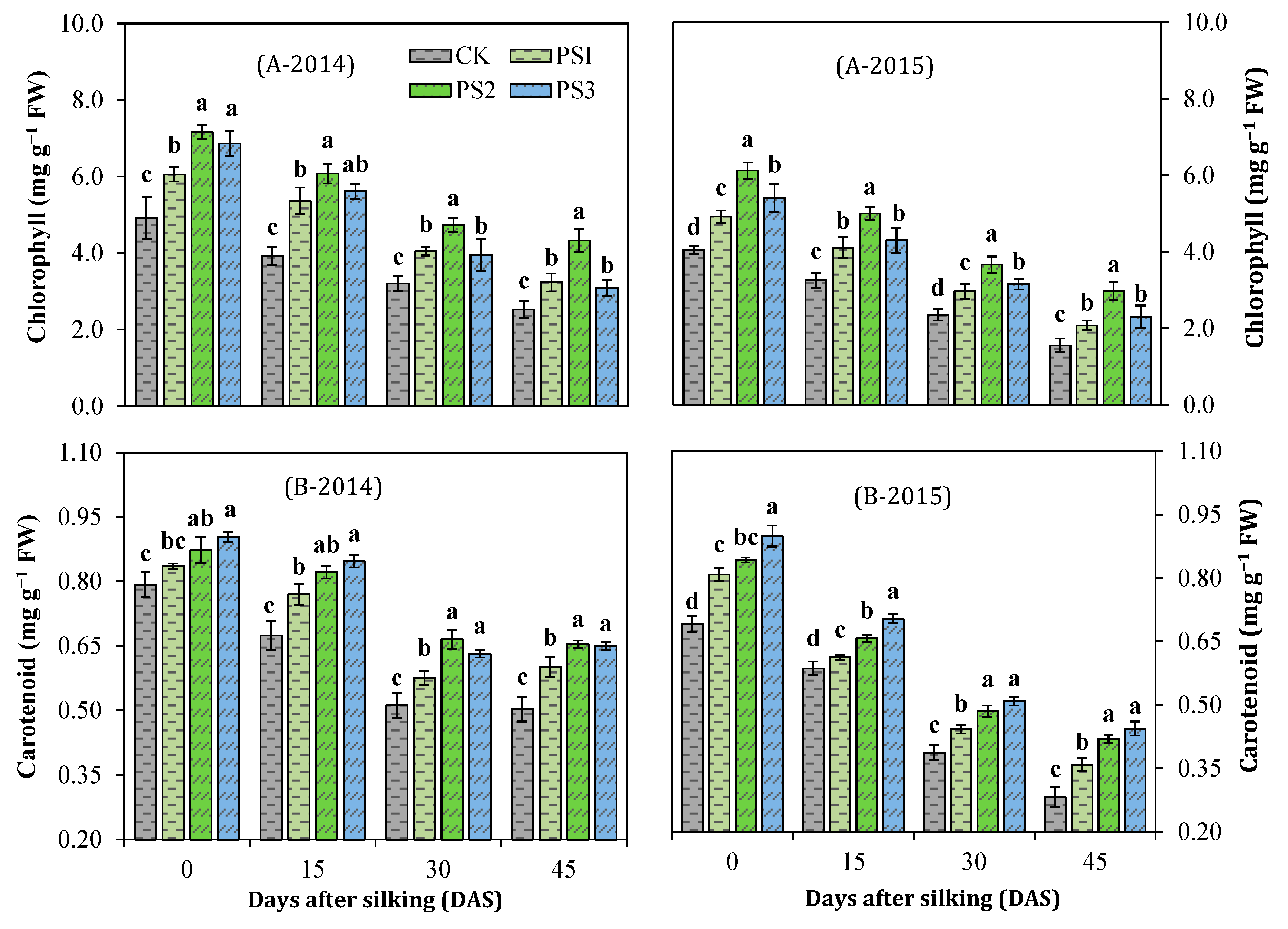
3.4. Effect of Paclobutrazol on Photosynthetic Pigments
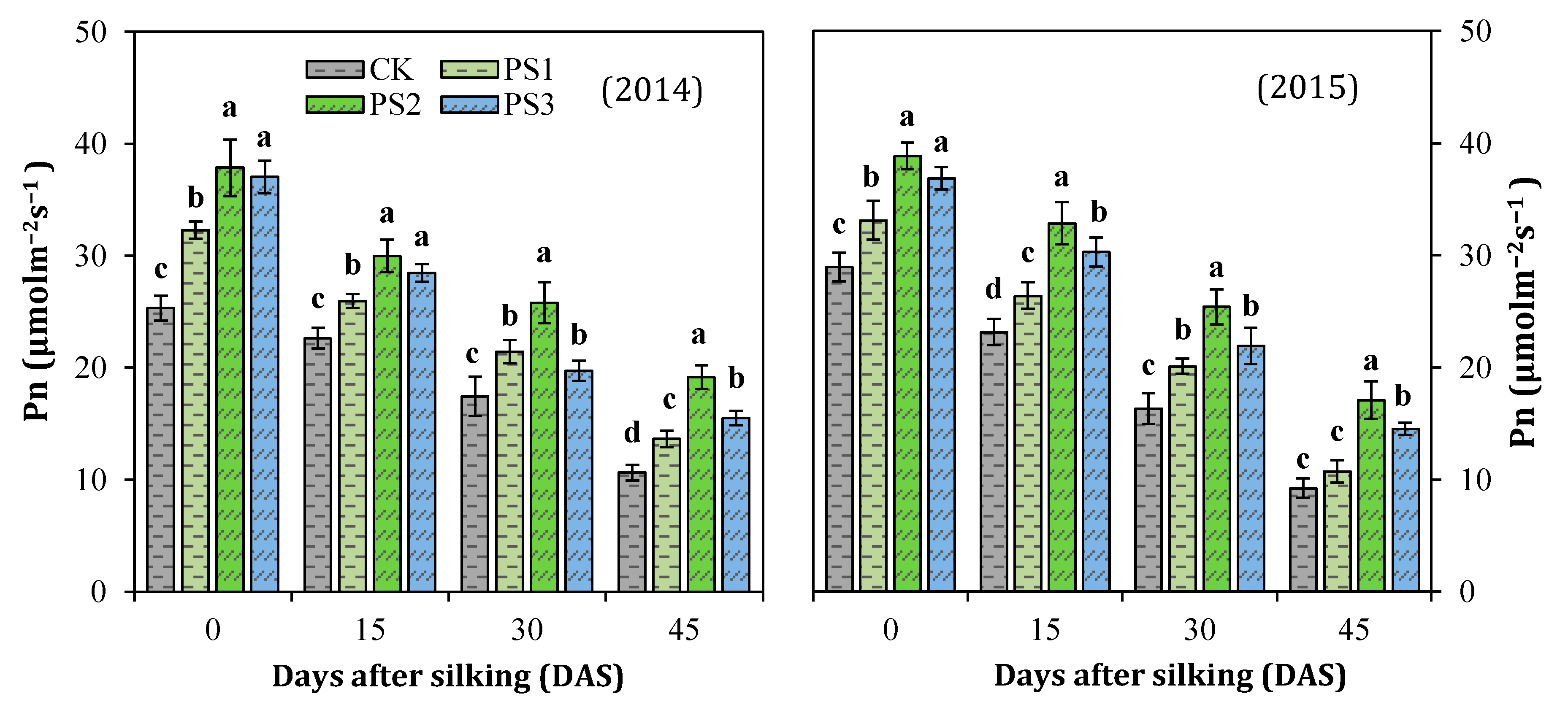
3.5. Net Photosynthesis Rate
3.6. Chlorophyll Fluorescence
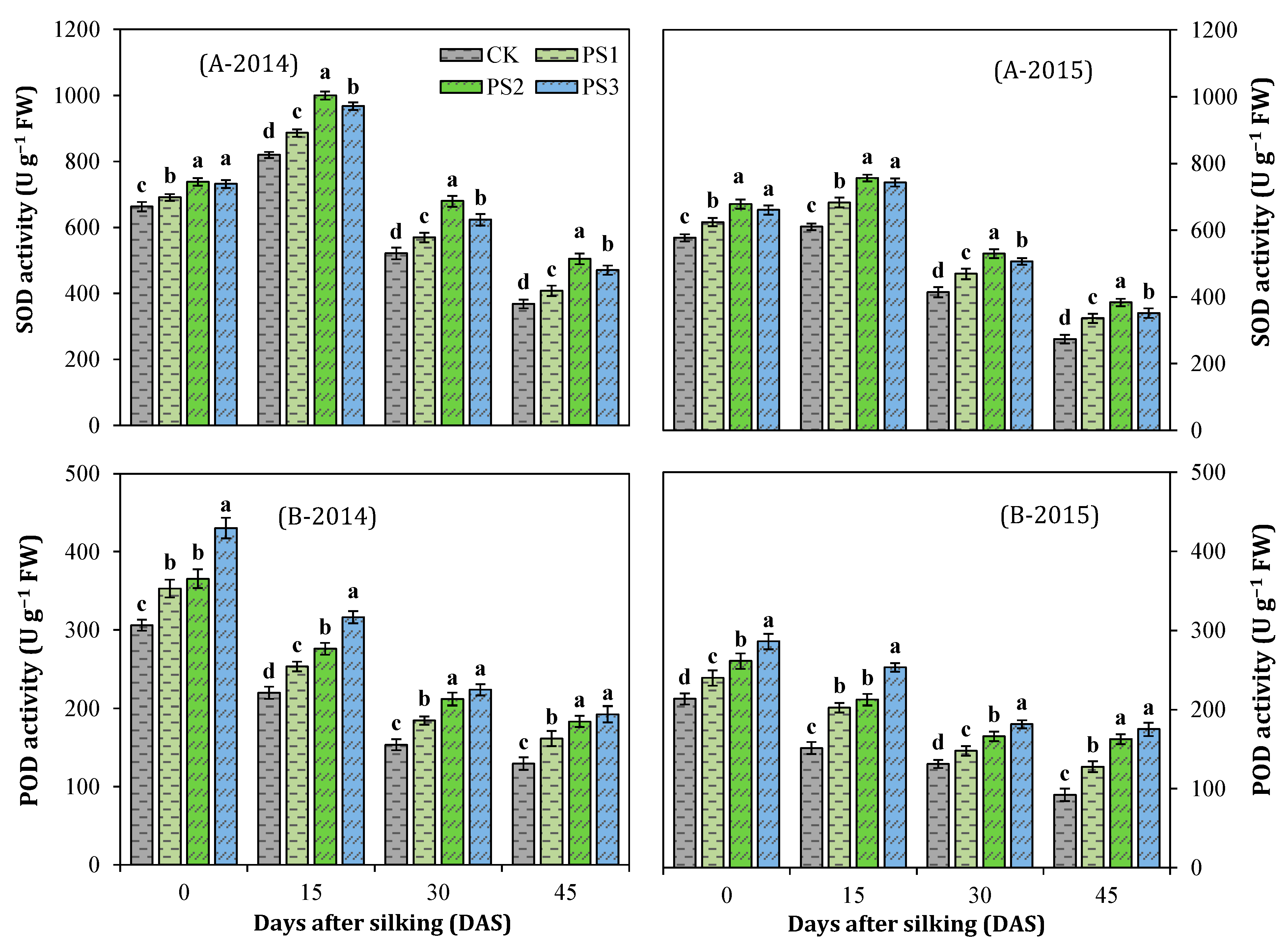
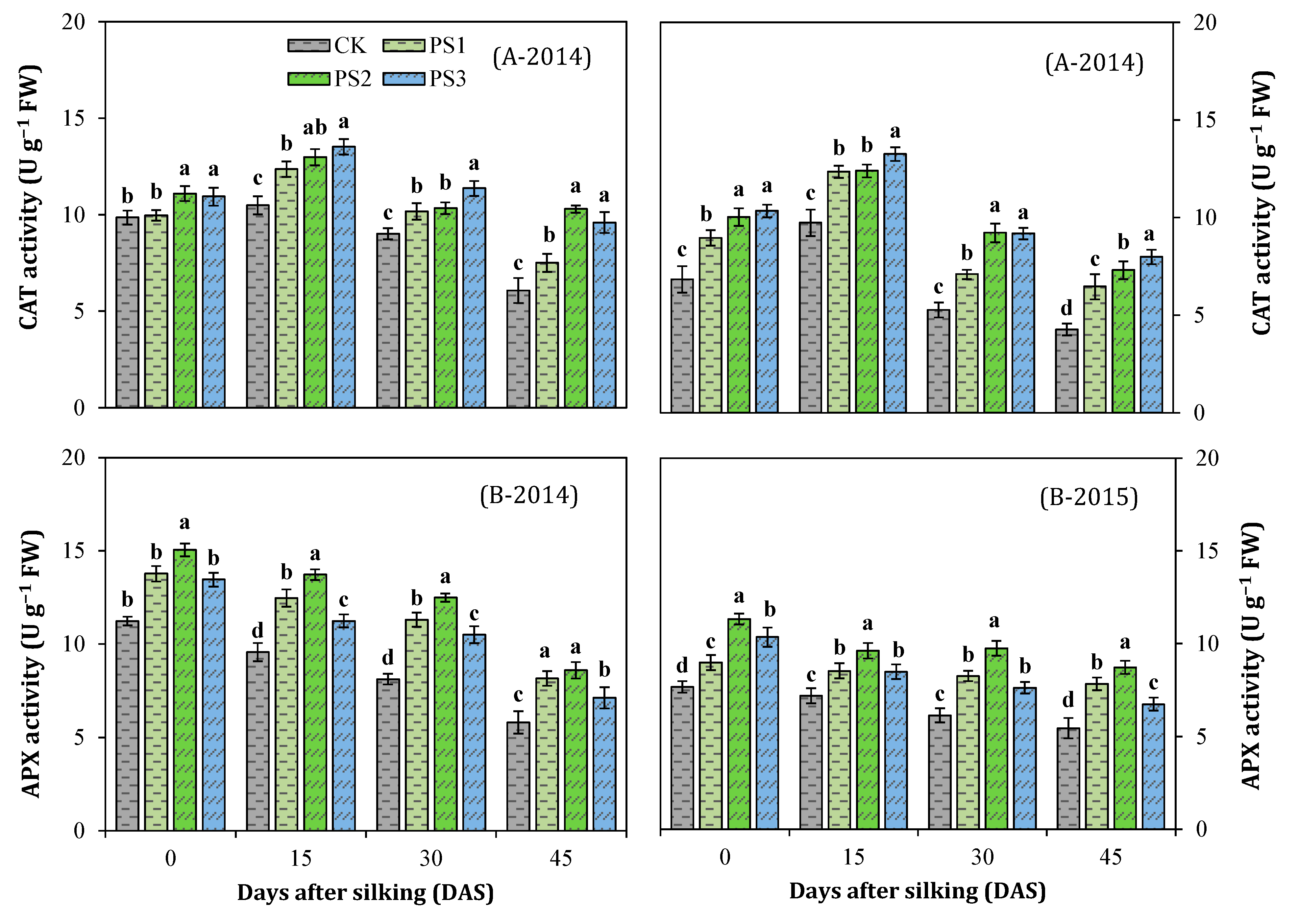
3.7. Activities of Antioxidant Enzymes
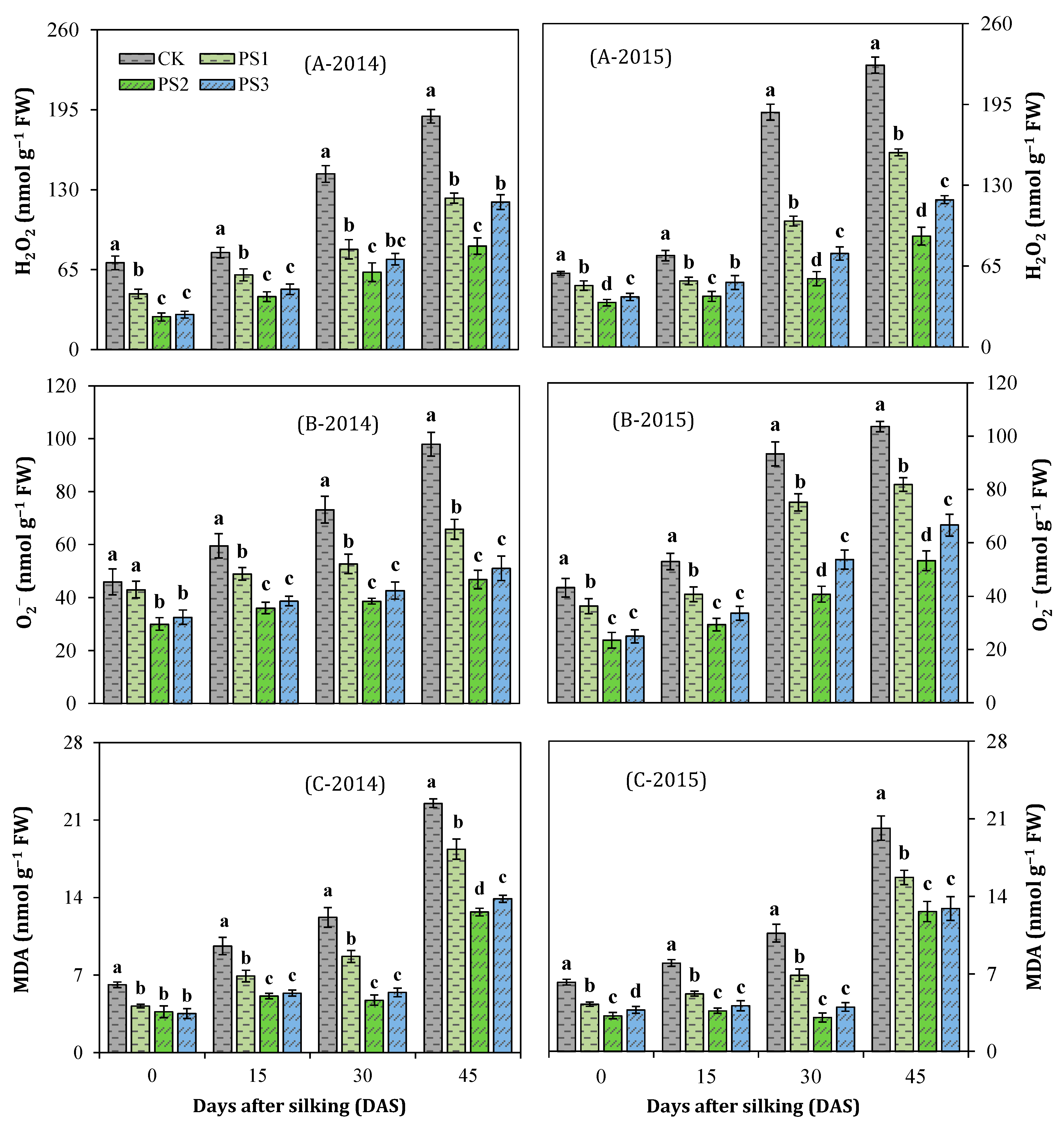
3.8. Reactive Oxygen Species (ROS) Accumulation and Lipid Peroxidation
3.9. Soluble Protein and Soluble Sugar
3.10. Correlation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Xu, C.; Gao, Y.; Tian, B.; Ren, J.; Meng, Q.; Wang, P. Effects of EDAH, a novel plant growth regulator, on mechanical strength, stalk vascular bundles and grain yield of summer maize at high densities. Field Crops Res. 2017, 200, 71–79. [Google Scholar] [CrossRef]
- Waqas, M.A.; Khan, I.; Akhter, M.J.; Noor, M.A.; Ashraf, U. Exogenous application of plant growth regulators (PGRs) induces chilling tolerance in short-duration hybrid maize. Env. Sci. Pollut. Res. 2017, 24, 11459–11471. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.P.; Shan, L.; Zhang, H.P.; Turner, N.C. Improving agricultural water use efficiency in arid and semiarid areas of China. Agric. Water Manag. 2006, 80, 23–40. [Google Scholar] [CrossRef]
- Anjum, S.A.; Wang, L.C.; Farooq, M.; Hussain, M.; Xue, L.L.; Zou, C.M. Brassinolide application improves the drought tolerance in maize through modulation of enzymatic antioxidants and leaf gas exchange. J. Agron. Crop Sci. 2011, 197, 177–185. [Google Scholar] [CrossRef]
- Ahmad, I.; Kamran, M.; Su, W.; Haiqi, W.; Ali, S.; Bilegjargal, B.; Ahmad, S.; Liu, T.; Cai, T.; Han, Q. Application of uniconazole improves photosynthetic efficiency of maize by enhancing the antioxidant defense mechanism and delaying leaf senescence in semiarid regions. J. Plant Growth Regul. 2019, 38, 855–869. [Google Scholar] [CrossRef]
- Gao, Z.; Liang, X.G.; Zhang, L.; Lin, S.; Zhao, X.; Zhou, L.L.; Shen, S.; Zhou, S.L. Spraying exogenous 6-benzyladenine and brassinolide at tasseling increases maize yield by enhancing source and sink capacity. Field Crops Res. 2017, 211, 1–9. [Google Scholar] [CrossRef]
- Ajigboye, O.O.; Murchie, E.; Ray, R.V. Foliar application of isopyrazam and epoxiconazole improves photosystem II efficiency, biomass and yield in winter wheat. Pestic. Biochem. Physiol. 2014, 114, 52–60. [Google Scholar] [CrossRef]
- Wang, Y.; Wanrong, G.; Tenglong, X.; Lijie, L.; Yang, S.; He, Z.; Jing, L.; Shi, W. Mixed compound of DCPTA and CCC increases maize yield by improving plant morphology and up-regulating photosynthetic capacity and antioxidants. Plos ONE 2016, 11, e0149404. [Google Scholar] [CrossRef]
- Wang, F.; Liu, J.; Zhou, L.; Pan, G.; Li, Z.; Zaidi, S.H.R.; Cheng, F. Senescence-specific change in ROS scavenging enzyme activities and regulation of various SOD isozymes to ROS levels in psf mutant rice leaves. Plant Physiol. Biochem. 2016, 109, 248–261. [Google Scholar] [CrossRef]
- Borrás, L.; Maddonni, G.A.; Otegui, M.E. Leaf senescence in maize hybrids: Plant population, row spacing and kernel set effects. Field Crops Res. 2003, 82, 13–26. [Google Scholar] [CrossRef]
- Zhao, H.; Dai, T.; Jing, Q.; Jiang, D.; Cao, W. Leaf senescence and grain filling affected by post-anthesis high temperatures in two different wheat cultivars. Plant Growth Regul. 2007, 51, 149–158. [Google Scholar] [CrossRef]
- Luo, Z.; Guan, H.; Zhang, X.; Liu, N. Photosynthetic capacity of senescent leaves for a subtropical broadleaf deciduous tree species Liquidambar formosana Hance. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yamori, W.; Noguchi, K.O.; Hikosaka, K.; Terashima, I. Phenotypic plasticity in photosynthetic temperature acclimation among crop species with different cold tolerances. Plant Physiol. 2010, 152, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.X.; Von Tiedemann, A. Physiological effects of azoxystrobin and epoxiconazole on senescence and the oxidative status of wheat. Pestic. Biochem. Physiol. 2001, 71, 1–10. [Google Scholar] [CrossRef]
- Dhindsa, R.S.; Plumb-dhindsa, P.; Thorpe, T.A. Leaf Senescence: Correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase content. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]
- Wang, P.; Sun, X.; Li, C.; Wei, Z.; Liang, D.; Ma, F. Long-term exogenous application of melatonin delays drought-induced leaf senescence in apple. J. Pineal Res. 2013, 54, 292–302. [Google Scholar] [CrossRef]
- Gill, S.S.; Khan, N.A.; Tuteja, N. Cadmium at high dose perturbs growth, photosynthesis and nitrogen metabolism while at low dose it up regulates sulfur assimilation and antioxidant machinery in garden cress (Lepidium sativum L.). Plant Sci. 2012, 182, 112–120. [Google Scholar] [CrossRef]
- Jaleel, C.A.; Gopi, R.; Manivannan, P.; Gomathinayagam, M.; Murali, P.V.; Panneerselvam, R. Soil applied propiconazole alleviates the impact of salinity on Catharanthus roseus by improving antioxidant status. Pestic. Biochem. Physiol. 2008, 90, 135–139. [Google Scholar] [CrossRef]
- Waqas, M.; Yaning, C.; Iqbal, H.; Shareef, M.; Rehman, H.; Yang, Y. Paclobutrazol improves salt tolerance in quinoa: Beyond the stomatal and biochemical interventions. J. Agron. Crop Sci. 2017, 203, 315–322. [Google Scholar] [CrossRef]
- Guoping, Z.; Jianxing, C.; Bull, D.A. The effects of timing of N application and plant growth regulators on morphogenesis and yield formation in wheat. Plant Growth Regul. 2001, 35, 239–245. [Google Scholar] [CrossRef]
- Peng, D.; Chen, X.; Yin, Y.; Lu, K.; Yang, W.; Tang, Y.; Wang, Z. Lodging resistance of winter wheat (Triticum aestivum L.): Lignin accumulation and its related enzymes activities due to the application of paclobutrazol or gibberellin acid. Field Crops Res. 2014, 157, 1–7. [Google Scholar] [CrossRef]
- Wang, C.; Hu, D.; Liu, X.; She, H.; Ruan, R.; Yang, H.; Yi, Z.; Wu, D. Effects of uniconazole on the lignin metabolism and lodging resistance of culm in common buckwheat (Fagopyrum esculentum M.). Field Crops Res. 2015, 180, 46–53. [Google Scholar] [CrossRef]
- Kuai, J.; Yang, Y.; Sun, Y.; Zhou, G.; Zuo, Q.; Wu, J.; Ling, X. Paclobutrazol increases canola seed yield by enhancing lodging and pod shatter resistance in Brassica napus L. Field Crops Res. 2015, 180, 10–20. [Google Scholar] [CrossRef]
- Kamran, M.; Ahmad, I.; Wu, X.; Liu, T.; Ding, R.; Han, Q. Application of paclobutrazol: a strategy for inducing lodging resistance of wheat through mediation of plant height, stem physical strength, and lignin biosynthesis. Env. Sci. Pollut. Res. 2018, 25, 29366–29378. [Google Scholar] [CrossRef]
- Kamran, M.; Cui, W.; Ahmad, I.; Meng, X.; Zhang, X.; Su, W.; Chen, J.; Ahmad, S.; Fahad, S.; Han, Q.; et al. Effect of paclobutrazol, a potential growth regulator on stalk mechanical strength, lignin accumulation and its relation with lodging resistance of maize. Plant Growth Regul. 2018, 84, 317–332. [Google Scholar] [CrossRef]
- Jaleel, C.A.; Gopi, R.; Manivannan, P.; Panneerselvam, R. Responses of antioxidant defense system of Catharanthus roseus (L.) G. Don. to paclobutrazol treatment under salinity. Acta Physiol. Plant. 2007, 29, 205–209. [Google Scholar] [CrossRef]
- Hajihashemi, S.; Kiarostami, K.; Saboora, A.; Enteshari, S. Exogenously applied paclobutrazol modulates growth in salt-stressed wheat plants. Plant Growth Regul. 2007, 53, 117–128. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts, polyphenol oxidase in Beta vulgaris L. Plant Physiol 1949, 24, 1–15. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide Dismutases: I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Ekmekci, Y.; Terzioglu, S. Effects of oxidative stress induced by paraquat on wild and cultivated wheats. Pestic. Biochem. Physiol. 2005, 83, 69–81. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Zhang, X.; Chen, C.J.; Zhou, M.G.; Wang, H.C. Effects of fungicides JS399-19, azoxystrobin, tebuconazloe, and carbendazim on the physiological and biochemical indices and grain yield of winter wheat. Pestic. Biochem. Physiol. 2010, 98, 151–157. [Google Scholar] [CrossRef]
- Borrás, L.; Slafer, G.A.; Otegui, M.E. Seed dry weight response to source-sink manipulations in wheat, maize and soybean: A quantitative reappraisal. F. Crop. Res. 2004, 86, 131–146. [Google Scholar] [CrossRef]
- Fletcher, R.A.; Gilley, A.; Sankhla, N.; Davis, T.D. Triazoles as plant growth regulators and stress protectants. Hortic. Rev.. 2000, 24, 55–138. [Google Scholar]
- Berova, M.; Zlatev, Z.; Stoeva, N. Effect of paclobutrazol on wheat seedlings under low temperature stress. Bulg. J. Plant Physiol. 2002, 28, 75–84. [Google Scholar]
- Khalil, I.A.; Rahman, H. ur Effect of paclobutrazol on growth, chloroplast pigments and sterol biosynthesis of maize (Zea mays L.). Plant Sci. 1995, 105, 15–21. [Google Scholar] [CrossRef]
- Tekalign, T. Growth, photosynthetic efficiency, rate of transpiration, lodging, and grain yield of Tef (Eragrostis Tef (Zucc.) Trotter ) as influenced by stage and rate of paclobutrazol application. East Afr. J. Sci. 2007, 1, 35–44. [Google Scholar] [CrossRef]
- Gopi, R.; Jaleel, C.A.; Sairam, R.; Lakshmanan, G.M.A.; Gomathinayagam, M.; Panneerselvam, R. Differential effects of hexaconazole and paclobutrazol on biomass, electrolyte leakage, lipid peroxidation and antioxidant potential of Daucus carota L. Colloids Surf. B: Biointerfaces 2007, 60, 180–186. [Google Scholar] [CrossRef]
- Rademacher, W. Growth Retardants: Effects on gibberellin biosynthesis and other metabolic pathways. Annu. Rev. Plant Physiol. Plant Mol. Biol 2000, 51, 501–531. [Google Scholar] [CrossRef]
- Xu, Z.; Huai, Z.; Pei, L.U.O.; Zheng, R.E.N. Effect of paclobutrazol on leaf senescence and yield of wheat. Acta Agric. Boreali-Sin. 2007, 22, 136–140. [Google Scholar]
- Elanchezhian, R.; Haris, A.A.; Kumar, S.; Singh, S.S. Positive impact of paclobutrazol on gas exchange, chlorophyll fluorescence and yield parameters under submergence stress in rice. Indian J. Plant Physiol. 2015, 20, 111–115. [Google Scholar] [CrossRef]
- Moradi, S.; Baninasab, B.; Gholami, M.; Ghobadi, C. Paclobutrazol application enhances antioxidant enzyme activities in pomegranate plants affected by cold stress. J. Hortic. Sci. Biotechnol. 2017, 92, 65–71. [Google Scholar] [CrossRef]
- Bora, K.K.; Ganesh, R.; Mathur, S.R. Paclobutrazol delayed dark-induced senescence of mung bean leaves. Biol. (Bratisl). 2007, 62, 185–188. [Google Scholar] [CrossRef]
- Wang, X.C.; Yang, W.Y.; Chen, G.; L I, Q.L.; Wang, X.-. Bin Effects of uniconazole on leaf senescence and yield of maize sprayed at late growth stage. J. Maize Sci. 2009, 17, 86–88. [Google Scholar]
- Panneerselvam, R.; Muthukumarasamy, M.; Karikalan, L. Triadimefon enhances growth and net photosynthetic rate in NaCl stressed plants of Raphanus sativus L. Photosynthetica 1997, 34, 605–609. [Google Scholar] [CrossRef]
- Gopi, R.; Sridharan, R.; Somasundaram, R.; Panneerselvam, R.; Lakshmanan, G.M.A.; Panneerselvam, R.; Lakshmanan, G.M.A.; Panneerselvam, R. Growth and photosynthetic characteristics as affected by triazoles in Amorphophallus campanulatus. Gen. Appl. Plant Physiol. 2005, 31, 171–180. [Google Scholar]
- Abdul Jaleel, C.; Manivannan, P.; Sankar, B.; Kishorekumar, A.; Sankari, S.; Panneerselvam, R. Paclobutrazol enhances photosynthesis and ajmalicine production in Catharanthus roseus. Process Biochem. 2007, 42, 1566–1570. [Google Scholar] [CrossRef]
- Yim, K.O.; Kwon, Y.W.; Bayer, D.E. Growth responses and allocation of assimilates of rice seedlings by paclobutrazol and gibberellin treatment. J. Plant Growth Regul. 1997, 16, 35–41. [Google Scholar] [CrossRef]
- Navarro, A.; Sánchez-Blanco, M.J.; Bañon, S. Influence of paclobutrazol on water consumption and plant performance of Arbutus unedo seedlings. Sci. Hortic. 2007, 111, 133–139. [Google Scholar] [CrossRef]
- Gilley, A.; Fletcher, R.A. Relative efficacy of paclobutrazol, propiconazole and tetraconazole as stress protectants in wheat seedlings. Plant Growth Regul. 1997, 21, 169–175. [Google Scholar] [CrossRef]
- Zhong, X.; Che, X.; Zhang, Z.; Li, S.; Li, Q.; Li, Y.; Gao, H. Slower development of PSI activity limits photosynthesis during Euonymus japonicus leaf development. Plant Physiol. Biochem. 2019, 136, 13–21. [Google Scholar] [CrossRef]
- Sheikh Mohammadi, M.H.; Etemadi, N.; Arab, M.M.; Aalifar, M.; Arab, M.; Pessarakli, M. Molecular and physiological responses of Iranian Perennial ryegrass as affected by Trinexapac ethyl, Paclobutrazol and Abscisic acid under drought stress. Plant Physiol. Biochem. 2017, 111, 129–143. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Jaleel, C.A.; Gopi, R.; Alagu Lakshmanan, G.M.; Panneerselvam, R. Triadimefon induced changes in the antioxidant metabolism and ajmalicine production in Catharanthus roseus (L.) G. Don. Plant Sci. 2006, 171. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Noctor, G.; Mhamdi, A.; Chaouch, S.; Han, Y.; Neukermans, J.; Marquez-Garcia, B.; Queval, G.; Foyer, C.H. Glutathione in plants: An integrated overview. PlantCell Env.. 2012, 35, 454–484. [Google Scholar] [CrossRef] [PubMed]
- Özmen, A.D.; Özdemir, F.; Türkan, I. Effects of paclobutrazol on response of two barley cultivars to salt stress. Biol. Plant. 2003, 46, 263–268. [Google Scholar] [CrossRef]
- Hu, Y.; Yu, W.; Liu, T.; Shafi, M.; Song, L.; du, X.; Huang, X.; Yue, Y.; Wu, J. Effects of paclobutrazol on cultivars of Chinese bayberry (Myrica rubra) under salinity stress. Photosynthetica 2017, 55, 1–11. [Google Scholar] [CrossRef]
- Akbari, G.A.; Hojati, M.; Modarres-Sanavy, S.A.M.; Ghanati, F. Exogenously applied hexaconazole ameliorates salinity stress by inducing an antioxidant defense system in Brassica napus L. plants. Pestic. Biochem. Physiol. 2011, 100, 244–250. [Google Scholar] [CrossRef]
- Reddy, A.R.; Chaitanya, K.V.; Vivekanandan, M. Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J. Plant Physiol. 2004, 161, 1189–1202. [Google Scholar] [CrossRef] [PubMed]
- Jaleel, C.A.; Gopi, R.; Manivannan, P.; Kishorekumar, A.; Gomathinayagam, M.; Panneerselvam, R. Changes in biochemical constituents and induction of early sprouting by triadimefon treatment in white yam (Dioscorea rotundata Poir.) tubers during storage. J. Zhejiang Univ. Sci. B 2007, 8, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Stern, R.A.; Raz, A.; Goldway, M. The growth retardant uniconazole is an effective blossom thinner for “Bing” cherry (Prunus avium). Acta Hortic. 2014, 1020, 513–520. [Google Scholar] [CrossRef]

| Effect | df | Ear Length | Ear Diameter | Kernels Ear-1 | TKW | Grain Yield |
|---|---|---|---|---|---|---|
| Year (Y) | 1 | 8.21 * | 28.57 ** | 12.10 ** | 14.62 ** | 25.58 ** |
| Paclobutrazol (P) | 3 | 72.57 ** | 96.86 ** | 114.42 ** | 174.56 ** | 183.60 ** |
| Y × P | 3 | 0.93 NS | 2.86 NS | 0.42 NS | 1.37 NS | 1.99 NS |
| Year | Treatments | Ear Length (cm) | Ear Diameter (cm) | Kernels ear −1 | TKW (g) | Grain Yield (t ha−1) |
|---|---|---|---|---|---|---|
| 2014 | CK | 14.1 ± 0.45 d | 4.3 ± 0.06 c | 403 ± 12.02 d | 264.6 ± 6.04 d | 6.50 ± 0.25 d |
| PS1 | 15.4 ± 0.56 c | 4.6 ± 0.07 b | 454 ± 9.38 c | 312.1 ± 2.40 c | 8.03 ± 0.30 c | |
| PS2 | 18.9 ± 0.71 a | 5.0 ± 0.07 a | 535 ± 8.35 a | 360.0 ± 7.38 a | 10.45 ± 0.29 a | |
| PS3 | 16.8 ± 0.47 b | 5.0 ± 0.09 a | 491 ± 13.21 b | 339.1 ± 6.71 b | 9.23 ± 0.23 b | |
| 2015 | CK | 13.5 ± 0.52 d | 4.2 ± 0.09 c | 390 ± 6.55 d | 257.2 ± 6.09 c | 6.37 ± 0.26 d |
| PS1 | 15.0 ± 0.49 c | 4.4 ± 0.05 b | 438 ± 11.93 c | 308.4 ± 9.23 b | 7.28 ± 0.19 c | |
| PS2 | 17.6 ± 0.72 a | 4.8 ± 0.05 a | 508 ± 9.07 a | 341.6 ± 7.62 a | 9.59 ± 0.37 a | |
| PS3 | 16.4 ± 0.38 b | 4.7 ± 0.06 a | 478 ± 14.80 b | 323.8 ± 9.45 b | 8.65 ± 0.33 b |
| Effect | df | Leaf Senescence | Chlorophyll | Carotenoid | Pn | Fo | Fv/Fm | YII | MDA |
| Year (Y) | 1 | 117.81 ** | 462.62 ** | 1325.96 ** | 0.56 NS | 30.67 ** | 70.02 ** | 29.91 ** | 91.70 ** |
| Paclobutrazol (P) | 3 | 408.27 ** | 225.11 ** | 298.44 ** | 221.39 ** | 300.07 ** | 628.96 ** | 171.12 ** | 477.82 ** |
| sampling time (T) | 3 | 4509.25 ** | 693.39 ** | 1837.87 ** | 1072.89 ** | 918.36 ** | 1498.3 ** | 184.41 ** | 1887.09 ** |
| Y × P | 3 | 3.73 * | 2.33 NS | 3.46 * | 1.48 NS | 3.27 * | 21.96 ** | 0.65 NS | 1.84 NS |
| Y × T | 3 | 53.24 ** | 0.59 NS | 102.64 ** | 8.57 ** | 9.14 ** | 18.92 ** | 3.68 * | 9.99 ** |
| P × T | 9 | 93.64 ** | 4.85 ** | 2.23 * | 4.86 ** | 4.88 ** | 7.67 ** | 0.93 NS | 27.96 ** |
| Y × P × T | 9 | 2.64 * | 1.23 NS | 4.75 ** | 1.73 NS | 3.16 ** | 1.36 NS | 0.50 NS | 1.69 NS |
| Effect | df | SOD | POD | CAT | APX | H2O | O2− | SP | SS |
| Year (Y) | 1 | 3695.57 ** | 1304.9 ** | 16.97 ** | 955.43 ** | 69.33 ** | 15.1 ** | 392.47 ** | 254.74 ** |
| Paclobutrazol (P) | 3 | 714.81 ** | 444.6 ** | 256.36 ** | 322.9 ** | 1011.75 ** | 363.71 ** | 204.17 ** | 6.54 ** |
| sampling time (T) | 3 | 7328.52 ** | 1736.32 ** | 504.36 ** | 464.7 ** | 1802 ** | 419.22 ** | 1782.02 ** | 3256.40 ** |
| Y × P | 3 | 11.05 ** | 2.16 NS | 9.24 ** | 6.72 ** | 13.12 ** | 3.70 * | 3.29 * | 31.26 ** |
| Y × T | 3 | 235.92 ** | 137.08 ** | 26.1 ** | 95.15 ** | 24.65 ** | 47.61 ** | 15.23 ** | 859.57 ** |
| P × T | 9 | 12.37 ** | 7.51 ** | 4.31 ** | 4.31 ** | 101.55 ** | 18.82 ** | 1.12 NS | 92.74 ** |
| Y × P × T | 9 | 3.24 ** | 4.93 ** | 4.86 ** | 2.62 * | 17.58 ** | 2.97 ** | 1.86 NS | 6.62 ** |
| Chl | Car | LS | SOD | POD | CAT | APX | H2O2 | O2− | MDA | Pn | Fv/Fm | Y(II) | SP | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Car | 0.861 ** | |||||||||||||
| LS | −0.962 ** | −0.948 ** | ||||||||||||
| SOD | 0.968 ** | 0.931 ** | −0.989 ** | |||||||||||
| POD | 0.752 ** | 0.973 ** | −0.868 ** | 0.863 ** | ||||||||||
| CAT | 0.870 ** | 0.988 ** | −0.945 ** | 0.922 ** | 0.946 ** | |||||||||
| APX | 0.959 ** | 0.750 ** | −0.880 ** | 0.870 ** | 0.608 * | 0.774 * | ||||||||
| H2O2 | −0.973 ** | −0.932 ** | 0.977 ** | −0.970 ** | −0.849 ** | −0.936 ** | −0.930 ** | |||||||
| O2− | −0.966 ** | −0.952 ** | 0.996 ** | −0.995 ** | −0.882 ** | −0.947 ** | −0.879 ** | 0.980 ** | ||||||
| MDA | −0.948 ** | −0.963 ** | 0.984 ** | −0.985 ** | −0.912 ** | −0.952 ** | −0.863 ** | 0.982 ** | 0.992 ** | |||||
| Pn | 0.983 ** | 0.898 ** | −0.985 ** | 0.985 ** | 0.806 ** | 0.904 ** | 0.911 ** | −0.974 ** | −0.985 ** | −0.965 ** | ||||
| Fv/Fm | 0.987 ** | 0.862 ** | −0.958 ** | 0.953 ** | 0.746 ** | 0.883 ** | 0.964 ** | −0.980 ** | −0.956 ** | −0.940 ** | 0.977 ** | |||
| Y(II) | 0.986 ** | 0.823 ** | −0.935 ** | 0.951 ** | 0.704 * | 0.843 ** | 0.934 ** | −0.938 ** | −0.939 ** | −0.909 ** | 0.966 ** | 0.972 ** | ||
| SP | 0.971 ** | 0.940 ** | −0.988 ** | 0.985 ** | 0.853 ** | 0.934 ** | 0.887 ** | −0.973 ** | −0.988 ** | −0.973 ** | 0.976 ** | 0.960 ** | 0.955 ** | |
| Yield | 0.961 ** | 0.886 ** | −0.974 ** | 0.980 ** | 0.805 ** | 0.893 ** | 0.869 ** | −0.951 ** | −0.973 ** | −0.951 ** | 0.993 ** | 0.955 ** | 0.952 ** | 0.959 ** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamran, M.; Ahmad, S.; Ahmad, I.; Hussain, I.; Meng, X.; Zhang, X.; Javed, T.; Ullah, M.; Ding, R.; Xu, P.; et al. Paclobutrazol Application Favors Yield Improvement of Maize Under Semiarid Regions by Delaying Leaf Senescence and Regulating Photosynthetic Capacity and Antioxidant System During Grain-Filling Stage. Agronomy 2020, 10, 187. https://doi.org/10.3390/agronomy10020187
Kamran M, Ahmad S, Ahmad I, Hussain I, Meng X, Zhang X, Javed T, Ullah M, Ding R, Xu P, et al. Paclobutrazol Application Favors Yield Improvement of Maize Under Semiarid Regions by Delaying Leaf Senescence and Regulating Photosynthetic Capacity and Antioxidant System During Grain-Filling Stage. Agronomy. 2020; 10(2):187. https://doi.org/10.3390/agronomy10020187
Chicago/Turabian StyleKamran, Muhammad, Shakeel Ahmad, Irshad Ahmad, Izhar Hussain, Xiangping Meng, Xudong Zhang, Tehseen Javed, Misbah Ullah, Ruixia Ding, Peizhi Xu, and et al. 2020. "Paclobutrazol Application Favors Yield Improvement of Maize Under Semiarid Regions by Delaying Leaf Senescence and Regulating Photosynthetic Capacity and Antioxidant System During Grain-Filling Stage" Agronomy 10, no. 2: 187. https://doi.org/10.3390/agronomy10020187
APA StyleKamran, M., Ahmad, S., Ahmad, I., Hussain, I., Meng, X., Zhang, X., Javed, T., Ullah, M., Ding, R., Xu, P., Gu, W., & Han, Q. (2020). Paclobutrazol Application Favors Yield Improvement of Maize Under Semiarid Regions by Delaying Leaf Senescence and Regulating Photosynthetic Capacity and Antioxidant System During Grain-Filling Stage. Agronomy, 10(2), 187. https://doi.org/10.3390/agronomy10020187






