Impact of Nonchemical Protection of Broad Bean on Epigeic and Soil Arthropodofauna—Analysis in Field-Realistic Conditions
Abstract
:1. Introduction
2. Materials and Methods
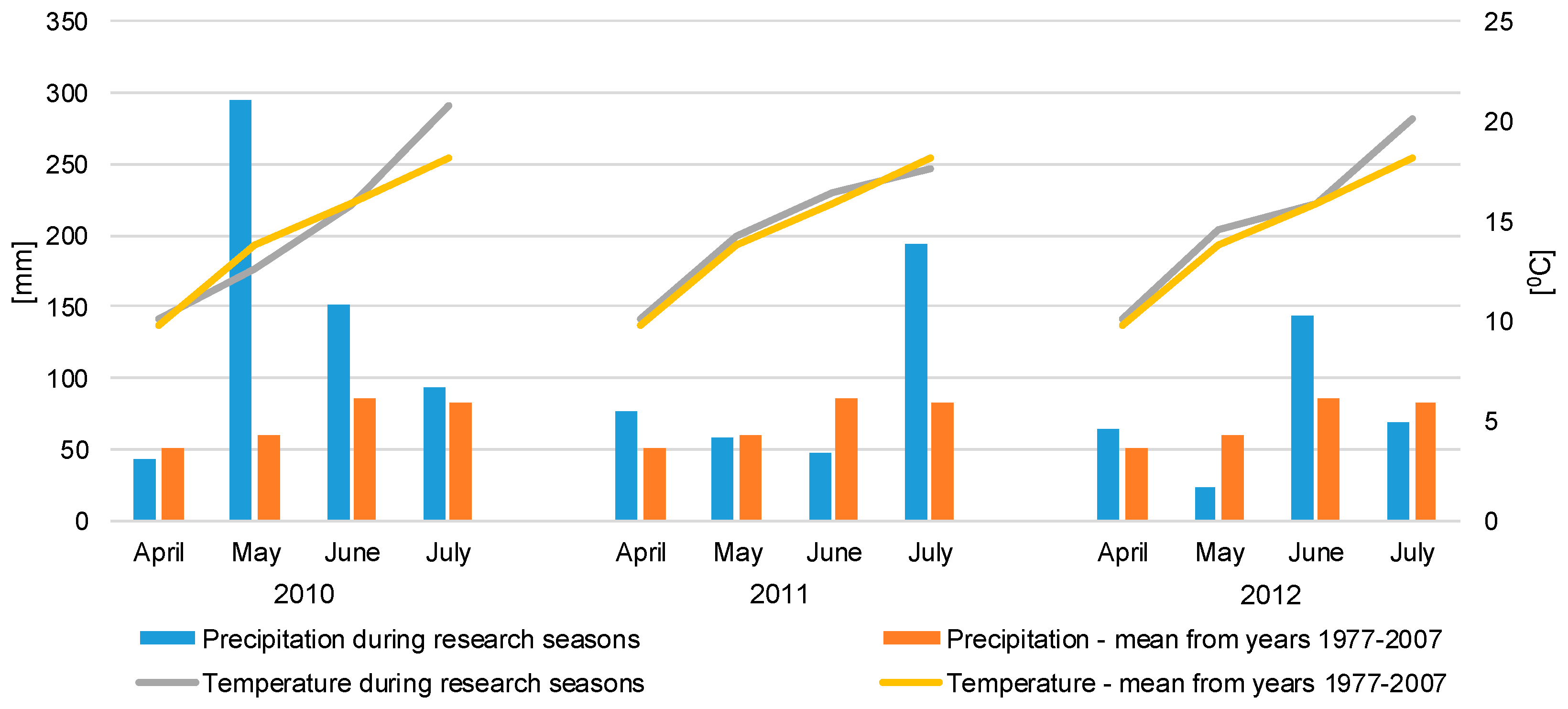
2.1. The Location and Design of the Experiment
2.2. Fauna Sampling
2.3. Selected Morphological Parameters of Broad Bean
2.4. Statistical Analysis of the Data
3. Results
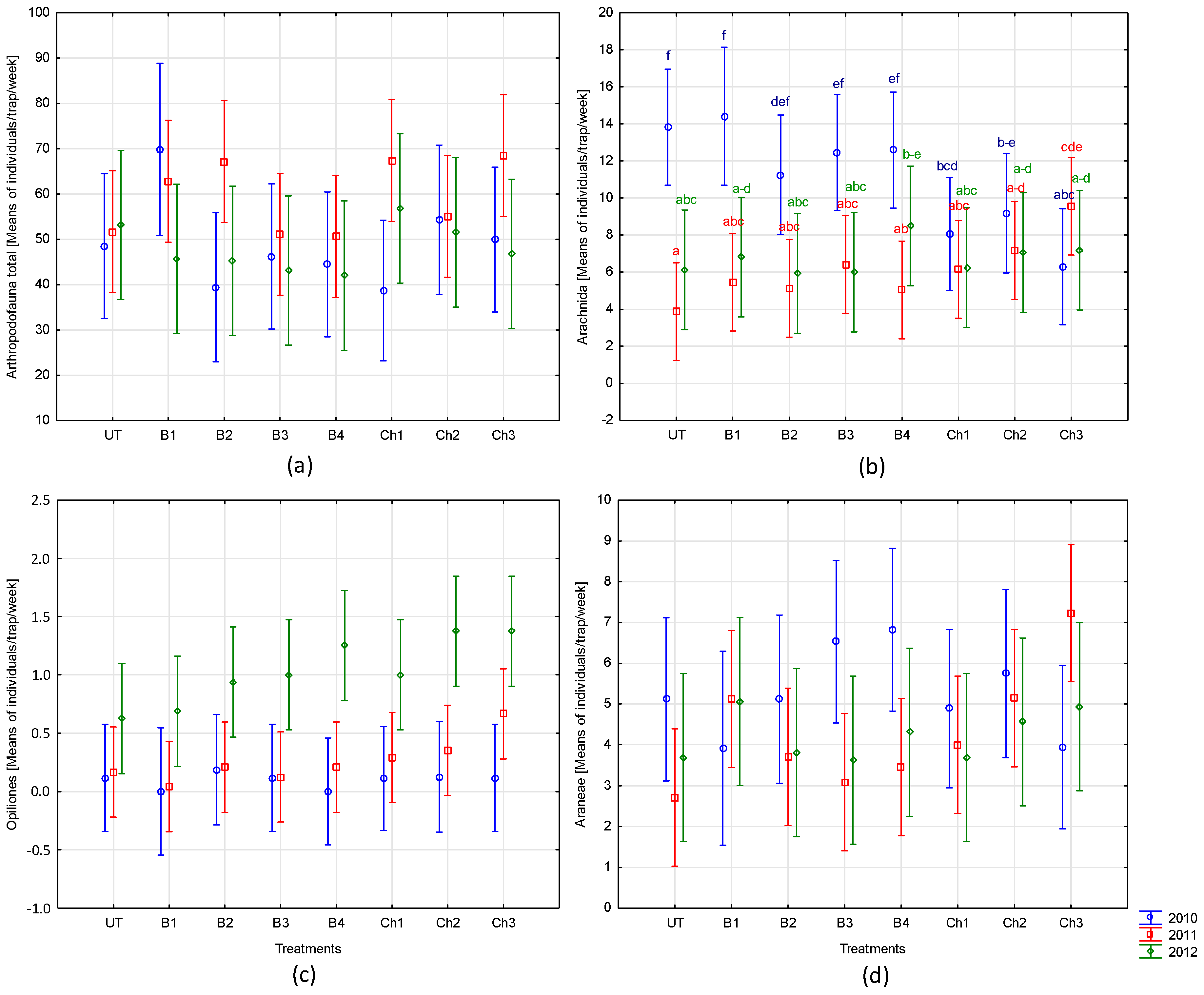
3.1. The Trapping Dynamics of Studied Arthropods
3.2. The Influence of the Protection Method and the Interaction of the Year of Study and Protection Method
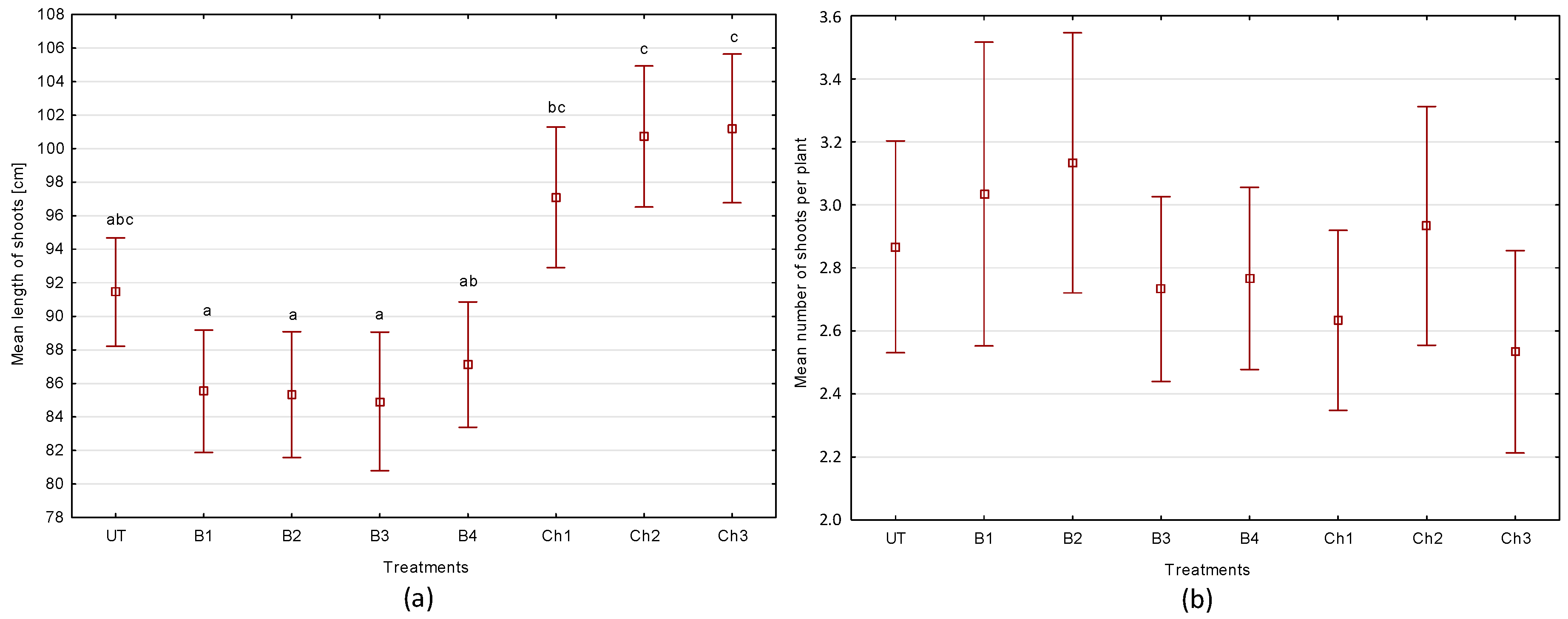
3.3. Selected Morphological Parameters of Broad Bean
4. Discussion
5. Conclusions
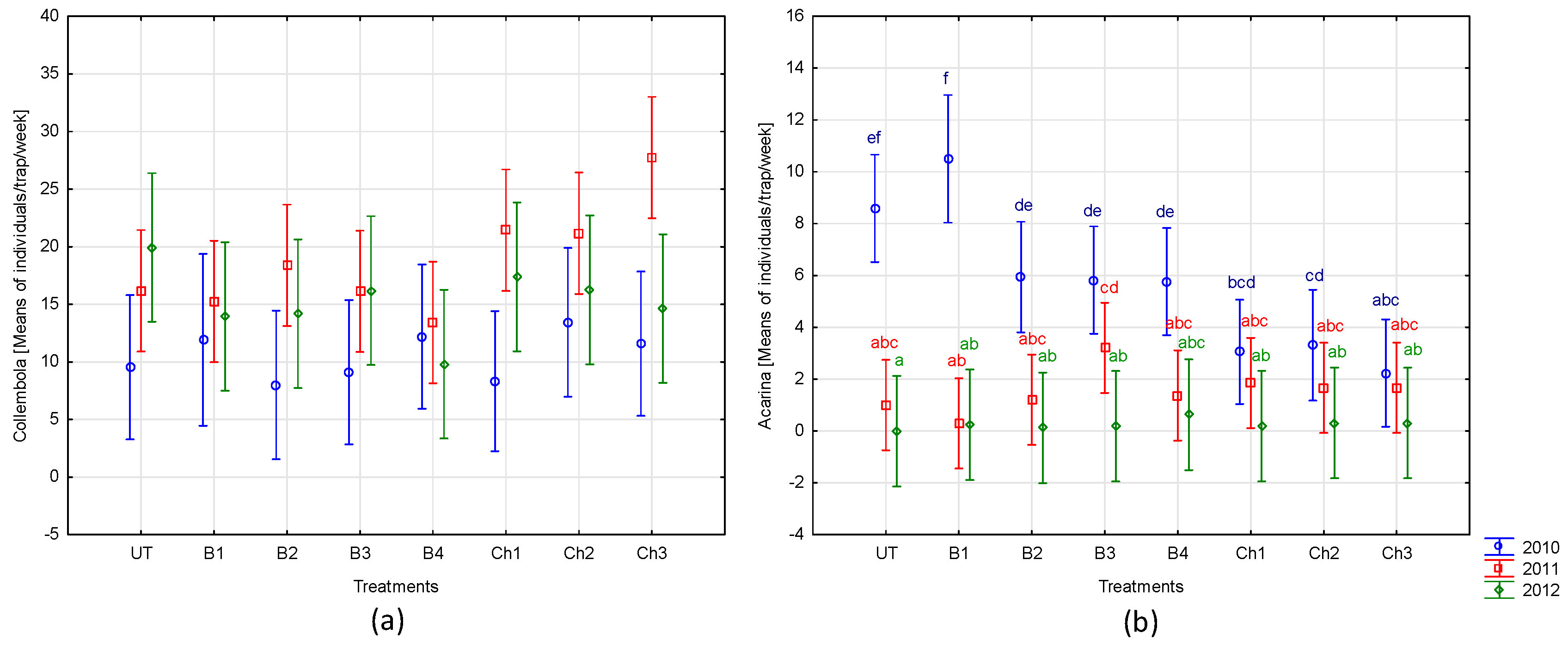
- Nonchemical protection measures applied to broad bean, such as the use of products based on garlic and grapefruit extracts and a preparation containing Pythium oligandrum, did not significantly affect the overall abundance of fauna and the major groups of invertebrates, such as Arachnida (except for Acarina), Carabidae, Staphylinidae, and Collembola. These methods (especially P. oligandrum dressing), however, may favor the occurrence of Formicidae.
- In comparison to the protection of broad beans with chemical products (carboxin, mancozeb, deltamethrin, and alpha-cypermethrin), nonchemical methods seemed to be safer for mites—they limited their abundance to a lesser degree. However, more detailed research is needed to evaluate the individual taxa response.
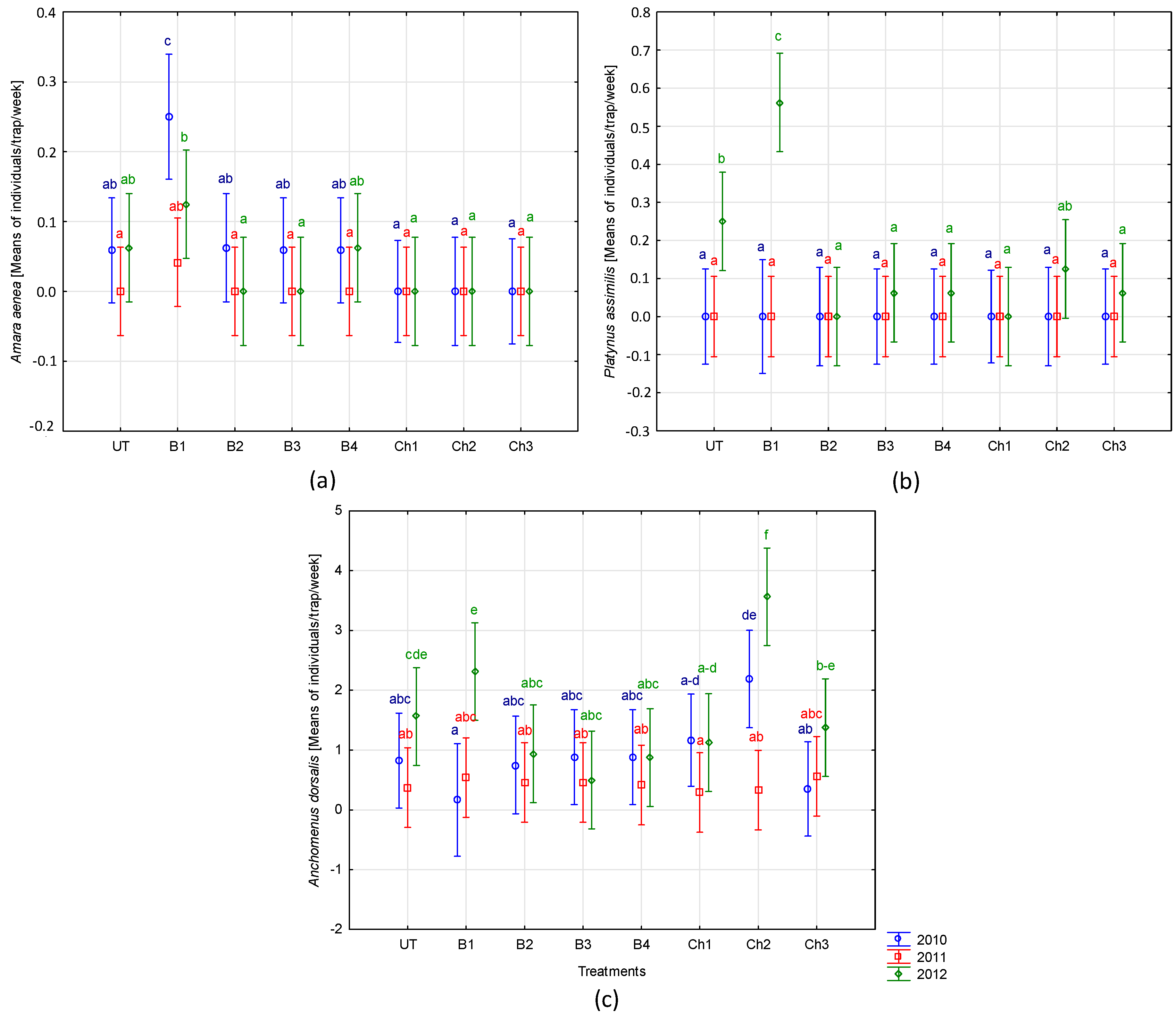
- Nonchemical protection measures facilitated a slight increase in biodiversity of beetles from the carabid family. Some individual carabid species showed differing responses to applied protection methods: Amara aenea occurred only in nonchemically protected areas, Platynus assimilis tends to prefer broad bean protected solely with P. oligandrum, while Anchomenus dorsalis preferred plants protected with chemicals in a moderate way (seed treatment and three-time spraying). This may be the effect of the different shading of the soil surface as a result of the influence of protection measures on plant growth or different vulnerability of species to preparations as well as indirect reaction to the occurrence of other taxa.
- Studied biological substances were demonstrated to be largely safe for nontarget organisms. Therefore, their use in plant-protection practice should be further recommended. However, significant impact of those substances was found on certain invertebrates (e.g., Acarina and Formicidae), which provides the basis for further research toward the accurate identification of affected species and an explanation of mechanisms behind such affection. In case of species belonging to the Carabidae family, in which a significant change in occurrence was observed, it is recommended to study their vulnerability to the tested active substances under laboratory conditions. Such studies could explain the causes of the observed phenomenon.
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hikal, W.M.; Baeshen, R.S.; Said-Al. Ahl, H.A.H. Botanical insecticide as simple extractives for pest control. Cogent Biol. 2017, 3, 1404274. [Google Scholar] [CrossRef]
- Gahukar, R.T. Plant-derived products in crop protection: Effects of various application methods on pests and diseases. Phytoparasitica 2016, 44, 379–391. [Google Scholar] [CrossRef]
- Kowalska, J. Organically grown Brassica napus—use of border strips and Trichoderma. Acta Agric. Scand. B Soil Plant Sci. 2014, 64, 529–536. [Google Scholar] [CrossRef]
- Gahukar, R.T. Potential and Utilization of Plant Products in Pest Control. In Integrated Pest Management, Current Concepts and Ecological Perspective; Elsevier: San Diego, CA, USA; London, UK; Waltham, MA, USA, 2014; pp. 125–139. [Google Scholar] [CrossRef]
- Hummel, R.L.; Walgenbach, J.F.; Hoyt, G.D.; Kennedy, G.G. Effects of vegetable production system on epigeal arthropod populations. Agric. Ecosyst. Environ. 2002, 93, 177–188. [Google Scholar] [CrossRef]
- Hurej, M.; Twardowski, J.P. The influence of yellow lupine intercropped with spring triticale on predatory carabid beetles (Coleoptera:Carabidae). Eur. J. Entomol. 2006, 103, 259–261. [Google Scholar] [CrossRef] [Green Version]
- Prasifka, J.R.; Schmidt, N.P.; Kohler, K.A.; O’Neal, M.E.; Hellmich, R.L.; Singer, J.W. Effects of living mulches on predator abundance and sentinel prey in a corn–soybean–forage rotation. Environ. Entomol. 2006, 35, 1423–1431. [Google Scholar] [CrossRef]
- Jezierska-Tys, S.; Rutkowska, A. Soil response to chemicals used in a field experiment. Int. Agrophys. 2013, 27, 151–158. [Google Scholar] [CrossRef]
- Wenda-Piesik, A.; Piesik, D. Efficacy of garlic extract. In the control of Sitona spp. on pea. Progr. Plant Prot. 2009, 49, 2038–2043, (In Polish, English abstract). [Google Scholar]
- Gospodarek, J.; Boligłowa, E.; Gleń, K. Comparison of the non-chemical and chemical method for broad bean protection against Sitona spp. Prog. Plant Prot./Post. Ochr. Roślin 2012, 52, 26–30, (In Polish, English abstract). [Google Scholar]
- Gospodarek, J.; Gleń, K.; Boligłowa, E. Effect of non-chemical preparations application in broad bean protection against harmfulness of broad bean seed beetle (Bruchus rufimanus Boh.) and seed yield. J. Res. Appl. Agric. Engin. 2012, 57, 124–128. [Google Scholar]
- Akyazi, R.; Soysal, M.; Altunc, E.Y.; Lisle, A.; Hassan, E.; Akyol, D. Acaricidal and sublethal effects of tobacco leaf and garlic bulb extract and soft soap on Tetranychus urticae Koch. (Acari: Trombidiformes: Tetranychidae). Syst. Appl. Acarol. UK 2018, 23, 2054–2069. [Google Scholar] [CrossRef]
- Ismail, M.S.M.; Soliman, M.F.M.; Abo-Ghalia, A.H.; Ghallab, M.M.A. The acaricidal activity of some essential and fixed oils against the two-spotted spider mite in relation to different temperatures. Int. J. Pest Manag. 2015, 61, 121–125. [Google Scholar] [CrossRef]
- Geng, S.; Chen, H.; Zhang, J.; Tu, H. Bioactivity of Garlic-Straw Extracts Against the Spider Mites, Tetranychus urticae and T-viennensis. J. Agric. Urban Entomol. 2014, 30, 38–48. [Google Scholar] [CrossRef]
- Rosa Pino-Otin, M.; Val, J.; Ballestero, D.; Navarro, E.; Sanchez, E.; Gonzalez-Coloma, A.; Mainar, A.M. Ecotoxicity of a new biopesticide produced by Lavandula luisieri on non-target soil organisms from different trophic levels. Sci. Total Environ. 2019, 671, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Rosa Pino-Otin, M.; Val, J.; Ballestero, D.; Navarro, E.; Sanchez, E.; Mainar, A.M. Impact of Artemisia absinthium hydrolate extracts with nematicidal activity on non-target soil organisms of different trophic levels. Ecotoxicol. Environ. Saf. 2019, 180, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Alves, P.R.L.; Cardoso, E.J.B.N.; Martines, A.M.; Sousa, J.P.; Pasini, A. Seed dressing pesticides on springtails in two ecotoxicological laboratory tests. Ecotoxicol. Environ. Saf. 2014, 105, 65–71. [Google Scholar] [CrossRef]
- Cloyd, R.A.; Herrick, N.J. Effects of Pesticides on the Survival of Rove Beetle (Coleoptera: Staphylinidae) and Insidious Flower Bug (Hemiptera: Anthocoridae) Adults. J. Econ. Entomol. 2018, 111, 78–88. [Google Scholar] [CrossRef]
- Frampton, G.K. RecoFvery responses of soil surface Collembola after spatial and temporal changes in long-term regimes of pesticide use. Pedobiologia 2000, 44, 489–501. [Google Scholar] [CrossRef]
- Huuselaveistola, E.; Kurppa, S.; Pihlava, J.M. Effects of fenvalerate and permethrin on soil arthropods and on residues in and decomposition of barley straw. Agric. Food Sci. 1994, 3, 213–223. [Google Scholar] [CrossRef] [Green Version]
- Kosewska, A. Effect of plant protection treatments on the occurrence of ground beetles (Col. Carabidae) in selected plant plantations. Progr. Plant Prot. 2012, 52, 529–534, (In Polish, English abstract). [Google Scholar]
- Vaj, C.; Van Gestel, C.A.M.; Vighi, M. Year-round behaviour of soil microarthropod communities under plant protection product application. Ecotoxicology 2014, 23, 898–913. [Google Scholar] [CrossRef] [PubMed]
- Chiverton, P.A. Pitfall-trap catches of the carabid beetle Pterostichus melanarius, in relation to gut contents and prey densities, in insecticide treated and untreated spring barley. Entomol. Exp. Appl. 1984, 36, 23–30. [Google Scholar] [CrossRef]
- Bel’skaya, E.A.; Zinov’ev, E.V.; Kozyrev, M.A. Carabids in a spring wheat agrocenosis to the south of Sverdlovsk oblast and the effect of insecticide treatment on their populations. Russ. J. Ecol. 2002, 33, 38–44. [Google Scholar] [CrossRef]
- Seidenglanz, M.; Huňady, I.; Poslušna, J.; Loes, A.K. Influence of intercropping with spring cereals on the occurrence of pea aphids (Acyrthosiphon pisum Harris, 1776) and their natural enemies in field pea (Pisum sativum L.). Plant Protect. Sci. 2011, 47, 25–36. [Google Scholar] [CrossRef] [Green Version]
- Holland, J.M.; Smith, S. Sampling epigeal arthropods: An evaluation of fenced pitfall traps using mark–release–recapture and comparisons to unfenced pitfall traps in arable crops. Entomol. Exp. Appl. 1999, 91, 347–357. [Google Scholar] [CrossRef] [Green Version]
- Hurka, K. Carabidae of the Czech and Slovak Republics; Kabourek: Zlin, Czech Republic, 1996; p. 565. [Google Scholar]
- Querner, P.; Bruckner, A. Combining pitfall traps and soil samples to collect Collembola for site scale biodiversity assessments. Appl. Soil Ecol. 2010, 45, 293–297. [Google Scholar] [CrossRef]
- Gonzalez, E.; Salvo, A.; Valladares, G. Natural vegetation cover in the landscape and edge effects: Differential responses of insect orders in a fragmented forest. Insect Sci. 2017, 24, 891–901. [Google Scholar] [CrossRef]
- Wasley, J.; Mooney, T.J.; King, C.K. Soil invertebrate community change over fuel–contaminated sites on a subantarctic island: An ecological field-based line of evidence for site risk assessment. Integr. Environ. Asses. 2016, 12, 306–314. [Google Scholar] [CrossRef]
- Gospodarek, J.; Petryszak, P.; Kołoczek, H. The effect of the bioremediation of soil contaminated with petroleum derivatives on the occurrence of epigeic and edaphic fauna. Bioremed. J. 2016, 20, 38–53. [Google Scholar] [CrossRef]
- Rusin, M.; Gospodarek, J. The occurrence of springtails (Collembola) and spiders (Araneae) as an effectiveness indicator of bioremediation of soil contaminated by petroleum-derived substances. Int. J. Environ. Res. 2016, 10, 449–458. [Google Scholar]
- Shannon, C.E.; Wiener, W. The Mathematical Theory of Communication; University of Illinois: Urbanna, VI, USA, 1949. [Google Scholar]
- Santos, S.; Cabanas, J.E.; Pereira, J.A. Abundance and diversity of soil arthropods in olive grove ecosystem (Portugal): Effect of pitfall trap type. Eur. J. Soil Biol. 2007, 43, 77–83. [Google Scholar] [CrossRef]
- Pruszyński, S. Plant protection in different cropping systems and biological diversity. Progr. Plant Prot. 2009, 49, 1092–1101, (In Polish, English abstract). [Google Scholar]
- Kos, T.; Bažok, R.; Drmić, Z.; Graša, Ž. Ground beetles (Coleoptera: Carabidae) in sugar beet fields as the base for conservation biological control. Insect Pathogens and Entomoparasitic Nematodes. Biological Control - its unique role in integrated and organic production. IOBC WPRS Bull. 2013, 90, 353–357. [Google Scholar]
- Prasifka, J.R.; Lopez, M.D.; Hellmich, R.L.; Prasifka, P.L. Effects of insecticide exposure on movement and population size estimates of predatory ground beetles (Coleoptera: Carabidae). Pest Manag. Sci. 2008, 64, 30–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eyre, M.D.; Labanowska-Bury, D.; Avayanos, J.G.; White, R.; Leifert, C. Ground beetles (Coleoptera, Carabidae) in an intensively managed vegetable crop landscape in eastern England. Agric. Ecosyst. Environ. 2009, 131, 340–346. [Google Scholar] [CrossRef]
- Navntoft, S.; Esbjerg, P.; Riedel, W. Effects of reduced pesticide dosages on carabids (Coleoptera:Carabidae) in winter wheat. Agric. Forest Entomol. 2006, 8, 57–62. [Google Scholar] [CrossRef]
- Kromp, B. Carabid beetles (Coleoptera, Carabidae) as bioindicators in biological and conventional farming in Austrian potato fields. Biol. Fertil. Soils 1990, 9, 182–187. [Google Scholar] [CrossRef]
- Gruss, I.; Twardowski, J.P.; Hurej, M.; Kozak, M. Effect of intercropping narrow-leafed lupin with spring triticale on the abundance and diversity of rove beetles. Biotechnol. Agron. Soc. 2018, 22, 220–229. [Google Scholar]
- Altieri, M.A.; Wilson, R.C.; Schmidt, L.L. The effects of living mulches and weed cover on the dynamics of foliage- and soil-arthropod communities in three crop systems. Crop Prot. 1985, 4, 201–213. [Google Scholar] [CrossRef]
- Silva, R.S.; Tomaz, A.C.; Lopes, M.C.; Martins, J.C.; Xavier, V.M.; Picanco, M.C. Toxicity of botanical insecticides on Diaphania hyalinata, their selectivity for the predatory ant Paratrechina sp., and their potential phytotoxicity on pumpkin. Int. J. Pest Manag. 2016, 62, 95–104. [Google Scholar] [CrossRef]
- Gospodarek, J. Occurrence of Black Bean Aphid (Aphis Fabae Scop.) and Its Natural Predators on Broad Bean Under the Conditions of Soil Pollution with Heavy Metals. Zesz Nauk UR W Krakowie 2012, 480, 207, (In Polish, English abstract). [Google Scholar]
- Powell, B.E.; Silverman, J. Impact of Linepithema humile and Tapinoma sessile (Hymenoptera: Formicidae) on three natural enemies of Aphis gossypii (Hemiptera: Aphididae). Biol. Control 2010, 54, 285–291. [Google Scholar] [CrossRef]


| Treatments | Protection Method | Preparation, Dose | Date of Application |
|---|---|---|---|
| UT | Untreated | Without protection | |
| B1 | Seed treatment | Polyversum WP, 10 g/kg of seeds | before sowing |
| B2 | Seed treatment and three-time spraying | Polyversum WP, 10 g/kg of seeds | before sowing |
| Bioczos BR, 4 briquettes/L of water (two times) | when first aphids appear (26 May 2010; 25 May 2011; 11 May 2012) by the end of flowering first inflorescences (15 June 2010; 11 June 2011; 31 May 2012) | ||
| Biosept 33 SL, 2 L/ha | before flowering (1 June 2010; 29 May 2011; 18 May 2012) | ||
| B3 | Seed treatment and four-time spraying | Polyversum WP, 10 g/kg of seeds | before sowing |
| Bioczos BR, 4 briquettes/L of water (three times) | when first aphids appear (26 May 2010; 25 May 2011; 11 May 2012) repeated after—7 days (2 June 2010; 1 June 2011; 18 May 2012) by the end of flowering first inflorescences (15 June 2010; 11 June 2011; 31 May 2012) | ||
| Biosept 33 SL, 2 L/ha | before flowering (1 June 2010; 29 May 2011; 18 May 2012) | ||
| B4 | Seed treatment and five-time spraying | Polyversum WP, 10 g/kg of seeds | before sowing |
| Bioczos BR, 4 briquettes/L of water (four times) | when first aphids appear (26 May 2010; 25 May 2011; 11 May 2012) repeated after—7 days (2 June 2010; 1 June 2011; 18 May 2012) by the end of flowering first inflorescences (15 June 2010; 11 June 2011; 31 May 2012) repeated after—7 days (22 June 2010; 18 June 2011; 10 June2012) | ||
| Biosept 33 SL, 2 L/ha | before flowering (1 June 2010; 29 May 2011; 18 May 2012) | ||
| Ch1 | Seed treatment | Vitavax 200 FS, 4 mL/1 kg of seeds | before sowing |
| Ch2 | Seed treatment and three-time spraying | Vitavax 200 FS, 4 mL/1 kg of seeds | before sowing |
| Decis 2.5 EC, 0.25 L/ha | when first aphids appear (26 May 2010; 25 May 2011; 11 May 2012) | ||
| Fastac 100 EC, 0.09 L/ha | by the end of flowering first inflorescences (15 June 2010; 11 June 2011; 31 May 2012) | ||
| Penncozeb 80 WP, 2 kg/ha | before flowering (1 June 2010; 29 May 2011; 18 May 2012) | ||
| Ch3 | Seed treatment and four-time spraying | Vitavax 200 FS, 4 mL/1 kg of seeds | before sowing |
| Decis 2.5 EC, 0.25 L/ha (two times) | when first aphids appear (26 May 2010; 25 May 2011; 11 May 2012) repeated after—7 days (2 June 2010; 1 June 2011; 18 May 2012) | ||
| Fastac 100 EC, 0.09 L/ha | by the end of flowering first inflorescences (15 June 2010; 11 June 2011; 31 May 2012) | ||
| Penncozeb 80 WP, 2 kg/ha | before flowering (1 June 2010; 29 May 2011; 18 May 2012) |
| Date | UT | B1 | B2 | B3 | B4 | Ch1 | Ch2 | Ch3 |
|---|---|---|---|---|---|---|---|---|
| 21 June 2010 | 58 ± 24 | 39 ± 7 | 56 ± 3 | 38 ± 14 | 39 ± 6 | 27 ± 14 | 27 ± 10 | 39 ± 3 |
| 25 June 2010 | 30 ± 3 | 49 ± 10 | 30 ± 6 | 42 ± 7 | 27 ± 18 | 11 ± 5 | 32 ± 11 | 38 ± 14 |
| 1 July 2010 | 51 ± 7 | 74 ± 40 | 48 ± 26 | 49 ± 13 | 41 ± 5 | 44 ± 10 | 67 ± 14 | 52 ± 9 |
| 8 July 2010 | 56 ± 5 | 45 ± 5 | 56 ± 4 | 60 ± 6 | 62 ± 11 | 58 ± 4 | 68 ± 20 | 70 ± 7 |
| 15 July 2010 | 98 ± 29 | 176 ± 133 | 56 ± 4 | 103 ± 42 | 87 ± 14 | 66 ± 13 | 144 ± 90 | 118 ± 27 |
| 22 July 2010 | 50 ± 14 | 66 ± 8 | 25 ± 6 | 25 ± 8 | 42 ± 4 | 48 ± 3 | 34 ± 11 | 39 ± 12 |
| 29 July 2010 | 29 ± 7 | 42 ± 19 | 35 ± 11 | 36 ± 5 | 37 ± 8 | 28 ± 3 | 23 ± 4 | 31 ± 6 |
| 4 August 2010 | 49 ± 10 | 66 ± 14 | 29 ± 2 | 24 ± 5 | 33 ± 5 | 29 ± 4 | 32 ± 7 | 29 ± 4 |
| 12 August 2010 | 29 ± 5 | 36 ± 7 | 13 ± 5 | 30 ± 5 | 29 ± 18 | 40 ± 4 | 50 ± 16 | 29 ± 7 |
| 26 May 2011 | 33 ± 7 | 59 ± 18 | 43 ± 16 | 49 ± 19 | 41 ± 7 | 59 ± 9 | 44 ± 13 | 51 ± 14 |
| 3 June 2011 | 29 ± 3 | 24 ± 3 | 34 ± 11 | 35 ± 10 | 28 ± 3 | 46 ± 8 | 40 ± 4 | 38 ± 8 |
| 11 June 2011 | 33 ± 3 | 39 ± 5 | 34 ± 11 | 37 ± 13 | 31 ± 5 | 51 ± 8 | 42 ± 2 | 51 ± 3 |
| 18 June 2011 | 89 ± 7 | 96 ± 25 | 80 ± 20 | 80 ± 36 | 89 ± 12 | 143 ± 26 | 66 ± 18 | 78 ± 8 |
| 25 June 2011 | 44 ± 17 | 71 ± 17 | 85 ± 26 | 53 ± 33 | 50 ± 5 | 57 ± 14 | 54 ± 7 | 82 ± 11 |
| 2 July 2011 | 42 ± 6 | 57 ± 8 | 77 ± 39 | 44 ± 6 | 27 ± 16 | 41 ± 18 | 16 ± 15 | 28 ± 18 |
| 9 July 2011 | 43 ± 5 | 72 ± 21 | 52 ± 9 | 44 ± 9 | 48 ± 10 | 55 ± 14 | 80 ± 23 | 115 ± 2 |
| 18 July 2011 | 99 ± 13 | 85 ± 5 | 133 ± 10 | 67 ± 10 | 92 ± 9 | 88 ± 12 | 99 ± 4 | 104 ± 5 |
| 1 June 2012 | 85 ± 4 | 54 ± 3 | 57 ± 23 | 65 ± 11 | 28 ± 6 | 81 ± 23 | 55 ± 23 | 60 ± 7 |
| 6 June 2012 | 42 ± 7 | 46 ± 1 | 24 ± 7 | 22 ± 3 | 25 ± 4 | 58 ± 7 | 21 ± 4 | 18 ± 7 |
| 15 June 2012 | 81 ± 19 | 60 ± 13 | 72 ± 36 | 73 ± 15 | 105 ± 14 | 125 ± 67 | 58 ± 27 | 53 ± 8 |
| 22 June 2012 | 47 ± 10 | 51 ± 24 | 57 ± 9 | 58 ± 21 | 46 ± 13 | 61 ± 10 | 92 ± 30 | 82 ± 12 |
| 29 June 2012 | 21 ± 13 | 18 ± 5 | 12 ± 3 | 16 ± 2 | 17 ± 2 | 15 ± 7 | 29 ± 17 | 23 ± 4 |
| 7 July 2012 | 24 ± 5 | 30 ± 3 | 23 ± 5 | 17 ± 5 | 18 ± 3 | 27 ± 14 | 37 ± 1 | 39 ± 10 |
| 17 July 2012 | 50 ± 17 | 38 ± 14 | 46 ± 7 | 28 ± 3 | 37 ± 11 | 27 ± 8 | 44 ± 7 | 32 ± 10 |
| 2 August 2012 | 78 ± 14 | 70 ± 7 | 73 ± 5 | 68 ± 27 | 62 ± 15 | 62 ± 4 | 77 ± 2 | 70 ± 10 |
| Effects | SS | df | MS | F | p |
|---|---|---|---|---|---|
| Arthropodofauna Total | |||||
| Year | 12,867.240 | 2 | 6433.620 | 5.722 | 0.004 |
| Protection method | 7448.318 | 7 | 1064.045 | 0.946 | 0.470 |
| Year × protection method | 15,608.999 | 14 | 1114.929 | 0.992 | 0.461 |
| Arachnida total | |||||
| Year | 2016.750 | 2 | 1008.375 | 23.345 | 0.000 |
| Protection method | 172.894 | 7 | 24.699 | 0.572 | 0.779 |
| Year × protection method | 1396.790 | 14 | 99.771 | 2.310 | 0.004 |
| Opiliones | |||||
| Year | 66.173 | 2 | 33.086 | 35.796 | 0.000 |
| Protection method | 8.851 | 7 | 1.264 | 1.368 | 0.217 |
| Year × protection method | 6.134 | 14 | 0.438 | 0.474 | 0.946 |
| Araneae | |||||
| Year | 90.933 | 2 | 45.467 | 2.593 | 0.076 |
| Protection method | 104.881 | 7 | 14.983 | 0.854 | 0.543 |
| Year × protection method | 374.796 | 14 | 26.771 | 1.527 | 0.098 |
| Formicidae | |||||
| Year | 1658.028 | 2 | 829.014 | 44.321 | 0.000 |
| Protection method | 795.403 | 7 | 113.629 | 6.075 | 0.000 |
| Year × protection method | 637.585 | 14 | 45.542 | 2.435 | 0.003 |
| Carabidae | |||||
| Year | 383.938 | 2 | 191.969 | 1.602 | 0.000 |
| Protection method | 190.982 | 7 | 27.283 | 1.507 | 0.163 |
| Year × protection method | 322.528 | 14 | 23.038 | 1.272 | 0.221 |
| Staphylinidae | |||||
| Year | 1254.054 | 2 | 627.027 | 39.926 | 0.000 |
| Protection method | 91.240 | 7 | 13.034 | 0.830 | 0.563 |
| Year × protection method | 174.534 | 14 | 12.467 | 0.794 | 0.676 |
| Effects | SS | df | MS | F | p |
|---|---|---|---|---|---|
| Amara aenea | |||||
| Year | 0.2416 | 2 | 0.121 | 4.856 | 0.008 |
| Protection method | 0.740 | 7 | 0.106 | 4.253 | 0.000 |
| Year × protection method | 0.326 | 14 | 0.023 | 0.936 | 0.520 |
| Platynus assimilis | |||||
| Year | 1.808 | 2 | 0.904 | 13.057 | 0.000 |
| Protection method | 1.377 | 7 | 0.197 | 2.840 | 0.007 |
| Year × protection method | 2.776 | 14 | 0.198 | 2.863 | 0.000 |
| Anchomenus dorsalis | |||||
| Year | 93.281 | 2 | 46.641 | 16.935 | 0.000 |
| Protection method | 77.326 | 7 | 11.047 | 4.011 | 0.000 |
| Year × protection method | 91.981 | 14 | 6.570 | 2.386 | 0.003 |
| Effects | SS | df | MS | F | p |
|---|---|---|---|---|---|
| Collembola | |||||
| Year | 5165.697 | 2 | 2582.849 | 14.969 | 0.000 |
| Protection method | 1560.555 | 7 | 222.936 | 1.292 | 0.253 |
| Year × protection method | 2864.642 | 14 | 204.617 | 1.1859 | 0.283 |
| Acarina | |||||
| Year | 2090.394 | 2 | 1045.197 | 55.423 | 0.000 |
| Protection method | 243.435 | 7 | 34.776 | 1.844 | 0.077 |
| Year × protection method | 791.847 | 14 | 56.560 | 2.999 | 0.000 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gospodarek, J.; Boligłowa, E.; Gleń-Karolczyk, K. Impact of Nonchemical Protection of Broad Bean on Epigeic and Soil Arthropodofauna—Analysis in Field-Realistic Conditions. Agronomy 2020, 10, 211. https://doi.org/10.3390/agronomy10020211
Gospodarek J, Boligłowa E, Gleń-Karolczyk K. Impact of Nonchemical Protection of Broad Bean on Epigeic and Soil Arthropodofauna—Analysis in Field-Realistic Conditions. Agronomy. 2020; 10(2):211. https://doi.org/10.3390/agronomy10020211
Chicago/Turabian StyleGospodarek, Janina, Elżbieta Boligłowa, and Katarzyna Gleń-Karolczyk. 2020. "Impact of Nonchemical Protection of Broad Bean on Epigeic and Soil Arthropodofauna—Analysis in Field-Realistic Conditions" Agronomy 10, no. 2: 211. https://doi.org/10.3390/agronomy10020211
APA StyleGospodarek, J., Boligłowa, E., & Gleń-Karolczyk, K. (2020). Impact of Nonchemical Protection of Broad Bean on Epigeic and Soil Arthropodofauna—Analysis in Field-Realistic Conditions. Agronomy, 10(2), 211. https://doi.org/10.3390/agronomy10020211






