Abstract
The objective was to evaluate aromatic plants’ effects on the acceptance, preference, egg load, and life span of females of the twospotted spider mite (TSSM) in laboratory experiments and TSSM population under aromatic plants’ intercropping in greenhouse experiments. The pseudofruits production was also evaluated. For the laboratory, basil’s, Chinese chives’, chives’, and garlic’s influence on TSSM were tested on strawberry leaves. Four laboratory experiments were conducted: (1) Multiple choice test; (2) T-shaped arena test; (3) host–plant acceptance on aromatic plant or strawberry leaves; and (4) performance of TSSMs on strawberry leaves under aromatic plant influence. For the greenhouse experiments, assessments of the TSSM populations were realized by observing TSSM with a 10× magnifying glass on strawberry leaves in a monocrop or intercropped with Chinese chives, chives, garlic, or onion. Pseudofruit production was evaluated. Our results show that strawberry leaves were strongly preferred by TSSM. The T-shaped arena test revealed that all aromatic plants repel the TSSM. The test with the performance of TSSM females revealed that aromatic plants affected the mite’s biological parameters. Chinese chives reduced the number of eggs laid per day by 33.22%, whereas garlic reduced the number by 17.30% and chives reduced it by 12.46%. The total number of eggs was reduced by 34.79% with Chinese chives and 25.65% with garlic. Greenhouse experiments showed that chives reduced TSSM populations on two cycles and Chinese chives and garlic reduced TSSM populations on the first cycle only. With our findings, we suggest that Chinese chives, chives, and garlic are the primary candidates for intercropping use against TSSM. Chinese chives and garlic reduced the total number of eggs, but only garlic reduced female mite longevity. However, none of the intercropping plants improved strawberry pseudofruit production.
1. Introduction
Plants are continuously releasing volatile organic compounds (VOCs) and these play an important role in plant–arthropods interactions in agroecosystems, including tritrophic communication among plant–pest–natural enemies, repelling pests and induced resistance [1]. Essential oils of plants from the Amaryllidaceae, Lamiaceae, Meliaceae, Rutaceae, and Solanaceae families have acaricidal and/or repellent effects against Tetranychus urticae Koch, twospotted spider mite (TSSM) [2]. Oviposition reduction of TSSM was achieved with 0.36 and 0.74 mg/L of garlic distillate in a laboratory conditions study [3]. Diallyl disulfide, a common organosulphur compound found in garlic, provided a 100% mortality rate at 100 μL/L in fumigation tests in pupae and adults of Tribolium confusum Jacquelin du Val [4]. Most of these essential oils are also volatiles, which supports the hypothesis that these VOCs emitted by aromatic plant leaves play an important role in pest population reductions in intercropping systems.
Aromatic plants in intercropping are reported in the literature as reducing pest populations or injuries to plants [5,6,7]. Basil (Ocimum basilicum L.), citronella (Cymbopogon sp.) (Poaceae), and coriander (Coriandrum sativum L.) repel Bemisia tabaci Genn. in a Y-shaped olfactometer and reduced its population when intercropped with tomato in field experiments [8]. The TSSM populations were reduced when Amaryllidaceae plants were intercropped in strawberry Fragaria × ananassa Duchesne [9,10]. However, we did not find studies elucidating how aromatic plants reduce mite populations. Mites may be repelled, or even oviposition and its longevity affected by aromatic plant VOCs. In addition, plants used as intercrop should be an unfavorable host for the pest to not increase its populations in the field. Strawberry leaf discs are more preferable than onion (Allium cepa L.), leek (Allium porrum L.), or parsley (Petroselinum crispum Mill.), and these aromatic plants are not a good host for TSSM because the fecundity of females fed on these plants was reduced compared to strawberry [11].
Intercropping may also affect plants’ productions because of restricted water, fertilizers, or reduced solar radiation source by shading. However, a study showed that strawberry pseudofruit yield is not reduced by intercropping garlic, and grower gross income was increased with garlic bulbs and strawberry pseudofruits’ commercialization [12]. Similarly, cucumber (Cucumis sativus L.), pepper (Capsicum annum L.), melon (Cucumis melo L.), summer squash (Cucumis pepo L.), lettuce (Lactuca sativa L.), onion, and radish (Raphanus sativus L.) yields are not affected by intercropping in strawberry crops [13,14]. Even if there is not an increase in plant yield, the commercialization of two or more crops produced in the same amount of area at the same time is advantageous because of higher land use efficiency, which is measured by the land equivalent ratio index (LER) [15]. If the LER index is above one, there is an advantage in producing two crops at the same time and area. The abovementioned studies with intercropping have higher than one LER indexes, which indicates that intercropping of those plants is better than growing in monocrops.
Strawberry, a plant species which belongs to the Rosaceae family, is one of the most important berries consumed in Brazil and around the world. Because of its high productivity in a relatively small area, this berry is a good option for small farmers. In Brazil, the total production area varies around 3500 hectares, with 130,000 ton of pseudofruits harvested per year [16]. Among the insects and mites that affect the strawberry crop, TSSM is considered a serious pest in several fields and greenhouses.
T. urticae is a polyphagous species, which has genomic adaptations that facilitate its feeding on a wide range of plants. Consequently, the TSSM can feed on more than 1100 plant species belonging to 140 plant families [17]. TSSM is an important mite pest in several crops, including beans (Phaseolus vulgaris L.), cotton (Gossypium sp. L.), rose (Rosa spp.), strawberry, etc. [18]. TSSM control is realized mostly with synthetic pesticides, and some of them can be highly toxic to predatory mites [19], and induce phytophagous mite population outbreaks [20]. As the use of biological control agents is increasing, methods that do not have negative effect on the predatory mites are desirable for agricultural use.
The objective was to evaluate aromatic plants’ effects on the egg load and life span of females of the twospotted spider mite (TSSM) in laboratory experiments and the TSSM population under aromatic plants’ intercropping in greenhouse experiments. The pseudofruits production was also evaluated. In the laboratory, we tested the TSSM acceptance and preference on the aromatic plants basil, Chinese chives, chives, and garlic or strawberry leaves; the effects of aromatic plants leaves on the egg load and life span of females’ TSSM mite reared on strawberry leaves; and the effects of intercropping Amaryllidaceae plants on the TSSM population on strawberry leaves and pseudofruit production in greenhouse.
2. Materials and Methods
Laboratory experiments were conducted in a climatized chamber (Temperature 26 ± 2 °C; Relative Humidity 70 ± 10%; L14:D10). Aromatic plants (AP) used were: Basil (B), Chinese chives (CC), chives (C), and garlic (G). Aromatic plants and strawberry (S) cv. Albion were cultivated and maintained in a greenhouse. Plants were not sprayed with insecticides, fungicides, or acaricides. TSSM used for tests were maintained on jack bean Canavalia ensiformis (L.) DC. plants.
2.1. Multiple-Choice Test
This experiment was conducted to identify if aromatic plant volatiles influence TSSM preference. We conducted a multiple-choice test to know the TSSM preference to strawberry vs. aromatic plants. Multiple-choice tests were conducted using square (4 cm2) pieces of young leaves in Petri dish arenas (10 cm diameter). Petri dishes were covered with humid filter paper to maintain moisture, and distillated water was added as needed. One piece of each of the aromatic plant and strawberry leaf were placed equidistant from the center of the Petri dish. Ten TSSM female adults (3–5 days) were released on the center of the arena. The numbers of females and eggs on each leaf were recorded 24 h later. Each Petri dish was considered as a replicate. Sixteen replicates were used.
2.2. T-Shaped Arena Test
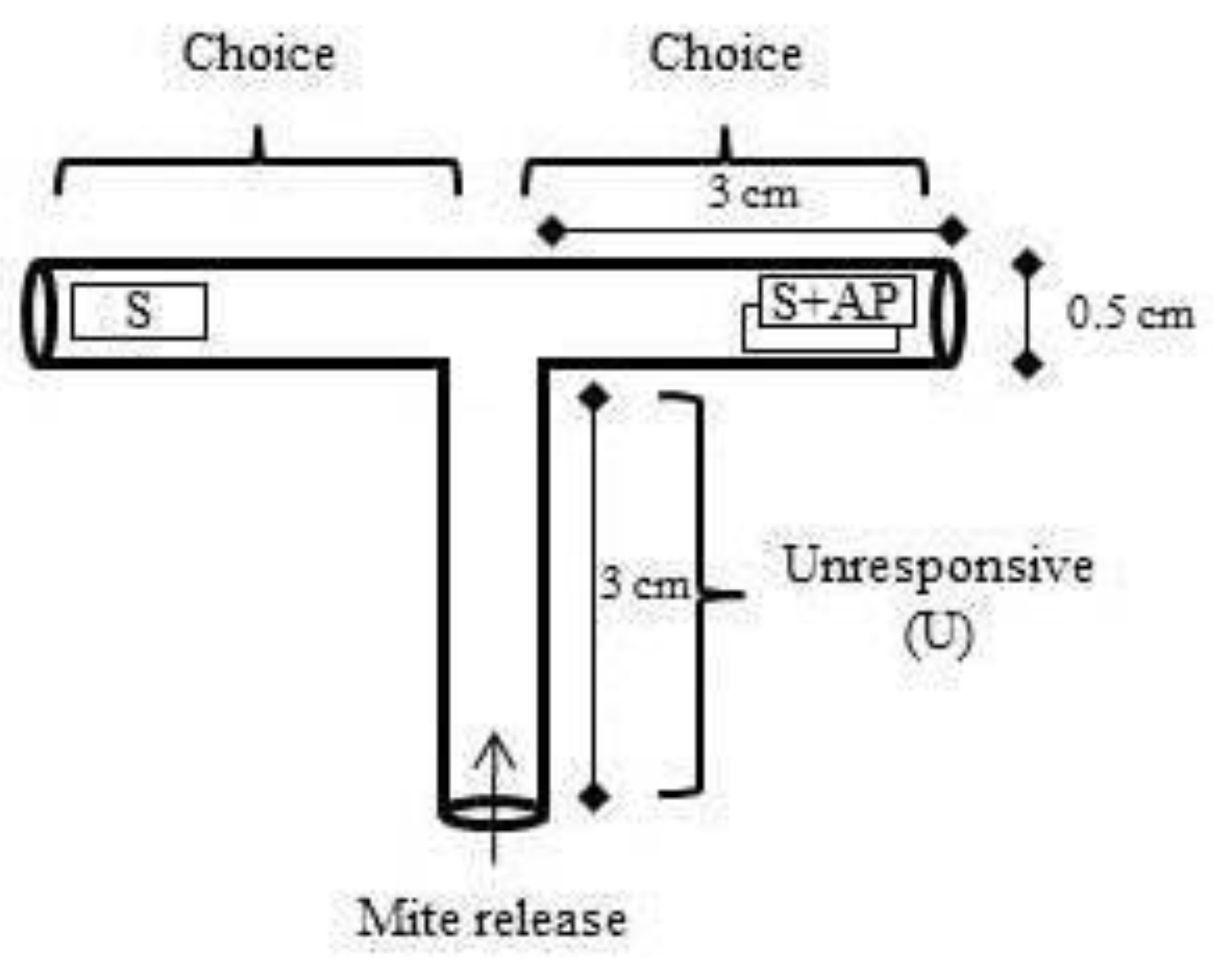
A two-choice T-shaped arena was built using drinking straws (Figure 1). Pieces of young leaves (1.00 cm × 0.25 cm) of strawberry (one piece) versus strawberry leaf (one piece) + aromatic plant leaf (basil, chives, Chinese chives, or garlic) (one piece) were compared and placed as shown in Figure 1. Then, on each replicate, five female adults (3–5 days) were placed at the releasing point. The first choice of mites was evaluated by observing which side they chose first. After three hours, the locations of females were quantified. Eleven replicates were used for each of the aromatic plants.
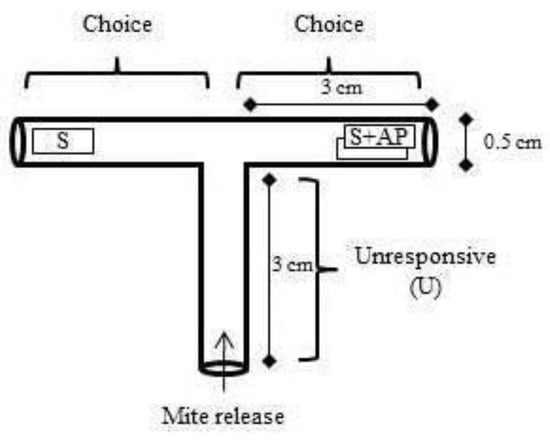
Figure 1.
T-shaped arena for studies with Tetranychus urticae female responses to a piece of strawberry leaf (S) versus a piece of strawberry leaf + aromatic plant (AP) piece of leaf (S + AP).
On every new aromatic plant test, tubes were washed with 70% alcohol and after washed 3× with distillated water.
2.3. Host–Plant Acceptance for Oviposition
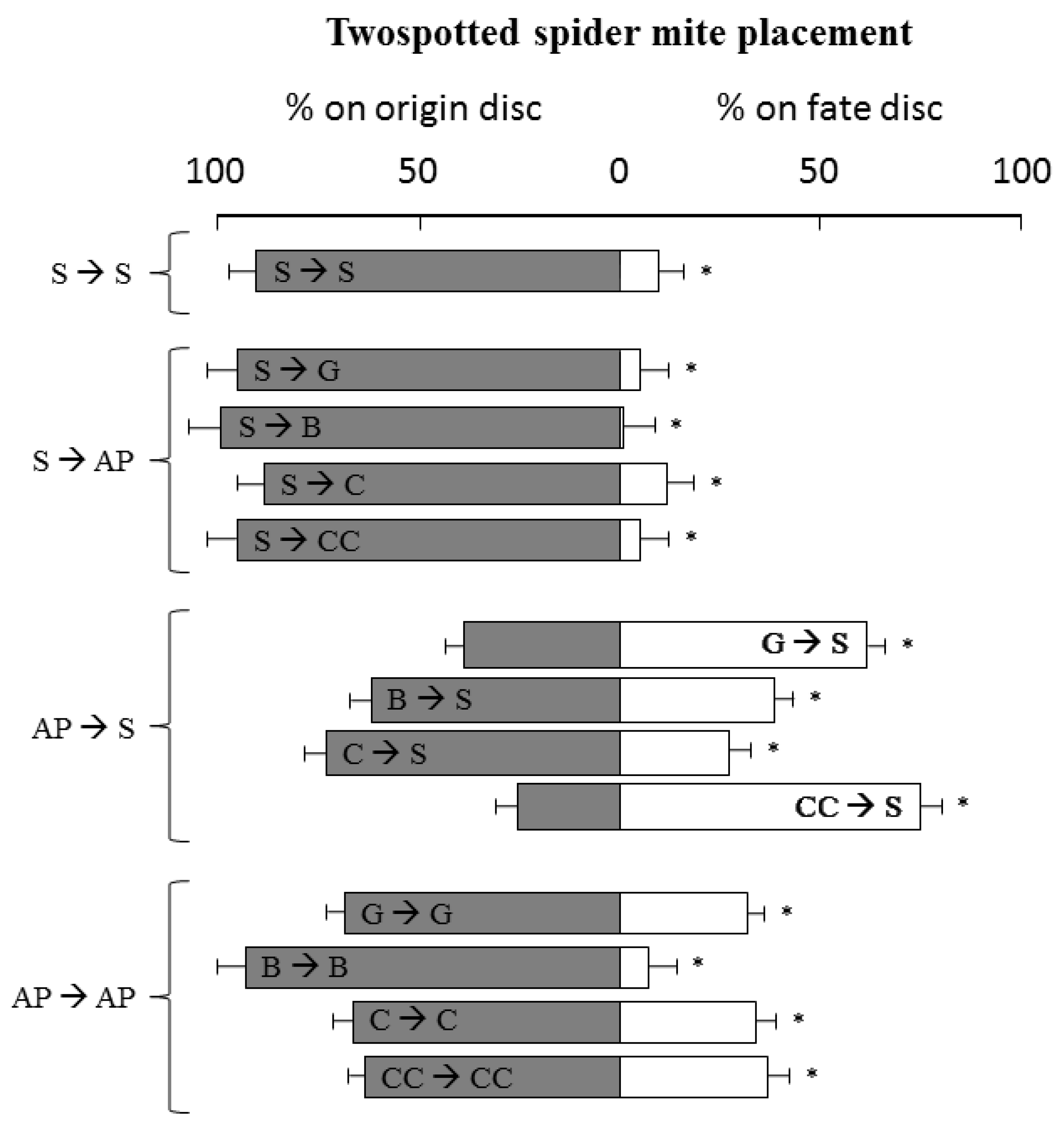
This experiment was conducted to know the acceptance of the oviposition of aromatic plant leaves. To study strawberry and aromatic plant host–plant acceptance for oviposition, the method described by Yano et al. (1998) [21] was adapted to ours. Young leaf discs (2 cm diameter) were first washed with distillated water and then disposed in Petri dishes. Two leaf discs linked by a vinyl bridge (5 cm) were placed in a Petri dish (10 cm diameter) covered with water-saturated filter paper to maintain moisture and avoid TSSM escape from leaves. Distillated water was added as needed. Ten adult females (3–5 days) were transferred to the test plant disc (origin). The number of mites remaining on the origin disc and those who were placed on the fate disc were quantified after 24 h. Then, after this time, if the mite was placed on the same disc that was first transferred, we counted it as being placed at the “origin disc”, if the female was placed on the other disc, we counted it as a “fate disc”.
Treatments were as follow (TSSM placed on origin disc → fate disc): S → S; S → B; S → CC; S → C; S → G; B → S; C→ S; G→ S; CC → S; B → B; C→ C; G → G and CC → CC. Twelve replications were used for each treatment.
2.4. TSSM Oviposition and Longevity
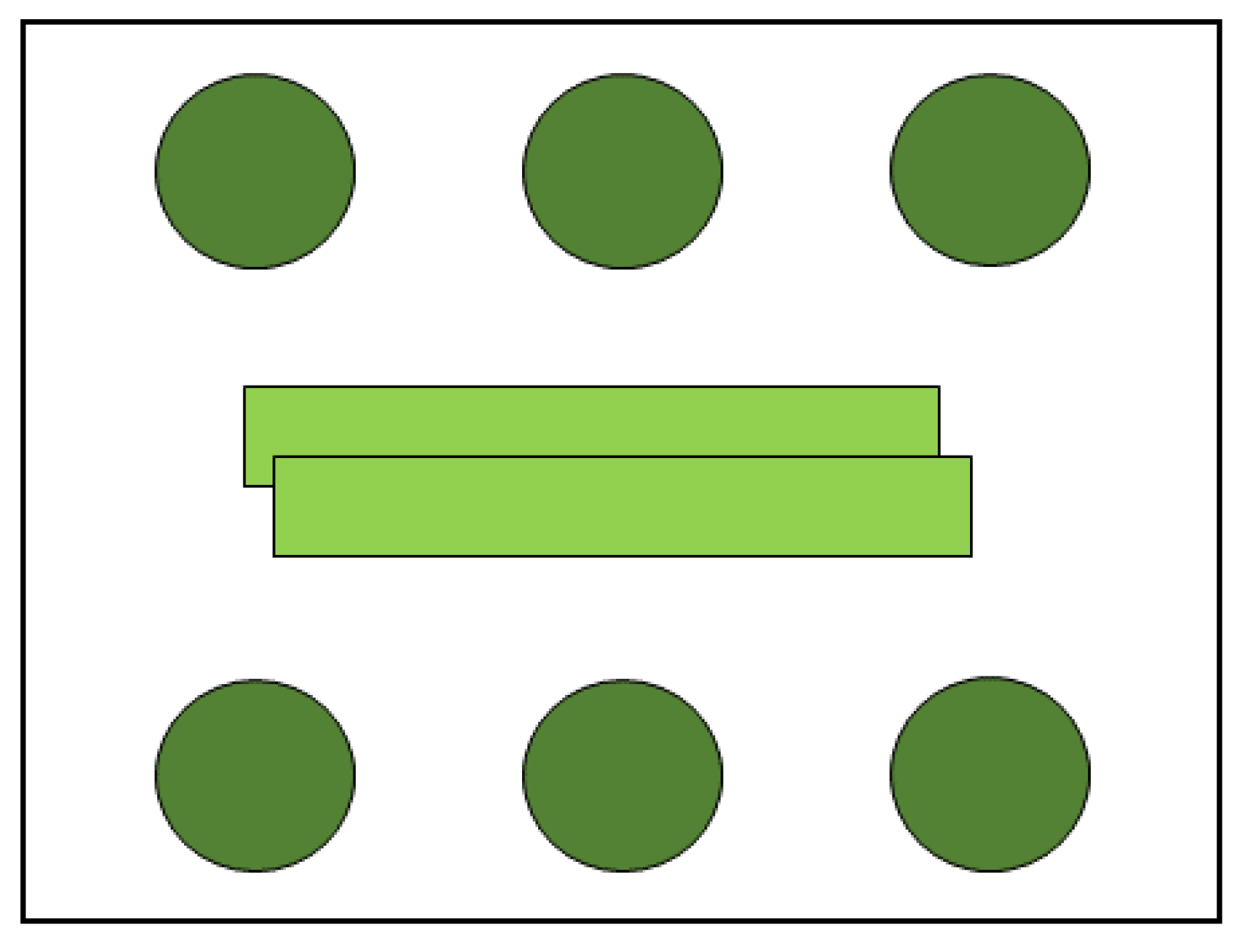
This experiment was conducted to test the influence of the aromatic plant leaf when put next to strawberry leaves with TSSM females. This was to try to simulate the aromatic plant effects in intercropping systems. Two leaves rectangles (8 cm × 1 cm) of aromatic plants were first washed with distillated water and then placed in the middle of acrylic boxes (11 cm × 11cm × 3.5 cm), and six strawberry young leaf discs (2 cm diameter) were washed in distillated water and then distributed on the periphery of the box (Figure 2) to study the oviposition and longevity of TSSM with the effect of aromatic plant young leaves’ VOCs.
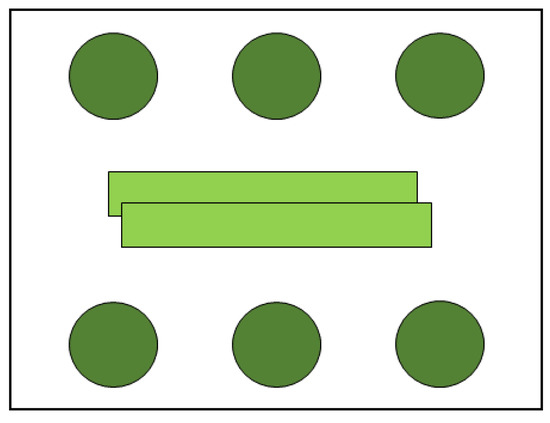
Figure 2.
Six strawberry leaf discs (2 cm diameter) with two leaf rectangles (8 cm × 1 cm) of the aromatic plant in a box (11 cm × 11 cm × 3.5 cm), for the Tetranychus urticae oviposition and longevity tests.
Strawberry and aromatic plant leaves were replaced every seven days or when it became dehydrated. Boxes were covered with humid filter paper to maintain moisture and distillated water was added as needed. Treatments were the control (strawberry leaves only) and strawberry together with leaf of one aromatic plant species: Basil, Chinese chives, chives, or garlic. Leaf discs were observed three times a day, and the performance of TSSM on strawberry leaves was assessed by the following variables: Number of eggs laid in 5 days (EFD); total number of eggs (TNE); eggs per day (EPD); and longevity of females (LON). Each strawberry disc received three TSSM eggs, and after hatching, one female was maintained in the leaves and the other mites were returned to jack bean leaves. Each box was considered as one experimental parcel. These treatments were replicated six times.
2.5. Experiments with Amaryllidaceae Plants’ Intercropping in the Greenhouse
Experiments were conducted in a greenhouse at Universidade Estadual de Londrina (23°20′28″ S, 51°12′34″ O; 548 m.), during two crop cycles, from July 2016 to November 2016 (first cycle) and July 2017 to November 2017 (second cycle) to evaluate the development of TSSM populations under the influence of Amaryllidaceae plants’ intercropping. The climate is classified as humid sub-tropical, according to Köppen.
Strawberry seedlings were acquired from a commercial nursery. The Dover and San Andreas cv. were used for the first and second cycles, respectively. Seedlings were planted in July 2016 and July 2017 for the first and second cycles, respectively, in 14-L pots containing 1/3 Oxisol (clay texture), 1/3 washed sand, and 1/3 organic compost. At the same day, aromatic plants were also planted.
Treatments were strawberry in a monocrop (control) or strawberry plus one aromatic plant cultivated together, on each pot: Chives cv. Hossonegui, Chinese chives, seedlings shared by grower Nelson Tamura, onion cv. Optima, and garlic cv. BRS Hozan. Each pot was considered as one plot, and a randomized complete block design, with four replications, was used. Each pot was separated by 2 m in the block and 2 m between blocks (the scheme is provided as Supplementary Material, Figure S1).
TSSM incidence occurred naturally. Strawberry leaves were observed by using a 10 × magnifying glass with an area of 1 cm2. In each strawberry plant (plot), five observations were realized on the abaxial surface, in the central area of young leaflets. Evaluations occurred between 10 October and 24 November 2016 and 02 October to 17 November 2017, for the first and second cycles, respectively.
Pseudofruit production was evaluated by harvesting commercial pseudofruit (3/4 mature and with no damage). Pseudofruits were collected twice a week and weighted. The harvesting period was between October 2016 and November 2016 and October 2017 and November 2017, for the first and second cycles, respectively.
2.6. Statistical Analysis
Raw data were checked for normality and homogeneity of variance tests using the Shapiro–Wilk and Hartley’s F max tests, respectively.
As the laboratory test data (means of longevity, number of eggs laid, etc.) did not consider the analysis of variance assumptions, for the multiple-choice, olfactometer, oviposition, and longevity tests, data were analyzed via the Kruskal–Wallis test using Student–Newman–Keuls (SNK) to compare means. For host–plant acceptance, data were analyzed using Mann–Whitney U-tests.
The mean number of TSSM and total plant pseudofruit production data of each plot were used for statistical analysis. The cumulative number of mites per day (CMD) was calculated to account for the cumulative effect of the mite on the strawberry leaves. CMD was calculated by using the following formula: ∑ 0.5 × (An + An+1)/D, where An is the number of mites on sample n, An+1 is the number of mites on the next sample date (n + 1), and D is the number of days between sample “n” and “n+1”. Data were submitted to analysis of variance assumptions tests; then, Tukey test was used to compare means (p < 0.05). If the assumption tests failed, Friedman test was used to compare treatments (p < 0.05).
The BioEstat 5.0 [22] and SASM-Agri [23] software package was used. In all the laboratory experiment data, the mean number of females or eggs was used for statistical analysis. The data in the results section were presented as a percentage for the T-shaped arena and host–plant acceptance tests.
3. Results
3.1. Preference and Acceptance for Oviposition Tests
After 24 h, from a total of 160 females released, 111 were found still walking on the filter paper, another 45 were found on host plants’ leaves, and 4 were missing. TSSM females were positioned mostly on strawberry leaves in the multiple-choice test (Table 1). Higher numbers of eggs were recorded in strawberry leaf discs than in those of aromatic plants (p < 0.01). TSSMs did not lay eggs in basil, garlic, or Chinese chives leaf discs.

Table 1.
Mean number (± Standard deviation) of adult females and number of eggs laid by Tetranychus urticae on strawberry or aromatic plant leaves during the multiple-choice test, after 24 h.
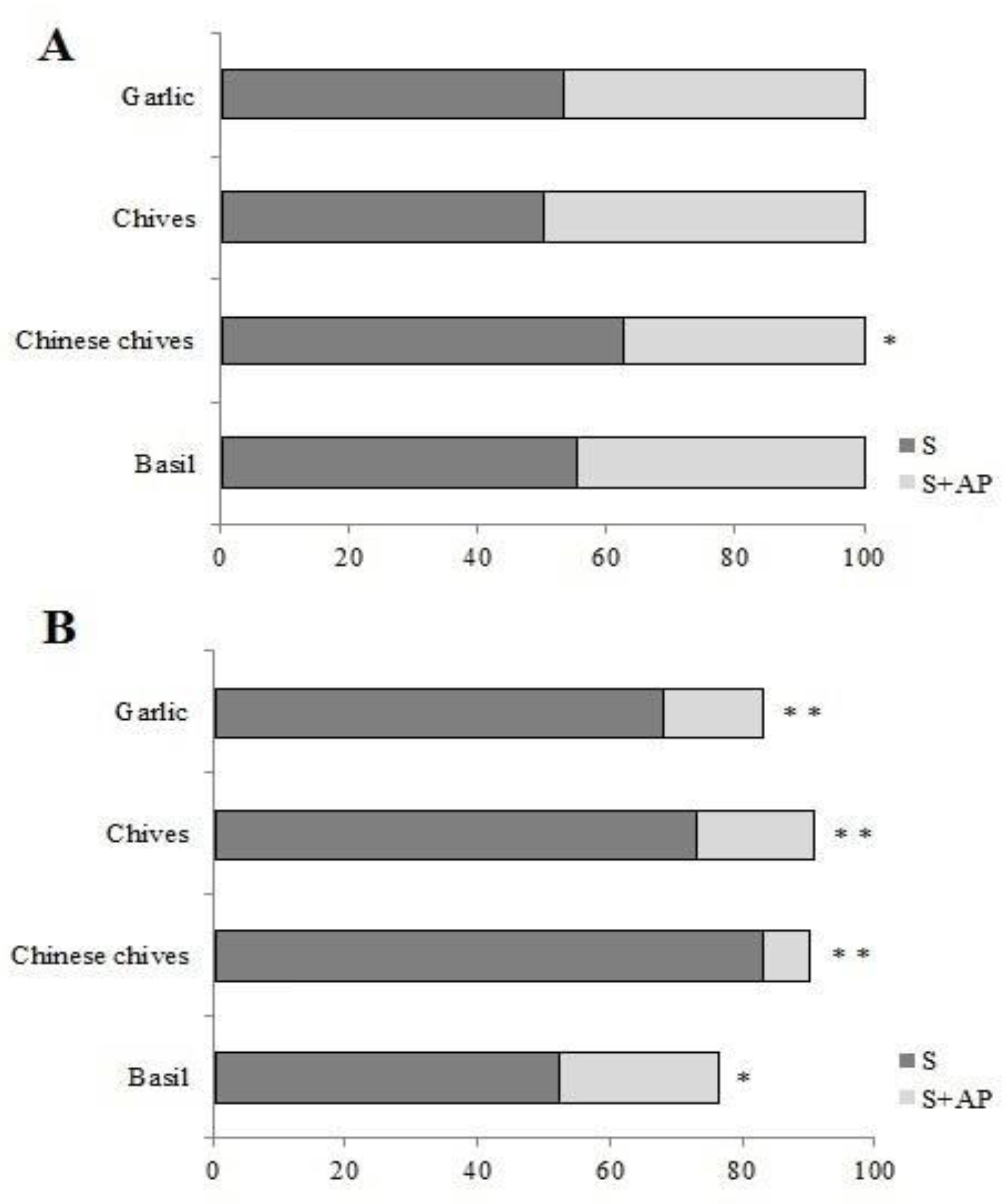
In the T-shaped arena test, the strawberry + Chinese chives treatment was chosen less (37.50%) than strawberry alone (62.50%) for the first choice (p < 0.05) (Figure 3A). Three hours after placement, most TSSM females were found positioned on strawberry leaves rather than on strawberry + basil, Chinese chives, chives, or garlic leaf pieces (52.63%, 83.33%, 73.33%, and 68.29% positive response to strawberry leaves alone, p < 0.05; p < 0.01; p < 0.01; p < 0.01, respectively) (Figure 3B). Note that on the second experiment, the sum is not 100% because some females were unresponsive (Figure 3B).
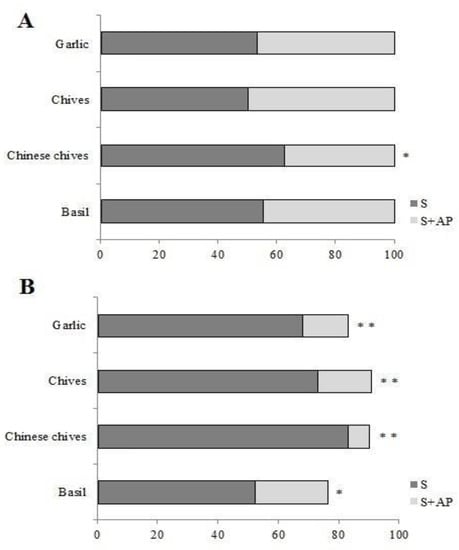
Figure 3.
(A) Tetranychus urticae first choice response percentage on T-shaped arena test. (B) T. urticae percentage response on the same test, 3 h after the test onset. S: in strawberry leaf; S + AP: Strawberry + Aromatic plant leaf. Bars followed by asterisk (* p < 0.05 or ** p < 0.01) denote significant differences based on Kruskal–Wallis and Student–Newman–Keuls (SNK) test statistics between strawberry × strawberry + AP leaves, in the same bar.
In the host–plant acceptance test, when TSSM females were released on strawberry plants, they predominantly remained in this leaf disc (99.17%, 95.83%, 89.17%, 95.83%, and 88.33% of permanence, for basil, Chinese chives, chives, garlic, and strawberry leaf, respectively) (p < 0.01) (Figure 4). When females were released on the Chinese chives and garlic plant, they tended to move to strawberry leaves (p < 0.01) (75.00% and 60.83% onto strawberry, respectively). Conversely, when females were released on basil and chives, a high percentages of them remained on the original disc (60.83% and 71.67% on basil and chives disc, respectively) (p < 0.01).
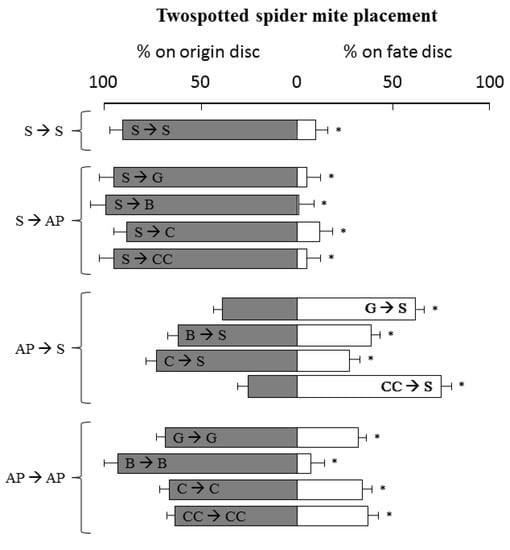
Figure 4.
Tetranychus urticae settlement on strawberry (S) or aromatic plants (AP) leaves (Basil—Ocimum basilicum (B), Chives—Allium schoenoprasum (C), Garlic—Allium sativum (G), Chinese chives—Allium tuberosum (CC)), 24 h after mite release. Bars (± Standard deviation) followed by an asterisk (*) denote significant differences by Mann–Whitney U-tests (p < 0.01) between % of TSSM on origin versus fate disc.
3.2. Oviposition and Longevity
In general, the number of eggs laid by TSSM females was reduced when Amaryllidaceae plants, i.e., chives, Chinese chives, or garlic, were placed next to strawberry leaf discs (p < 0.01) (Table 2). A lower longevity of TSSM females was observed on strawberry discs in the treatment with garlic leaves than in the treatment with only the strawberry leaf disc (p < 0.01).

Table 2.
Number of eggs laid by Tetranychus urticae females on first five days (EFD), total number of eggs (TNE), eggs per day (EPF), and female longevity (LON) (days) means (± Standard deviation) in control, solely containing strawberry leaves or those where strawberry leaves were placed with aromatic plants in boxes.
3.3. Experiments with Amaryllidaceae Plants Intercropping in Greenhouse
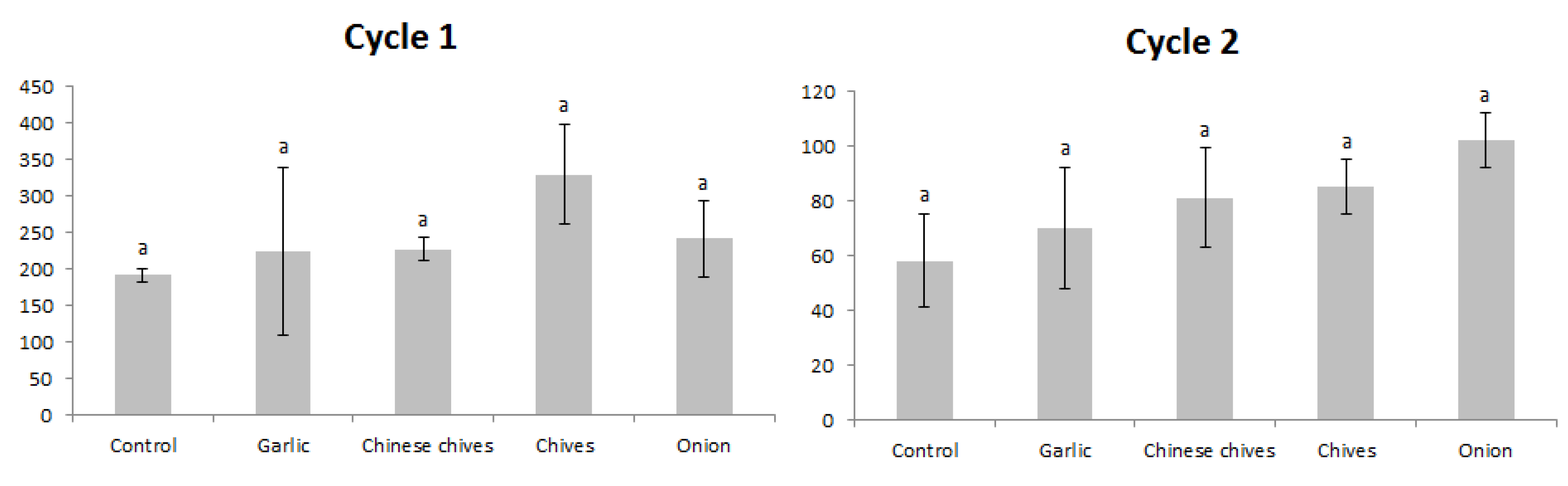
For the first cycle (Table 3), the intercropping of garlic, Chinese chives, and chives presented significantly lower TSSM mean numbers compared to the control on 10 October 2016, and Chinese chives and chives presented lower populations compared to the control on 26 October 2016 (p < 0.05). Taking into account the CMD data, the garlic, Chinese chives, and chives treatments reduced TSSM populations compared to the control (p < 0.05).

Table 3.
Mean number (± Standard deviation) of Tetranychus urticae mobile forms and cumulated mite days (CMD) per cm2, on strawberry leaves, submitted to Amaryllidaceae plants intercropping, in the first cycle. Londrina, Paraná, Brazil, October–November 2016.
For the second cycle (Table 4), garlic, Chinese chives, and chives intercropping presented significantly lower TSSM mean numbers compared to the control on 2 October 2017 (p < 0.05). Chinese chives and chives presented higher populations compared to chives on 17 October 2017 (p < 0.05). Onion intercropping presented higher TSSM populations compared to the control, Chinese chives, and chives (p < 0.05). Taking into account the CMD data, only the chives treatment reduced TSSM populations compared to the control (p < 0.05).

Table 4.
Mean number (± Standard deviation) of Tetranychus urticae mobile forms and cumulated mite days (CMD) per cm2, on strawberry leaves, submitted Amaryllidaceae plants intercropping in second cycle. Londrina, Paraná, Brazil, October–November 2017.
For the production experiment, the mean strawberry pseudofruit production per plant did not vary significantly for the first and second cycles (p > 0.05) (Figure 5).
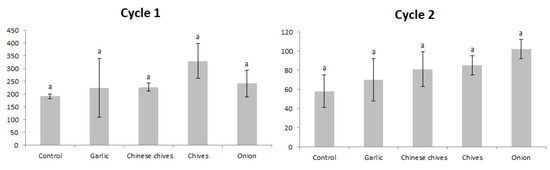
Figure 5.
Mean total pseudofruit production (g) (± Standard deviation) of each strawberry plant under Amaryllidaceae plants’ intercropping, in two crop cycles. Londrina, Paraná, Brazil, October 2016 to November 2017. Bars followed by the same letter are not significantly different by Tukey test (p > 0.05).
4. Discussion
In general, the results presented in our study showed that aromatic plants are not a suitable host for TSSM, and aromatic plant leaves affect its oviposition and life span. Effects were mostly observed on the three Amaryllidaceae plants (Chinese chives, chives, and garlic) used in the tests. These results are important to understand how aromatic plant leaves from these plants affect TSSM in intercropping systems or how essential oils may affect TSSM host plant acceptance for oviposition and life span.
Where TSSMs have multiple choices they critically preferred strawberry leaves (Table 1), and when females were released in strawberry leaves, they remained in the same host and did not move to the aromatic plant (Figure 4), providing evidence for the suitability and preference of the strawberry as a plant host. This corroborates with a previous study, which showed a higher acceptance of TSSM for strawberry leaves than onion, leek, or parsley [11]. Although TSSM females exhibit a weak preference for strawberry than strawberry + Chinese chives in the first-choice assessment (Figure 3A), host selection effectively occurred by changing the host after arriving on the plant (Figure 3B and Figure 4). This pattern held even when females reached strawberry leaves (preferred host plant) + aromatic plant but changed to another branch of the T-shaped arena containing just strawberry (Figure 1B). In a former study, TSSMs also tended to walk away from unfavorable host plants [21]. Therefore, the presence of an aromatic plant in the vicinity of strawberry plants makes strawberry a less favorable host plant than strawberry alone.
When TSSMs were released in garlic or Chinese chives leaves, they predominantly changed to strawberry leaves, and when TSSMs were released on basil and chives, a portion of females changed to strawberry leaves, but the majority remained in these aromatic plants. TSSMs remained mostly in aromatic plants when an option to change to another leaf of the same plant was provided. No change from chives + strawberry to strawberry leaf alone indicates that chives may also be suitable for TSSM development, and this observation corroborates a previous field study, in which TSSM mobile forms were found in high numbers in chives plants intercropped in a strawberry field, probably for shelter [9]. Therefore, despite our promissory greenhouse results for chives uses in intercropping, because of lower TSSM populations when this aromatic plant was used (Table 3 and Table 4), we suggest that its use must occur with parsimony, and TSSM control on chives leaves may be realized as needed.
The presence of Chinese chives or garlic in the vicinity of strawberry leaf diminished TSSM oviposition compared to strawberry leaves alone; however, only garlic diminished TSSM female longevity. Chives reduced the number of eggs laid per day by TSSM females (Table 2). Lower preference and performance may explain the present results obtained in the greenhouse (Table 3 and Table 4) and the results obtained in previous study in which intercropping garlic reduced the number of TSSM mobile forms and eggs in strawberry when garlic was intercropped in the field [9]. Leaf and fruit damage caused by red spider mites (Tetranychus evansi Baker and Pritchard) was also reduced by intercropping garlic in tomato fields [24]. Nevertheless, as shown in cycle 2 (Table 4), Chinese chives or garlic alone could not control TSSM populations. Thus, complementary management as a biological control must be done for TSSM control.
TSSM’s lower preference may be related with the repellence or even antifeedant of aromatic plant; as strawberry plants were available to be associated with the aromatic plant, TSSMs may have been repelled because they moved to leaves without garlic or Chinese chives in the vicinity (Figure 3B). Laboratory tests with garlic bulbs or straw extracts were found to repel TSSMs [25,26]. Chinese chives have also been previously reported as a repellent to arthropods, with essential oils or crushed plants acting against Diaphorina citri Kuwayama (Hemiptera: Psyllidae) [27] and plant extracts acting against Myzus persicae Sulzer (Homoptera: Aphididae) [28]. Aromatic plants may also make strawberry leaf less preferable by TSSM, since diallyl disulfide, which has an antifeedant effect [29], may be absorbed by strawberry leaves [30].
Similar to previous behavioral evidence of the aromatic plants’ negative effects on the TSSM, Amaryllidaceae plant also disrupted the oviposition and life duration of female mites (Table 2). Spraying garlic distillate caused a reduction in TSSM oviposition and high mortality [3]. Similarly, onion and garlic essential oils caused mortality of TSSM adults (67% and 61%, respectively) [31]. A previous study showed that diallyl disulfide added in an Ephestia kuehiella Zeller artificial diet reduces the protein and glycogen content in their fat bodies and decreased activity of digestive enzymes [32].
An intensive effort has been performed to find botanical insecticides/acaricides, i.e., essential oils, esters and fatty acids, glycosides, etc., and studies with Asteraceae, Lamiaceae, Lauraceae, and Myrtaceae plants are highlighted in the literature [33,34]. Garlic is the most studied Amaryllidaceae species for pest control. Our data show a potential use of Chinese chives and chives for TSSM control, and studies with other pests may be conducted. A previous study showed Chinese chives in intercropping reduced Neopamera bilobata Say, an emerging pest of strawberry [7]. In a chemical characterization study, it was revealed that dimethyl disulphide, allyl methyl disulfide, and dimethyl trisulphide were the major essential oils present in Chinese chives and dipropyl disulfide, dipropyl trisulfide, and methyl propyl trisulfide in chives composition [35]. Hence, studies with these Amaryllidaceae plants may present a promissory resource of compounds and essential oils for use in natural pesticide formulations or volatile releasers, as used with garlic oil blend [6], for pest management.
Including two or more plant species dividing the same space and time may reduce one or another plant production. However, in the present study, strawberry pseudofruit productions were not reduced by aromatic plants’ intercropping (Figure 5). These results are in agreement with previous studies with strawberry intercropping [12,14]. The tested Amaryllidaceae plants may have a particular root architecture and depth that exploits nutrients and water without causing a reduction in strawberry yield. In addition, these Amaryllidaceae have thin leaves, which did not reduce the light availability to strawberry plants. The intercropping plants did not provide augmentation in strawberry production. The main advantage is the possibility of the commercialization of aromatic plants, which increases the grower’s income without increasing the production area. Beside this, a reduction in TSSM populations was observed, and an increase of environmental services, such as augmentation of pollinators in the crop area, may also occur as Allium species flowers release floral volatiles that may attract beneficial pollinators [36,37].
5. Conclusions
To summarize, our results showed that TSSM negatively respond to basil, Chinese chives, chives, and garlic leaves. Chinese chives and garlic, which are not well accepted by TSSM, reduce this mite’s total number of eggs and number of eggs laid per day, but only garlic reduces its longevity. Chives reduce the number of eggs laid per day only. For intercropping experiment, chives reduced TSSM populations on strawberry leaves in the two crop cycles and Chinese chives or garlic reduced on the first cycle only. We thus suggest that in an intercropping system, Chinese chives, chives, or garlic may repel TSSM and spoil its oviposition and life span, triggering a lower population of this pest without reducing strawberry pseudofruit production.
Intercropping may be included as one of the tools for integrated pest management of strawberry pests. As Chinese chives and garlic were already tested as an intercrop for the control of N. bilobata, a new pest species in Brazilian strawberry crops [7], this approach may be tested for other pests, such as Drosophila suzukii Matsumura and Duponchelia fovealis Zeller, species with a high potential for causing damage to strawberry. Future studies may also combine sustainable management practices, such as the reduction of the source of fertilization to optimal levels [38,39], use of low impact insecticides and entomopathogenic agents [38,40,41], and incorporation of plants that maintain or enhance natural enemy populations in the crop production landscape [42,43], to build an organic strawberry production protocol that may be used even for conventional strawberry growers or incorporated into others IPM crop protocols.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4395/10/2/193/s1, Figure S1: Greenhouse experimental layout.
Author Contributions
F.T.H.; M.U.V. Performed the experiments and statistical analysis and prepared the manuscript. V.L.B.; F.d.S.G.; J.E.P.d.S.; R.R.M.; V.S. Performed the experiments and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
We would like to thank the research funding agencies Conselho Nacional de Desenvolvimento Científico e Tecnológico—CNPq and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—CAPES, for the scholarships granted.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kortbeek, R.W.J.; van der Gragt, M.; Bleeker, P.M. Endogenous plant metabolites against insects. Eur. J. Plant Pathol. 2018, 154, 67–90. [Google Scholar] [CrossRef]
- Attia, S.; Grissa, K.L.; Lognay, G.; Bitume, E.; Hance, T.; Mailleux, A.C. A review of the major biological approaches to control the worldwide pest Tetranychus urticae (Acari: Tetranychidae) with special reference to natural pesticides. J. Pest Sci. 2013, 86, 361–386. [Google Scholar] [CrossRef]
- Attia, S.; Grissa, K.L.; Mailleux, A.C.; Lognay, G.; Heuskin, S.; Mayoufi, S.; Hance, T. Effective concentrations of garlic distillate (Allium sativum) for the control of Tetranychus urticae (Tetranychidae). J. Appl. Entomol. 2012, 136, 302–312. [Google Scholar] [CrossRef]
- Sağlam, O.; Özder, N. Fumigant toxicity of monoterpenoid compounds against the confused flour beetle, Tribolium confusum Jacquelin du Val. (Coleoptera: Tenebrionidae). Turk. Entomol. Derg. 2013, 37, 457–466. [Google Scholar]
- Sarker, P.K.; Rahman, M.M.; Das, B.C. Effect of intercropping of mustard with onion and garlic on aphid population and yield. J. Bio-Sci. 2007, 15, 35–40. [Google Scholar] [CrossRef]
- Zhou, H.; Chen, J.; Liu, Y.; Francis, F.; Haubruge, E.; Bragard, C.; Sun, J.; Cheng, D. Influence of garlic intercropping or active emitted volatiles in releasers on aphid and related beneficial in wheat fields in China. J. Integr. Agric. 2013, 12, 467–473. [Google Scholar] [CrossRef]
- Hata, F.T.; Ventura, M.U.; Béga, V.L.; Camacho, I.M.; de Paula, M.T. Chinese chives and garlic in intercropping in strawberry high tunnels for Neopamera bilobata Say (Hemiptera: Rhyparochromidae) control. Bull. Entomol. Res. 2018, 109, 419–425. [Google Scholar] [CrossRef]
- Carvalho, M.G.; Bortolotto, O.C.; Ventura, M.U. Aromatic plants affect the selection of host tomato plants by Bemisia tabaci biotype B. Entomol. Exp. Appl. 2017, 162, 86–92. [Google Scholar] [CrossRef]
- Hata, F.T.; Ventura, M.U.; Carvalho, M.G.; Miguel, A.L.; Souza, M.S.; Paula, M.T.; Zawadneak, M.A.C. Intercropping garlic plants reduces Tetranychus urticae in strawberry crop. Exp. Appl. Acarol. 2016, 69, 311–321. [Google Scholar] [CrossRef]
- Hata, F.T.; Ventura, M.U.; Béga, V.L.; Camacho, I.M.; de Paula, M.T. Effects of undercropping Allium tuberosum Rottler ex Sprengel (Amaryllidaceae) on Tetranychus urticae Koch (Acari: Tetranychidae) populations in strawberry. EntomoBrasilis 2017, 10, 178–182. [Google Scholar] [CrossRef]
- Greco, N.M.; Pereyra, P.C.; Guillade, A. Host-plant acceptance and performance of Tetranychus urticae (Acari, Tetranychidae). J. Appl. Entomol. 2006, 130, 32–36. [Google Scholar] [CrossRef]
- Hata, F.T.; Ventura, M.U.; Paula, M.T.; Shimizu, G.D.; de Paula, J.C.B.; Kussaba, D.A.O.; de Souza, N.V. Intercropping garlic in strawberry fields improves land equivalent ratio and gross income. Ciência Rural 2019, 49, e20190338. [Google Scholar] [CrossRef]
- Duval, J.R. Relay-intercropping does not reduce strawberry yield in an annual-hill production system. HortTechnology 2005, 15, 907–908. [Google Scholar] [CrossRef]
- Karlidag, H.; Yildirim, E. Strawberry intercropping with vegetables for proper utilization of space and resources. J. Sustain. Agric. 2009, 33, 107–116. [Google Scholar] [CrossRef]
- Mead, R.; Willey, R.W. The concept of land equivalent ratio and advantages in yields from intercropping. Exp. Agric. 1980, 16, 217–228. [Google Scholar] [CrossRef]
- Emater—Empresa de Assistência Técnica e Extensão Rural do Estado de Minas Gerais—Departamento Técnico; Technical Report; Emater: Belo Horizonte, MG, Brazil, December 2012.
- Grbic, M.; van Leeuwen, T.; Clark, R.M.; Rombauts, S.; Rouze, P.; Grbic, V.; Osborne, E.J.; Dermauw, W.; Ngoc, P.C.T.; Ortego, F.; et al. The genome of Tetranychus urticae reveals herbivorous pest adaptations. Nature 2011, 479, 487–492. [Google Scholar] [CrossRef]
- Moraes, G.J.; Flechtmann, C.H.W. Manual de Acarologia: Acarologia Básica E Ácaros de Plantas Cultivadas No Brasil; Holos: Ribeirão Preto, Brazil, 2008. [Google Scholar]
- Cheng, S.; Lin, R.; Zhang, N.; Yuan, S.; Zhou, X.; Huang, J.; Ren, X.; Wang, S.; Juang, H.; Yu, C. Toxicity of six insecticides to predatory mite Amblyseius cucumeris (Oudemans) (Acari: Phytoseiidae) in-and off-field. Ecotoxicol. Environ. Saf. 2018, 161, 715–720. [Google Scholar] [CrossRef]
- Zanardi, O.Z.; Bordini, G.P.; Franco, A.A.; de Morais, M.R.; Yamamoto, P.T. Spraying pyrethroid and neonicotinoid insecticides can induce outbreaks of Panonychus citri (Trombidiformes: Tetranychidae) in citrus groves. Exp. Appl. Acarol. 2018, 76, 339–354. [Google Scholar] [CrossRef]
- Yano, S.; Wakabayashi, M.; Takabayashi, J.; Takafuji, A. Factors determining the host plant range of the phytophagous mite, Tetranychus urticae (Acari: Tetranychidae): A method for quantifying host plant acceptance. Exp. Appl. Acarol. 1998, 22, 595–601. [Google Scholar] [CrossRef]
- Ayres, M. BioEstat 5.0: Aplicações estatísticas nas áreas das ciências biológicas e médicas; Sociedade Civil Mamirauá: Belém, Brazil, 2007. [Google Scholar]
- Canteri, M.G.; Althaus, R.A.; Virgens Filho, J.S.; Giglioti, E.A.; Godoy, C.V. SASM-Agri: Sistema para análise e separação de médias em experimentos agrícolas pelos métodos Scott-Knott, Tukey e Duncan. Rev. Braz. Agrocomputação 2001, 1, 18–24. [Google Scholar]
- Mtambo, C.C.; Zeledon, I.H. The development of integrated control methods for the tomato red spider mite (Tetranychus evansi) in Malawi. In Agricultural technologies for sustainable development in Malawi, Proceedings of the first annual scientific conference held at the Malawi Institute of Management, Lilongwe, Malawi, 6–10 September, 2000; Phiri, I.M.G., Saka, A.R., Chilembwe, E.H.C., Eds.; CABI: Wallingford, UK; pp. 139–147.
- Hincapié, C.A.; López, G.E.; Torres, R. Comparison and characterization of garlic (Allium sativum L.) bulbs extracts and their effect on mortality and repellency of Tetranychus urticae Koch (Acari: Tetranychidae). Chilean J. Agric. Res. 2008, 68, 317–327. [Google Scholar] [CrossRef]
- Geng, S.; Chen, H.; Zhang, J.; Tu, H. Bioactivity of garlic-straw extracts against the spider mites, Tetranychus urticae and T. viennensis. J. Agric. Urban Entomol. 2014, 30, 38–48. [Google Scholar] [CrossRef]
- Mann, R.S.; Rouseff, R.L.; Smoot, J.M.; Castle, W.S.; Stelinski, L.L. Sulfur volatiles from Allium spp. affect Asian citrus psyllid, Diaphorina citri Kuwayama (Hemiptera: Psyllidae), response to citrus volatiles. Bull. Entomol. Res. 2011, 101, 89–97. [Google Scholar] [CrossRef]
- Amarawardana, L.; Bandara, P.; Kumar, V.; Pettersson, J.; Ninkovic, V.; Glinwood, R. Olfactory response of Myzus persicae (Homoptera: Aphididae) to volatiles from leek and chive: Potential for intercropping with sweet pepper. Agric. Scand. Sect. B—Soil Plant Sci. 2007, 57, 87–91. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, S.X.; Ho, S.H. Bioactivities of methyl allyl disulfide and diallyl trisulfide from essential oil of garlic to two species of stored-product pests, Sitophilus zeamais (Coleoptera: Curculionidae) and Tribolium castaneum (Coleoptera: Tenebrionidae). J. Econ. Entomol. 2000, 93, 537–543. [Google Scholar] [CrossRef]
- Himanen, S.J.; Blande, J.D.; Klemola, T.; Pulkkinen, J.; Heijari, J.; Holopainen, J.K. Birch (Betula spp.) leaves adsorb and re-release volatiles specific to neighbouring plants-a mechanism for associational herbivore resistance? New Phytol. 2010, 186, 722–732. [Google Scholar] [CrossRef]
- Attia, S.; Grissa, K.L.; Ghrabi, Z.G.; Mailleux, A.C.; Lognay, G.; Hance, T. Acaricidal activity of 31 essential oils extracted from plants collected in Tunisia. J. Essent. Oil Res. 2012, 24, 279–288. [Google Scholar] [CrossRef]
- Shahriari, M.; Sahebzadeh, N. Effect of diallyl disulfide on physiological performance of Ephestia kuehniella Zeller (Lepidoptera: Pyralidae). Arch. Phytopathol. Plant Prot. 2017, 50, 33–46. [Google Scholar] [CrossRef]
- Regnault-Roger, C.; Vincent, C.; Arnason, J.T. Essential oils in insect control: Low-risk products in a high-stakes world. Annu. Rev. Entomol. 2012, 57, 405–424. [Google Scholar] [CrossRef]
- Hikal, W.M.; Baeshen, R.S.; Said-Al Ahl, H.A.H. Botanical insecticides as simple extractives for pest control. Cogent Biol. 2017, 3, 1404274. [Google Scholar] [CrossRef]
- Mnayer, D.; Fabiano-Tixier, A.S.; Petitcolas, E.; Hamieh, T.; Nehme, N.; Ferrant, C.; Fernandez, X.; Chemat, F. Chemical composition, antibacterial and antioxidant activities of six essentials oils from the Alliaceae family. Molecules 2014, 19, 20034–20053. [Google Scholar] [CrossRef]
- Zito, P.; Tavella, F.; Pacifico, D.; Campanella, V.; Sajeva, M.; Carimi, F.; Ebmer, A.W.; Dötterl, S. Interspecifc variation of inforescence scents and insect visitors in Allium (Amaryllidaceae: Allioideae). Plant Syst. Evol. 2019, 305, 727–741. [Google Scholar] [CrossRef]
- Karuppaiah, V.; Soumia, P.S.; Wagh, P.D. Diversity and foraging behaviour of insect pollinators in onion. Indian J. Entomol. 2018, 80, 1366–1369. [Google Scholar] [CrossRef]
- Marques-Francovig, C.R.; Mikami, A.Y.; Dutra, V.; Carvalho, M.G.; Picareli, B.; Ventura, M.U. Organic fertilization and botanical insecticides to control two-spotted spider mite in strawberry. Ciência Rural 2014, 44, 1908–1914. [Google Scholar] [CrossRef]
- Hata, F.T.; Ventura, M.U.; de Souza, M.S.D.J.; de Sousa, N.V.; Oliveira, B.G.; da Silva, J.B. Mineral and organic fertilization affects Tetranychus urticae, pseudofruit production and leaf nutrient content in strawberry. Phytoparasitica 2019, 47, 513–521. [Google Scholar] [CrossRef]
- Amatuzzi, R.F.; Cardoso, N.; Poltronieri, A.S.; Poitevin, C.G.; Dalzoto, P.; Zawadeneak, M.A.C.; Pimentel, I.C. Potential of endophytic fungi as biocontrol agents of Duponchelia fovealis (Zeller) (Lepidoptera:Crambidae). Braz. J. Biol. 2018, 78, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Poitevin, C.G.; Porsani, M.V.; Poltronieri, A.S.; Zawadneak, M.A.C.; Pimentel, I.C. Fungi isolated from insects in strawberry crops act as potential biological control agents of Duponchelia fovealis (Lepidoptera: Crambidae). Appl. Entomol. Zool. 2018, 53, 323–331. [Google Scholar] [CrossRef]
- Ottaviano, M.F.G.; Cédola, C.V.; Sánchez, N.E.; Greco, N.M. Conservation biological control in strawberry: Effect of different pollen on development, survival, and reproduction of Neoseiulus californicus (Acari: Phytoseiidae). Exp. Appl. Acarol. 2018, 67, 507–521. [Google Scholar] [CrossRef]
- Peñalver-Cruz, A.; Alvarez-Baca, J.K.; Alfaro-Tapia, A.; Gontijo, L.; Lavandero, B. Manipulation of agricultural habitats to improve conservation biological control in South America. Neotrop. Entomol. 2019, 48, 875–898. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).