Efficiency of an Integrated Purification System for Pig Slurry Treatment under Mediterranean Climate
Abstract
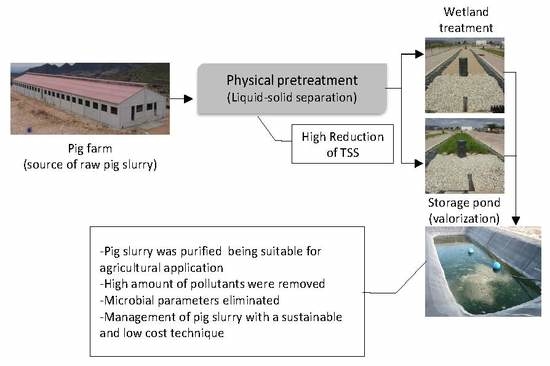
:1. Introduction
2. Materials and Methods
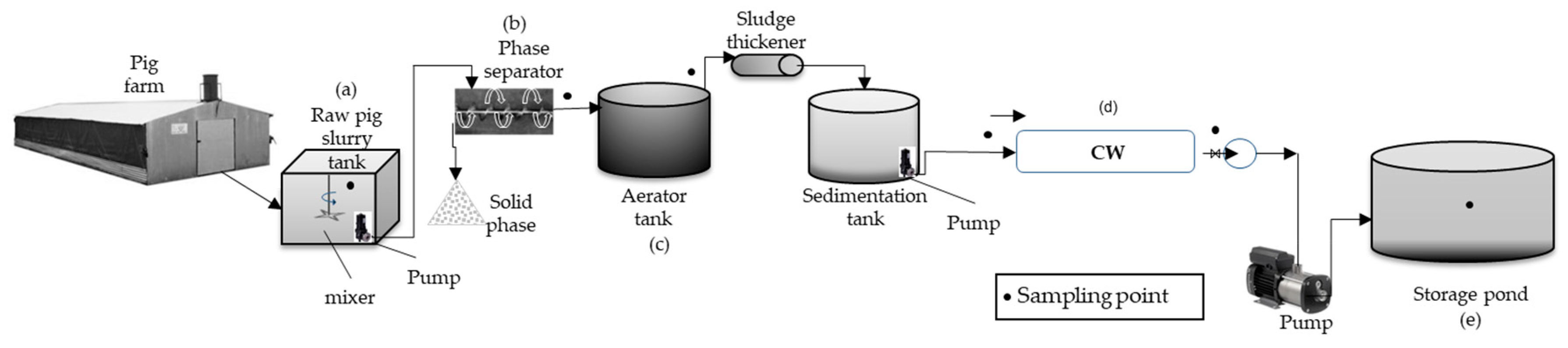
2.1. Location of the Integrated Purification System
2.2. Experimental Design and Sampling
2.3. Pretreatment Module
2.4. Horizontal Subsurface Flow Constructed Wetland Characteristics
2.5. Storage Pond
2.6. Sampling Design
2.7. Physical, Chemical, and Microbial Parameters and Analytical Methods
2.8. Statistical Analysis
3. Results
3.1. Physico-Chemical Parameters
3.1.1. Pretreatment
3.1.2. Horizontal Subsurface Flow Constructed Wetland
3.1.3. Storage Pond
3.2. Microbial Parameters
4. Discussion
4.1. Physic−Chemical Parameters
4.1.1. Pretreatment
4.1.2. Horizontal Subsurface Flow Constructed Wetland
4.1.3. Storage Pond
4.2. Microbial parameters
4.3. Treatment Efficiency
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Daudén, A.; Quílez, D. Pig slurry versus mineral fertilization on corn yield and nitrate leaching in a Mediterranean irrigated environment. Eur. J. Agron. 2004, 21, 7–19. [Google Scholar] [CrossRef]
- Cavanagh, A.; Gasser, M.O.; Labrecque, M. Pig slurry as fertilizer on willow plantation. Biomass Bioenergy 2011, 35, 4165–4173. [Google Scholar] [CrossRef]
- Christensen, M.L.; Hjorth, M.; Keiding, K. Characterization of pig slurry with reference to flocculation and separation. Water Res. 2009, 43, 773–783. [Google Scholar] [CrossRef] [PubMed]
- Ten Hoeve, M.; Hutchings, N.J.; Peters, G.M.; Svanström, M.; Jensen, L.S.; Bruun, S. Life cycle assessment of pig slurry treatment technologies for nutrient redistribution in Denmark. J. Environ. Manag. 2014, 132, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Suresh, A.; Choi, H.L.; Oh, D.I.; Moon, O.K. Prediction of the nutrients value and biochemical characteristics of swine slurry by measurement of EC—Electrical conductivity. Bioresour. Technol. 2009, 100, 4683–4689. [Google Scholar] [CrossRef]
- Cotta, M.A.; Whitehead, T.R.; Zeltwanger, R.L. Isolation, characterization and comparison of bacteria from swine faeces and manure storage pits. Environ. Microbiol. 2003, 5, 737–745. [Google Scholar] [CrossRef] [Green Version]
- Caballero-Lajarín, A.; Zornoza, R.; Faz, A.; Lobera, J.B.; Muñoz, M.A.; Domínguez-Oliver, S.G. Combination of Low-Cost Technologies for Pig Slurry Purification under Semiarid Mediterranean Conditions. Water Air Soil Pollut. 2015, 226, 341. [Google Scholar] [CrossRef]
- Yuan, Z.; Pan, X.; Chen, T.; Liu, X.; Zhang, Y.; Jiang, S.; Sheng, H.; Zhang, L. Evaluating environmental impacts of pig slurry treatment technologies with a life-cycle perspective. J. Clean. Prod. 2018, 188, 840–850. [Google Scholar] [CrossRef]
- Shin, S.-R.; Im, S.; Mostafa, A.; Lee, M.-K.; Yun, Y.-M.; Oh, S.-E.; Kim, D.-H. Effects of pig slurry acidification on methane emissions during storage and subsequent biogas production. Water Res. 2019, 152, 234–240. [Google Scholar] [CrossRef]
- Directive 91/676/EEC of 12 December 1991. Concerning the protection of waters against pollution caused by nitrates from agricultural sources. Off. J. Eur. Commun. 1991, 375, 1–8. [Google Scholar]
- Brockmann, D.; Hanhoun, M.; Négri, O.; Hélias, A. Environmental assessment of nutrient recycling from biological pig slurry treatment—Impact of fertilizer substitution and field emissions. Bioresour. Technol. 2014, 163, 270–279. [Google Scholar] [CrossRef]
- Karakashev, D.; Schmidt, J.E.; Angelidaki, I. Innovative process scheme for removal of organic matter, phosphorus and nitrogen from pig manure. Water Res. 2008, 42, 4083–4090. [Google Scholar] [CrossRef] [PubMed]
- Andreo-Martínez, P.; García-Martínez, N.; Quesada-Medina, J.; Almela, L. Domestic wastewaters reuse reclaimed by an improved horizontal subsurface-flow constructed wetland: A case study in the southeast of Spain. Bioresour. Technol. 2017, 233, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Vymazal, J. Long-term performance of constructed wetlands with horizontal sub-surface flow: Ten case studies from the Czech Republic. Ecol. Eng. 2011, 37, 54–63. [Google Scholar] [CrossRef]
- Barco, A.; Borin, M. Treatment performance and macrophytes growth in a restored hybrid constructed wetland for municipal wastewater treatment. Ecol. Eng. 2017, 107, 160–171. [Google Scholar] [CrossRef]
- Liang, Y.; Zhu, H.; Bañuelos, G.; Yan, B.; Zhou, Q.; Yu, X.; Cheng, X. Constructed wetlands for saline wastewater treatment: A review. Ecol. Eng. 2017, 98, 275–285. [Google Scholar] [CrossRef]
- Saeed, T.; Muntaha, S.; Rashid, M.; Sun, G.; Hasnat, A. Industrial wastewater treatment in constructed wetlands packed with construction materials and agricultural by-products. J. Clean. Prod. 2018, 189, 442–453. [Google Scholar] [CrossRef]
- Huang, X.; Ye, G.; Yi, N.; Lu, L.; Zhang, L.; Yang, L.; Xiao, L.; Liu, J. Effect of plant physiological characteristics on the removal of conventional and emerging pollutants from aquaculture wastewater by constructed wetlands. Ecol. Eng. 2019, 135, 45–53. [Google Scholar] [CrossRef]
- Vymazal, J. Horizontal sub-surface flow constructed wetlands Ondřejov and Spálené Poříčí in the Czech Republic—15 years of operation. Desalination 2009, 246, 226–237. [Google Scholar] [CrossRef]
- Meers, E.; Tack, F.M.G.; Tolpe, I.; Michels, E. Application of a Full-scale Constructed Wetland for Tertiary Treatment of Piggery Manure: Monitoring Results. Water Air Soil Pollut. 2008, 193, 15–24. [Google Scholar] [CrossRef]
- Muñoz, M.A.; Rosales, R.M.; Gabarrón, M.; Faz, A.; Acosta, J.A. Effects of the Hydraulic Retention Time on Pig Slurry Purification by Constructed Wetlands and Stabilization Ponds. Water Air Soil Pollut. 2016, 227, 293. [Google Scholar] [CrossRef]
- Wu, S.; Lei, M.; Lu, Q.; Guo, L.; Dong, R. Treatment of pig manure liquid digestate in horizontal flow constructed wetlands: Effect of aeration. Eng. Life Sci. 2016, 16, 263–271. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Zhu, J. Characteristics of solids, BOD5 and VFAs in liquid swine manure treated by short-term low-intensity aeration for long-term storage. Bioresour. Technol. 2006, 97, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Chiumenti, A. Complete nitrification–denitrification of swine manure in a full-scale, non-conventional composting system. Waste Manag. 2015, 46, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Scholz, M. Piggery wastewater treatment with integrated constructed wetlands. In Wetlands for Water Pollution Control; Elsevier: Amsterdam, The Netherlands, 2015; pp. 419–432. ISBN 9780444636072. [Google Scholar]
- Borin, M.; Politeo, M.; De Stefani, G. Performance of a hybrid constructed wetland treating piggery wastewater. Ecol. Eng. 2013, 51, 229–236. [Google Scholar] [CrossRef]
- Fernandes, J.P.; Almeida, C.M.R.; Pereira, A.C.; Ribeiro, I.L.; Reis, I.; Carvalho, P.; Basto, M.C.P.; Mucha, A.P. Microbial community dynamics associated with veterinary antibiotics removal in constructed wetlands microcosms. Bioresour. Technol. 2015, 182, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zhang, J.; Ngo, H.H.; Guo, W.; Hu, Z.; Liang, S.; Fan, J.; Liu, H. A review on the sustainability of constructed wetlands for wastewater treatment: Design and operation. Bioresour. Technol. 2015, 175, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Licciardello, F.; Milani, M.; Consoli, S.; Pappalardo, N.; Barbagallo, S.; Cirelli, G. Wastewater tertiary treatment options to match reuse standards in agriculture. Agric. Water Manag. 2018, 210, 232–242. [Google Scholar] [CrossRef]
- Duchaufour, P. Precis de Pedologie; Masson: Paris, France, 1970. [Google Scholar]
- Popovic, O.; Gioelli, F.; Dinuccio, E.; Balsari, P. Improved pig slurry mechanical separation using chitosan and biochar. Biosyst. Eng. 2014, 127, 115–124. [Google Scholar] [CrossRef]
- Vymazal, J. Does clogging affect long-term removal of organics and suspended solids in gravel-based horizontal subsurface flow constructed wetlands? Chem. Eng. J. 2018, 331, 663–674. [Google Scholar] [CrossRef]
- Burton, C.H. The potential contribution of separation technologies to the management of livestock manure. Livest. Sci. 2007, 112, 208–216. [Google Scholar] [CrossRef]
- Gómez-Garrido, M.; Faz-Cano, Á.; Martínez-Martínez, S.; Carmona-Garcés, D.M.; Büyükkiliç-Yanardag, A. Nitrogen dynamic and leaching in calcareous soils amended with pig slurry. In Soil Management and Climate Change: Effects on Organic Carbon, Nitrogen Dynamics, and Greenhouse Gas Emissions; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 171–187. ISBN 9780128121290. [Google Scholar]
- Møller, H.B.; Sommer, S.G.; Ahring, B.K. Separation efficiency and particle size distribution in relation to manure type and storage conditions. Bioresour. Technol. 2002, 85, 189–196. [Google Scholar] [CrossRef]
- Hjorth, M.; Christensen, K.V.; Christensen, M.L.; Sommer, S.G. Soli-liquid separation of animal slurry in therory and practice. A review. Agron. Sustain. Dev. 2010, 30, 153–180. [Google Scholar] [CrossRef] [Green Version]
- Reilly, J.F.; Horne, A.J.; Miller, C.D. Nitrate removal from a drinking water supply with large free-surface constructed wetlands prior to groundwater recharge. Ecol. Eng. 2000, 14, 33–47. [Google Scholar] [CrossRef]
- Chung, A.K.; Wu, Y.; Tam, N.F.; Wong, M.H. Nitrogen and phosphate mass balance in a sub-surface flow constructed wetland for treating municipal wastewater. Ecol. Eng. 2008, 32, 81–89. [Google Scholar] [CrossRef]
- Knight, R.L.; Payne, V.W., Jr.; Borer, R.E.; Clarke, R.A., Jr.; Pries, J.H. Constructed wetlands for livestock wastewater management. Ecol. Eng. 2000, 15, 41–55. [Google Scholar] [CrossRef]
- Bernal, M.P.; Alburquerque, J.A.; Moral, R. Composting of animal manures and chemical criteria for compost maturity assessment. A review. Bioresour. Technol. 2009, 100, 5444–5453. [Google Scholar] [CrossRef]
- Lopez-Ridaura, S.; Werf, H.; van der Paillat, J.M.; Le Bris, B. Environmental evaluation of transfer and treatment of excess pig slurry by life cycle assessment. J. Environ. Manag. 2009, 90, 1296–1304. [Google Scholar] [CrossRef]
- Akratos, C.S.; Tsihrintzis, V.A. Effect of temperature, HRT, vegetation and porous media on removal efficiency of pilot-scale horizontal subsurface flow constructed wetlands. Ecol. Eng. 2007, 29, 173–191. [Google Scholar] [CrossRef]
- Knowles, P.; Dotro, G.; Nivala, J.; García, J. Clogging in subsurface-flow treatment wetlands: Occurrence and contributing factors. Ecol. Eng. 2011, 37, 99–112. [Google Scholar] [CrossRef]
- Bayley, M.L.; Davison, L.M.; Headley, T.R. Nitrogen removal from domestic effluent using subsurface flow constructed wetlands: Influence of depth, hydraulic residence time and pre-nitrification. Water Sci. Technol. 2003, 48, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Kaseva, M.E. Performance of a sub-surface flow constructed wetland in polishing pre-treated wastewater—A tropical case study. Water Res. 2004, 38, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Brix, H. Do macrophytes play a role in constructed treatment wetlands? Water Sci. Technol. 1997, 35, 11–17. [Google Scholar] [CrossRef]
- Caselles-Osorio, A.; Villafañe, P.; Caballero, V.; Manzano, Y. Efficiency of Mesocosm-Scale Constructed Wetland Systems for Treatment of Sanitary Wastewater under Tropical Conditions. Water Air Soil Pollut. 2011, 220, 161–171. [Google Scholar] [CrossRef]
- Wang, R.; Baldy, V.; Périssol, C.; Korboulewsky, N. Influence of plants on microbial activity in a vertical-downflow wetland system treating waste activated sludge with high organic matter concentrations. J. Environ. Manag 2012, 95, S158–S164. [Google Scholar] [CrossRef]
- Bachand, P.A.; Horne, A.J. Denitrification in constructed free-water surface wetlands: I. Very high nitrate removal rates in a macrocosm study. Ecol. Eng. 2000, 14, 9–15. [Google Scholar] [CrossRef]
- Tsalkatidou, M.; Gratziou, M.; Kotsovinos, N. Combined stabilization ponds-constructed wetland system. Desalination 2009, 248, 988–997. [Google Scholar] [CrossRef]
- Polprasert, C.; Kittipongvises, S. Constructed Wetlands and Waste Stabilization Ponds. Treatise Water Sci. 2011, 4, 277–299. [Google Scholar]
- Mbuligwe, S.E. Comparative effectiveness of engineered wetland systems in the treatment of anaerobically pre-treated domestic wastewater. Ecol. Eng. 2004, 23, 269–284. [Google Scholar] [CrossRef]
- Kadlec, H.R.; Wallace, D.S. Treatment Wetlands, 2nd ed.; Taylor & Francis Group: Boca Ratón, FL, USA, 2009; ISBN 9781566705264. [Google Scholar]
- Gill, L.W.; Ring, P.; Casey, B.; Higgins, N.M.P.; Johnston, P.M. Long term heavy metal removal by a constructed wetland treating rainfall runoff from a motorway. Sci. Total Environ. 2017, 601, 32–44. [Google Scholar] [CrossRef]
- Terzakis, S.; Fountoulakis, M.S.; Georgaki, I.; Albantakis, D.; Sabathianakis, I.; Karathanasis, A.D.; Kalogerakis, N.; Manios, T. Constructed wetlands treating highway runoff in the central Mediterranean region. Chemosphere 2008, 72, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.; Suthar, S. Performance assessment of horizontal and vertical surface flow constructed wetland system in wastewater treatment using multivariate principal component analysis. Ecol. Eng. 2018, 116, 121–126. [Google Scholar] [CrossRef]
- Vohla, C.; Kõiv, M.; Bavor, H.J.; Chazarenc, F.; Mander, Ü. Filter materials for phosphorus removal from wastewater in treatment wetlands—A review. Ecol. Eng. 2011, 37, 70–89. [Google Scholar] [CrossRef]
- Kadlec, R.H.; Knight, R.L. Treatment Wetland. Ecol. Eng. 1997, 8, 173–175. [Google Scholar]
- Vymazal, J. Constructed wetlands for treatment of industrial wastewaters: A review. Ecol. Eng. 2014, 73, 724–751. [Google Scholar] [CrossRef]
- Massé, D.; Gilbert, Y.; Topp, E. Pathogen removal in farm-scale psychrophilic anaerobic digesters processing swine manure. Bioresour. Technol. 2011, 102, 641–646. [Google Scholar] [CrossRef]
- Ottová, V.; Balcarová, J.; Vymazal, J. Microbial characteristics of constructed wetlands. Water Sci. Technol. 1997, 35, 117–123. [Google Scholar] [CrossRef]
- Møller, H.B.; Lund, I.; Sommer, S.G. Solid-liquid separation of livestock slurry: Efficiency and cost. Bioresour. Technol. 2000, 74, 223–229. [Google Scholar] [CrossRef]
- Xu, X.; Mills, G.L. Do constructed wetlands remove metals or increase metal bioavailability? J. Environ. Manag 2018, 218, 245–255. [Google Scholar] [CrossRef]


| Parameters * | Module (n = 3) | Efficiency (%) | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RST | PHS | AT | IW1 | OW1 | SP | ||||||||||||||||||||||||
| PHS | AT | IW1 | OW | SP | |||||||||||||||||||||||||
| T (°C) | 16.4 | ± | 1.1 | a | 16.4 | ± | 0.4 | a | 15.2 | ± | 0.4 | a | 18.1 | ± | 1.8 | a | 18.5 | ± | 0.7 | a | 15.5 | ± | 1.6 | a | 0 | 8 | −19 | −2 | 16 |
| TSS (g L−1) | 60.2 | ± | 0.8 | c | 38.9 | ± | 0.6 | bc | 40.5 | ± | 1.8 | bc | 26.1 | ± | 26.2 | ab | 2.1 | ± | 0.6 | a | 2.1 | ± | 0.7 | a | 35 | −4 | 36 | 92 | 4 |
| pH | 7.7 | ± | 0.0 | ab | 7.8 | ± | 0.0 | ab | 8.2 | ± | 0.0 | b | 8.2 | ± | 0.4 | b | 7.6 | ± | 0.1 | a | 7.8 | ± | 0.0 | ab | −1 | −6 | 0 | 7 | −3 |
| EC (dS m−1) | 19.5 | ± | 0.3 | b | 21.2 | ± | 0.5 | b | 21.0 | ± | 0.3 | b | 19.7 | ± | 2.8 | b | 16.9 | ± | 2.1 | b | 12.2 | ± | 0.1 | a | −9 | 1 | 6 | 14 | 28 |
| TN (g L−1) | 4.3 | ± | 0.1 | b | 3.8 | ± | 0.1 | b | 3.8 | ± | 0.2 | b | 3.5 | ± | 1.1 | ab | 3.1 | ± | 0.9 | ab | 2.0 | ± | 0.4 | a | 11 | −1 | 10 | 10 | 37 |
| KN (g L−1) | 4.2 | ± | 0.1 | b | 3.7 | ± | 0.1 | b | 3.7 | ± | 0.1 | b | 3.4 | ± | 1.2 | b | 1.4 | ± | 0.2 | a | 0.5 | ± | 0.0 | a | 11 | −1 | 10 | 58 | 64 |
| NH4+ (g L−1) | 2.8 | ± | 0.2 | b | 2.6 | ± | 0.1 | b | 2.5 | ± | 0.1 | b | 2.3 | ± | 0.6 | b | 1.1 | ± | 0.2 | a | 0.4 | ± | 0.0 | a | 6 | 4 | 10 | 50 | 63 |
| ON (g L−1) | 1.4 | ± | 0.3 | b | 1.1 | ± | 0.1 | b | 1.2 | ± | 0.2 | b | 1.1 | ± | 0.6 | b | 0.3 | ± | 0.1 | a | 0.1 | ± | 0.0 | a | 23 | −12 | 9 | 73 | 66 |
| BOD5 (g L−1) | 4.4 | ± | 0.5 | d | 4.2 | ± | 0.4 | cd | 4.2 | ± | 0.5 | d | 2.6 | ± | 1.2 | bc | 1.0 | ± | 0.1 | ab | 0.6 | ± | 0.2 | a | 5 | −1 | 39 | 61 | 36 |
| COD (g L−1) | 37.0 | ± | 2.0 | b | 32.3 | ± | 3.2 | b | 34.7 | ± | 2.3 | b | 26.6 | ± | 19.7 | ab | 4.9 | ± | 0.9 | a | 2.8 | ± | 0.1 | a | 13 | −7 | 23 | 82 | 42 |
| TP (mg L−1) | 1662 | ± | 335 | b | 1608 | ± | 60.3 | b | 1728 | ± | 321 | b | 1093 | ± | 1044 | ab | 59.4 | ± | 19.0 | a | 21.8 | ± | 1.9 | a | 3 | −7 | 37 | 95 | 63 |
| Cu (mg L−1) | 2.5 | ± | 0.5 | b | 1.3 | ± | 0.1 | a | 1.3 | ± | 0.2 | a | 1.5 | ± | 0.8 | ab | 0.6 | ± | 0.3 | a | 0.7 | ± | 0.1 | a | 49 | 2 | −19 | 60 | −21 |
| Fe (mg L−1) | 0.7 | ± | 0.4 | a | 13.6 | ± | 2.0 | cd | 11.9 | ± | 1.7 | c | 18.1 | ± | 1.8 | d | 7.0 | ± | 2.8 | b | 2.4 | ± | 0.1 | a | −1852 | 12 | −52 | 61 | 66 |
| Mn (mg L−1) | 12.1 | ± | 2.1 | d | 2.8 | ± | 0.2 | cd | 2.6 | ± | 0.5 | c | 2.2 | ± | 0.4 | c | 0.3 | ± | 0.2 | b | 0.1 | ± | 0.0 | a | 77 | 7 | 14 | 85 | 67 |
| Zn (mg L−1) | 1.6 | ± | 0.1 | ab | 6.1 | ± | 0.8 | ab | 6.9 | ± | 1.1 | ab | 8.2 | ± | 5.0 | b | 3.0 | ± | 2.0 | ab | 1.1 | ± | 0.1 | a | −275 | −12 | −19 | 64 | 62 |
| Cl− (mg L−1) | 1000 | ± | 68 | b | 1028 | ± | 51.8 | b | 985 | ± | 32.8 | b | 954 | ± | 77.2 | ab | 903 | ± | 91.7 | ab | 797 | ± | 54.2 | a | −3 | 4 | 3 | 5 | 12 |
| Br− (mg L−1) | 12.1 | ± | 0.2 | ab | 12.2 | ± | 0.1 | ab | 13.3 | ± | 0.9 | ab | 14.3 | ± | 2.2 | b | 12.3 | ± | 0.8 | ab | 11.9 | ± | 0.2 | a | −1 | −9 | −7 | 14 | 4 |
| SO4−2 (mg L−1) | 88.9 | ± | 9.3 | a | 157 | ± | 29.3 | a | 74.5 | ± | 18.2 | a | 152 | ± | 159 | a | 196 | ± | 181 | a | 607 | ± | 20.6 | b | −77 | 53 | −104 | −29 | −209 |
| NO3− (mg L−1) | 35.1 | ± | 1.2 | a | 37.1 | ± | 4.2 | a | 34.9 | ± | 1.0 | a | 36.5 | ± | 1.8 | a | 36.1 | ± | 5.8 | a | 28.4 | ± | 1.1 | a | −6 | 6 | −5 | 1 | 21 |
| NO2− (mg L−1) | 65.7 | ± | 17.6 | a | 86.2 | ± | 9.2 | a | 49.7 | ± | 9.9 | a | 78.7 | ± | 64.7 | a | 1464 | ± | 692 | b | 1200 | ± | 30.3 | b | −31 | 42 | −58 | −1761 | 18 |
| F− (mg L−1) | 2.8 | ± | 0.1 | a | 2.6 | ± | 0.9 | a | 3.0 | ± | 1.0 | a | 2.4 | ± | 0.7 | a | 1.9 | ± | 0.1 | a | 2.0 | ± | 0.0 | a | 8 | −18 | 19 | 21 | −1 |
| Ca+2 (mg L−1) | 14.5 | ± | 3.4 | a | 57.7 | ± | 1.6 | ab | 54.4 | ± | 8.4 | ab | 90.6 | ± | 45.7 | b | 15.7 | ± | 8.5 | a | 27.3 | ± | 1.1 | a | −297 | 6 | −66 | 83 | −74 |
| Mg+2 (mg L−1) | 122 | ± | 25.6 | ab | 204 | ± | 2.1 | b | 229 | ± | 54.9 | b | 149 | ± | 87.0 | ab | 43.2 | ± | 20.0 | a | 119 | ± | 37.1 | ab | −67 | −12 | 35 | 71 | −176 |
| Na+ (mg L−1) | 421 | ± | 66.2 | bc | 262 | ± | 64.1 | ab | 173 | ± | 61.2 | a | 347 | ± | 111 | abc | 455 | ± | 46.2 | c | 430 | ± | 17.8 | bc | 38 | 34 | −100 | −31 | 5 |
| K+ (mg L−1) | 1504 | ± | 143 | b | 1307 | ± | 65.1 | ab | 1230 | ± | 274 | ab | 1316 | ± | 109 | ab | 1110 | ± | 164 | ab | 936 | ± | 9.0 | a | 13 | 6 | −7 | 16 | 16 |
| Parameters * | Module (n = 3) | Efficiency (%) | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RST | PHS | AT | IW2 | OW2 | SP | ||||||||||||||||||||||||
| PHS | AT | IW2 | OW2 | SP | |||||||||||||||||||||||||
| T (°C) | 18.2 | ± | 0.0 | b | 19.9 | ± | 0.0 | bc | 20.3 | ± | 0.6 | c | 19.3 | ± | 0.1 | bc | 19.8 | ± | 1.5 | bc | 16.0 | ± | 0.4 | a | −9 | −2 | 5 | −3 | 19 |
| TSS (g L−1) | 56.3 | ± | 9.3 | c | 40.2 | ± | 1.0 | b | 36.8 | ± | 3.4 | b | 29.5 | ± | 8.4 | b | 2.2 | ± | 0.7 | a | 1.5 | ± | 0.1 | a | 29 | 8 | 20 | 93 | 34 |
| pH | 7.8 | ± | 0.1 | ab | 7.9 | ± | 0.1 | ab | 8.3 | ± | 0.0 | b | 8.1 | ± | 0.3 | b | 7.6 | ± | 0.2 | a | 8.0 | ± | 0.0 | ab | −1 | −5 | 2 | 7 | −5 |
| EC (dS m−1) | 22.2 | ± | 2.0 | bc | 24.0 | ± | 0.7 | c | 23.5 | ± | 0.5 | c | 22.5 | ± | 1.0 | bc | 20.4 | ± | 0.9 | b | 14.4 | ± | 0.2 | a | −8 | 2 | 4 | 9 | 29 |
| TN (g L−1) | 5.1 | ± | 0.3 | b | 4.5 | ± | 0.2 | b | 4.3 | ± | 0.4 | b | 4.0 | ± | 0.5 | b | 1.9 | ± | 0.4 | a | 1.7 | ± | 1.1 | a | 11 | 3 | 7 | 52 | 14 |
| KN (g L−1) | 5.0 | ± | 0.3 | c | 4.5 | ± | 0.2 | bc | 4.3 | ± | 0.4 | bc | 4.0 | ± | 0.5 | b | 1.9 | ± | 0.4 | a | 1.0 | ± | 0.0 | a | 11 | 3 | 7 | 52 | 48 |
| NH4+ (g L−1) | 3.6 | ± | 0.2 | c | 3.6 | ± | 0.2 | c | 3.0 | ± | 0.1 | c | 3.0 | ± | 0.3 | c | 1.6 | ± | 0.5 | b | 0.8 | ± | 0.0 | a | 1 | 17 | 0 | 45 | 49 |
| ON (g L−1) | 1.4 | ± | 0.1 | b | 1.1 | ± | 0.1 | b | 1.3 | ± | 0.3 | b | 1.0 | ± | 0.3 | b | 0.3 | ± | 0.1 | a | 0.2 | ± | 0.0 | a | 22 | −23 | 22 | 73 | 38 |
| BOD5 (g L−1) | 1.2 | ± | 0.3 | ab | 2.5 | ± | 0.2 | bc | 3.0 | ± | 0.5 | c | 3.3 | ± | 1.0 | c | 1.5 | ± | 0.4 | ab | 0.9 | ± | 0.0 | a | −103 | −20 | −10 | 54 | 41 |
| COD (g L−1) | 35.3 | ± | 7.8 | b | 35.7 | ± | 2.1 | b | 27.0 | ± | 7.0 | b | 28.7 | ± | 5.4 | b | 6.5 | ± | 1.0 | a | 5.6 | ± | 0.1 | a | −1 | 24 | −6 | 77 | 13 |
| TP (mg L−1) | 1953 | ± | 292 | d | 1697 | ± | 184 | cd | 1272 | ± | 67.5 | b | 1398 | ± | 43.3 | b | 44.1 | ± | 11.1 | a | 31.4 | ± | 0.5 | a | 13 | 25 | −10 | 97 | 29 |
| Cu (mg L−1) | 1.5 | ± | 0.4 | a | 2.3 | ± | 0.4 | a | 2.7 | ± | 0.2 | a | 1.6 | ± | 0.8 | a | 2.3 | ± | 0.9 | a | 1.8 | ± | 0.0 | a | −58 | −16 | 41 | −46 | 24 |
| Fe (mg L−1) | 13.9 | ± | 2.4 | b | 20.9 | ± | 3.1 | cd | 22.4 | ± | 0.6 | d | 17.3 | ± | 2.2 | bc | 8.5 | ± | 0.2 | a | 8.7 | ± | 0.4 | a | −51 | −7 | 23 | 51 | −2 |
| Mn (mg L−1) | 2.3 | ± | 0.5 | bc | 2.9 | ± | 0.4 | c | 2.7 | ± | 0.3 | c | 1.8 | ± | 0.3 | b | 0.3 | ± | 0.2 | a | 0.2 | ± | 0.0 | a | −29 | 7 | 35 | 82 | 27 |
| Zn (mg L−1) | 6.5 | ± | 1.1 | abc | 10.3 | ± | 1.5 | cd | 12.4 | ± | 0.8 | d | 9.8 | ± | 1.7 | bcd | 5.9 | ± | 2.3 | ab | 5.4 | ± | 0.2 | a | −59 | −21 | 21 | 40 | 8 |
| Cl− (mg L−1) | 1359 | ± | 33.2 | a | 1369 | ± | 18.7 | a | 1425 | ± | 31.2 | ab | 1277 | ± | 44.1 | a | 1572 | ± | 145 | b | 1365 | ± | 39.4 | a | −1 | −4 | 10 | −23 | 13 |
| Br− (mg L−1) | 12.3 | ± | 0.7 | a | 11.9 | ± | 0.8 | a | 12.4 | ± | 0.0 | a | 13.8 | ± | 1.9 | a | 13.1 | ± | 0.8 | a | 13.3 | ± | 0.2 | a | 4 | −5 | −11 | 5 | −1 |
| SO4−2 (mg L−1) | 52.1 | ± | 3.9 | a | 43.5 | ± | 4.5 | a | 46.0 | ± | 2.3 | a | 202 | ± | 16.9 | b | 327 | ± | 98.5 | c | 97.1 | ± | 0.2 | ab | 16 | −6 | −339 | −62 | 70 |
| NO3− (mg L−1) | 30.4 | ± | 0.8 | a | 30.3 | ± | 0.5 | a | 30.8 | ± | 1.4 | a | 33.5 | ± | 5.6 | a | 28.0 | ± | 2.0 | a | 31.6 | ± | 1.8 | a | 0 | −2 | −9 | 16 | −13 |
| NO2− (mg L−1) | 4.5 | ± | 0.4 | a | 4.7 | ± | 0.5 | a | 4.8 | ± | 0.5 | a | 4.5 | ± | 0.4 | a | 4.2 | ± | 0.5 | a | 4.1 | ± | 0.2 | a | −5 | −3 | 7 | 7 | 2 |
| F− (mg L−1) | 3.1 | ± | 1.0 | a | 3.3 | ± | 0.6 | a | 3.1 | ± | 0.8 | a | 2.1 | ± | 0.3 | a | 2.0 | ± | 0.1 | a | 2.0 | ± | 0.1 | a | −5 | 6 | 32 | 6 | −2 |
| Ca+2 (mg L−1) | 98.9 | ± | 17.9 | b | 106 | ± | 27.4 | b | 112 | ± | 19.6 | b | 107 | ± | 13.6 | b | 53 | ± | 36.1 | a | 33.1 | ± | 0.3 | a | −7 | −5 | 4 | 51 | 37 |
| Mg+2 (mg L−1) | 212 | ± | 41.2 | cd | 269 | ± | 16.0 | d | 192 | ± | 0.2 | cd | 158 | ± | 12.5 | bc | 82 | ± | 50.3 | ab | 80.7 | ± | 8.6 | a | −27 | 29 | 18 | 48 | 2 |
| Na+ (mg L−1) | 670 | ± | 141 | b | 188 | ± | 5.5 | a | 219 | ± | 61.3 | a | 371 | ± | 66.9 | a | 720 | ± | 25.2 | b | 802 | ± | 13.3 | b | 72 | −16 | −69 | −94 | −11 |
| K+ (mg L−1) | 1907 | ± | 234 | bc | 2202 | ± | 63.2 | c | 2192 | ± | 32.7 | c | 1855 | ± | 105 | b | 738 | ± | 115 | a | 827 | ± | 8.9 | a | −15 | 0 | 15 | 60 | −12 |
| Parameter * | Modules (n = 3) | Efficiency (%) | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RST | PHS | AT | IW1 | OW1 | SP | PHS | AT | W1 | SP | Total | |||||||||||||
| FS (UFC L−1) | 6.1 × 104 | ± | 9.2 × 103 | 6.6 × 104 | ± | 1.6 × 104 | 1.9 × 104 | ± | 1.9 × 104 | 7.5 × 104 | ± | 8.4 × 104 | 5.1 × 103 | ± | 4.2 × 103 | 7.7 × 103 | ± | 8.5 × 103 | −8 | 71 | 93 | −50 | 87 |
| MA (UFC L−1) | 1.5 × 106 | ± | 3.5 × 105 | 2.1 × 106 | ± | 6.0 × 105 | 4.6 × 106 | ± | 1.7 × 106 | 3.7 × 106 | ± | 2.2 × 106 | 2.3 × 106 | ± | 2.0 × 106 | 3.2 × 106 | ± | 5.5 × 105 | −40 | −119 | 38 | −42 | −113 |
| FC (UFC L−1) | 1.7 × 105 | ± | 4.6 × 104 | 2.0 × 105 | ± | 0 | 5.7 × 104 | ± | 1.6 × 104 | 1.4 × 104 | ± | 1.2 × 104 | 5.5 × 103 | ± | 1.1 × 104 | 2.7 × 104 | ± | 7.6 × 103 | −18 | 72 | 62 | −391 | 84 |
| TC (UFC L−1) | 1.2 × 104 | ± | 3.1 × 103 | 1.2 × 104 | ± | 3.2 × 103 | 1.1 × 104 | ± | 7.6 × 103 | 4.4 × 103 | ± | 3.2 × 103 | 0 | ± | 0 | 0 | ± | 0 | 0 | 8 | 100 | 0 | 100 |
| E. coli (UFC L−1) | 8.5 × 104 | ± | 7.6 × 103 | 1.3 × 105 | ± | 1.5 × 104 | 1.2 × 105 | ± | 1.5 × 104 | 1.2 × 104 | ± | 1.5 × 104 | 1.7 × 103 | ± | 4.2 × 102 | 0 | ± | 0 | −53 | 8 | 87 | 100 | 100 |
| Parameter * | Modules (n = 3) | Efficiency (%) | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RST | PHS | AT | IW2 | OW2 | SP | PHS | AT | W2 | SP | Total | |||||||||||||
| FS (UFC L−1) | 1.7 × 104 | ± | 1.7 × 103 | 1.4 × 104 | ± | 5.6 × 103 | 3.5 × 103 | ± | 1.0 × 103 | 3.5 × 104 | ± | 7.6 × 104 | 5.2 × 102 | ± | 7.6 × 102 | 0 | ± | 0 | 19 | 75 | 99 | 100 | 100 |
| MA (UFC L−1) | 1.1 × 106 | ± | 3.9 × 105 | 1.6 × 106 | ± | 2.9 × 105 | 8.9 × 105 | ± | 5.2 × 105 | 1.0 × 106 | ± | 6.6 × 105 | 8.1 × 105 | ± | 4.3 × 105 | 1.6 × 106 | ± | 7.4 × 105 | −55 | 46 | 22 | −97 | −52 |
| FC (UFC L−1) | 1.0 × 103 | ± | 1.5 × 103 | 2.0 × 103 | ± | 4.0 × 102 | 0 | ± | 0 | 4.7 × 102 | ± | 1.2 × 102 | 9.0 × 102 | ± | 9.3 × 102 | 0 | ± | 0 | −95 | 100 | −93 | 100 | 100 |
| TC (UFC L−1) | 1.7 × 103 | ± | 2.6 × 103 | 1.1 × 104 | ± | 5.2 × 103 | 0 | ± | 0 | 1.6 × 103 | ± | 7.3 × 102 | 2.5 × 103 | ± | 2.3 × 103 | 0 | ± | 0 | −557 | 100 | −57 | 100 | 100 |
| E. coli (UFC L−1) | 0 | ± | 0 | 0 | ± | 0 | 0 | ± | 0 | 2.9 × 102 | ± | 1.1 × 102 | 0 | ± | 0 | 0 | ± | 0 | 0 | 0 | 100 | 0 | 0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terrero, M.A.; Muñoz, M.Á.; Faz, Á.; Gómez-López, M.D.; Acosta, J.A. Efficiency of an Integrated Purification System for Pig Slurry Treatment under Mediterranean Climate. Agronomy 2020, 10, 208. https://doi.org/10.3390/agronomy10020208
Terrero MA, Muñoz MÁ, Faz Á, Gómez-López MD, Acosta JA. Efficiency of an Integrated Purification System for Pig Slurry Treatment under Mediterranean Climate. Agronomy. 2020; 10(2):208. https://doi.org/10.3390/agronomy10020208
Chicago/Turabian StyleTerrero, Martire Angélica, María Ángeles Muñoz, Ángel Faz, María Dolores Gómez-López, and Jose A. Acosta. 2020. "Efficiency of an Integrated Purification System for Pig Slurry Treatment under Mediterranean Climate" Agronomy 10, no. 2: 208. https://doi.org/10.3390/agronomy10020208
APA StyleTerrero, M. A., Muñoz, M. Á., Faz, Á., Gómez-López, M. D., & Acosta, J. A. (2020). Efficiency of an Integrated Purification System for Pig Slurry Treatment under Mediterranean Climate. Agronomy, 10(2), 208. https://doi.org/10.3390/agronomy10020208