A Generic Model Accounting for the Interactions among Pathogens, Host Plants, Biocontrol Agents, and the Environment, with Parametrization for Botrytis cinerea on Grapevines
Abstract
:1. Introduction
2. Model Description
2.1. State Variables and Connecting Flows
2.2. Driving Variables for the Pathogen
2.3. Driving Variables for the BCA
2.4. Model Output
3. Model Parametrization
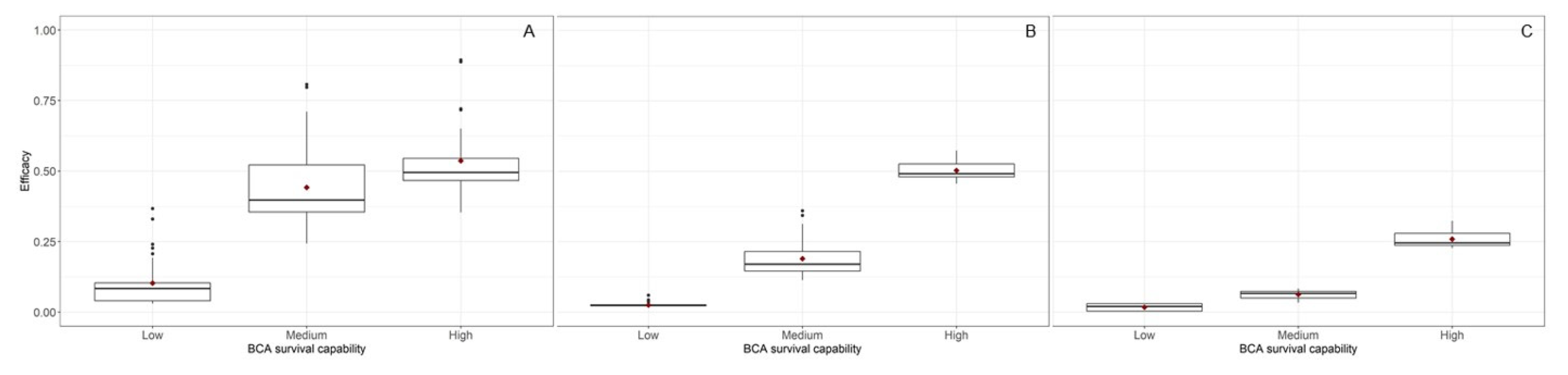
4. Model Running
5. Discussion and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Elad, Y.; Freeman, S. Biological control of fungal plant pathogens. In Agricultural Applications; Kempken, F., Ed.; Springer Berlin Heidelberg: Berlin/Heidelberg, Germany, 2002; pp. 93–109. [Google Scholar]
- Elmer, P.A.G.; Hoyte, S.M.; Vanneste, J.L.; Reglinski, T.; Wood, P.N.; Parry, F.J. Biological control of fruit pathogens. New Zeal. Plant Prot. 2005, 54, 47–54. [Google Scholar] [CrossRef] [Green Version]
- Harman, G.E. Myths and dogmas of biocontrol: Changes in perceptions derived from research on Trichoderma harzianum T-22. Biol. Control 2000, 377–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tracy, E.F. The promise of biological control for sustainable agriculture: A stakeholder- based analysis. J. Sci. Poly Goverance 2014, 5. [Google Scholar]
- Alavanja, M.C.R.; Hoppin, J.A.; Kamel, F. Health effects of chronic pesticide exposure: Cancer and neurotoxicity. Annu. Rev. Public Health 2004, 25, 155–197. [Google Scholar] [CrossRef] [PubMed]
- Epstein, L. Fifty years since Silent Spring. Annu. Rev. Phytopathol. 2014, 52, 377–402. [Google Scholar] [CrossRef] [Green Version]
- Hahn, M. The rising threat of fungicide resistance in plant pathogenic fungi: Botrytis as a case study. J. Chem. Biol. 2014, 7, 133–141. [Google Scholar] [CrossRef] [Green Version]
- Guetsky, R.; Shtienberg, D.; Elad, Y.; Dinoor, A. Combining biocontrol agents to reduce the variability of biological control. Phytopathology 2001, 91, 621–627. [Google Scholar] [CrossRef] [Green Version]
- Paulitz, T.C.; Belanger, R.R. Biological control in greenhouse systems. Annu. Rev. Phytopathol. 2001, 39, 103–133. [Google Scholar] [CrossRef]
- Rosenheim, J.A.; Kaya, H.K.; Ehler, L.E.; Marois, J.J.; Jaffee, B.A. Intraguild predation among biological-control agents: Theory and evidence. Biol. Control 1995, 5, 303–335. [Google Scholar] [CrossRef]
- Fedele, G.; González-Domínguez, E.; Rossi, V. Influence of environment on the biocontrol of Botrytis cinerea: A systematic literature review. In Strategies to Develop Successful Biocontrol Agents; De Cal, A., Melgarejo, P., Magan, N., Eds.; Springer International Publishing: Cham, Switzerland, 2019; In press. [Google Scholar]
- Kredics, L.; Antal, Z.; Manczinger, L.; Szekeres, A.; Kevei, F.; Nagy, E. Influence of environmental parameters on Trichoderma strains with biocontrol potential. Food Technol. Biotechnol. 2003, 41, 37–42. [Google Scholar]
- Xu, X.; Robinson, J.; Jeger, M.; Jeffries, P. Using combinations of biocontrol agents to control Botrytis cinerea on strawberry leaves under fluctuating temperatures. Biocontrol Sci. Technol. 2010, 20, 359–373. [Google Scholar] [CrossRef]
- Dik, A.J.; Elad, Y. Comparison of antagonists of Botrytis cinerea in greenhouse-grown cucumber and tomato under different climatic conditions. Eur. J. Plant Pathol. 1999, 105, 123–137. [Google Scholar] [CrossRef]
- Elad, Y.; Zimand, G.; Zaqs, Y.; Zuriel, S.; Chet, I. Use of Trichoderma harzianum in combination or alternation with fungicides to control cucumber grey mould (Botrytis cinerea) under commercial greenhouse conditions. Plant Pathol. 1993, 42, 324–332. [Google Scholar] [CrossRef]
- Hannusch, D.; Boland, G.J. Interactions of air temperature, relative humidity and biological control agents on grey mold of bean. Phytopathology 1996, 86, 156. [Google Scholar] [CrossRef]
- Jackson, M.; Whipps, M.; Lynch, M. Effects of temperature, pH and water potential on growth of four fungi with disease biocontrol potential. World J. Microbiol. Biotechnol. 1991, 7, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.K.; Jeger, J.M.; Taber, R.A. The effect of temperature on colonization of Cercosporidium personatum leafspot of peanuts by the hyperparasite Dicyma pulvinata. Agric. Ecosyst. Environ. 1987, 18, 325–332. [Google Scholar] [CrossRef]
- Deacon, J.W.; Berry, L.A. Biocontrol of soil-borne plant pathogens: Concepts and their application. Pestic. Sci. 1993, 37, 417–426. [Google Scholar] [CrossRef]
- Whipps, J.M. Developments in the Biological Control of Soil-Borne Plant Pathogens. In Advances in Botanical Research; Callow, J.A., Ed.; Academic Press: Cambridge, MA, USA, 1997; Volume 26, pp. 1–134. [Google Scholar]
- Cabrefiga, J.; Montesinos, E. Analysis of aggressiveness of Erwinia amylovora using disease-dose and time relationships. Phytopathology 2005, 95, 1430–1437. [Google Scholar] [CrossRef] [Green Version]
- Johnson, K.B. Dose-response relationships and inundative biological control. Phytopathology 1994, 780–784. [Google Scholar] [CrossRef]
- Montesinos, E.; Bonaterra, A. Dose-response models in biological control of plant pathogens: An empirical verification. Phytopathology 1996, 464–472. [Google Scholar] [CrossRef]
- Smith, K.P.; Handelsman, J.; Goodman, R.M. Modeling dose-response relationships in biological control: Partitioning host responses to the pathogen and biocontrol agent. Phytopathology 1997, 87, 720–729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cunniffe, N.J.; Gilligan, C.A. A theoretical framework for biological control of soil-borne plant pathogens: Identifying effective strategies. J. Theor. Biol. 2011, 278, 32–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knudsen, G.R.; Hudler, G.W. Use of a computer simulation model to evaluate a plant disease biocontrol agent. Ecol. Modell. 1987, 35, 45–62. [Google Scholar] [CrossRef]
- Kessel, G.J.T.; Köhl, J.; Powell, J.A.; Rabbinge, R.; van der Werf, W. Modeling spatial characteristics in the biological control of fungi at leaf scale: Competitive substrate colonization by Botrytis cinerea and the saprophytic antagonist Ulocladium atrum. Phytopathology 2005, 95, 439–448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeger, M.J.; Jeffries, P.; Elad, Y.; Xu, X.M. A generic theoretical model for biological control of foliar plant diseases. J. Theor. Biol. 2009, 256, 201–214. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.-M.; Salama, N.; Jeffries, P.; Jeger, M.J. Numerical studies of biocontrol efficacies of foliar plant pathogens in relation to the characteristics of a biocontrol agent. Phytopathology 2010, 100, 814–821. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.M.; Jeffries, P.; Pautasso, M.; Jeger, M.J. A numerical study of combined use of two biocontrol agents with different biocontrol mechanisms in controlling foliar pathogens. Phytopathology 2011, 101, 1032–1044. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.-M.; Jeger, M.J. Combined use of two biocontrol agents with different biocontrol mechanisms most likely results in less than expected efficacy in controlling foliar pathogens under fluctuating conditions: A modeling study. Phytopathology 2013, 103, 108–116. [Google Scholar] [CrossRef] [Green Version]
- Elad, Y.; Vivier, M.; Fillinger, S. Botrytis, the Good, the Bad and the Ugly. In Botrytis—The Fungus, the Pathogen and its Management in Agricultural Systems; Fillinger, S., Elad, Y., Eds.; Springer International Publishing: Basel, Switzerland, 2016; pp. 1–15. [Google Scholar]
- Jarvis, W.R. Botryotinia and Botrytis Species: Taxonomy, Physiology, and Pathogenicity; Research Branch, Canada Department of Agricolture: Ottawa, ON, Canada, 1977.
- Williamson, B.; Tudzynski, B.; Tudzynski, P.; Van Kan, J.A.L. Botrytis cinerea: The cause of grey mould disease. Mol. Plant Pathol. 2007, 8, 561–580. [Google Scholar] [CrossRef]
- Hethcote, H.W. Three basic epidemiological models. In Applied Mathematical Ecology; Levin, S.A., Hallam, T.G., Grossi, L.J., Eds.; Springer Berlin Heidelberg: Berlin/Heidelberg, Germany, 1989; Volume 18, pp. 119–144. [Google Scholar]
- Leffelaar, P.A.; Ferrari, T.J. Some elements of dynamic simulation. In Simulation and Systems Management in Crop Protection; Van Laar, H.H., Ed.; Pudoc: Wageningen, The Netherlands, 1989; Volume 66, pp. 37–39. [Google Scholar]
- Isee Systems, I. STELLA. System Thinking for Education and Research. Available online: https://www.iseesystems.com/ (accessed on 10 June 2019).
- Forrester, J.W. Industrial Dynamics; M. I. T. Press: Cambridge, UK, 1961. [Google Scholar]
- Rabbinge, R.; de Wit, C.T. Systems, model and simulation. Tetrahedron Lett. 1989, 23, 4461–4464. [Google Scholar]
- Campbell, C.L.; Madden, L.V. Introduction to plant disease epidemiology; John Wiley & Sons.: New York, NY, USA, 1990. [Google Scholar]
- Analytis, S. Über die relation zwischen biologischer entwicklung und temperatur bei phytopathogenen pilzen. J. Phytopathol. 1977, 90, 64–76. [Google Scholar] [CrossRef]
- Reed, K.L.; Hamerly, E.R.; Dinger, B.E.; Jarvis, P.G. An analytical model for field measurement of photosynthesis. J. Appl. Ecol. 1976, 13, 925. [Google Scholar] [CrossRef]
- Wadia, K.D.R.; Butler, D.R. Relationships between temperature and latent periods of rust and leaf-spot diseases of groundnut. Plant Pathol. 1994, 43, 121–129. [Google Scholar] [CrossRef] [Green Version]
- Lorenz, D.H.; Eichhorn, K.W.; Bleiholder, H.; Klose, R.; Meier, U.; Weber, E. Phenological growth stages of the grapevine (Vitis vinifera L. ssp. vinifera)- Codes and descriptions according to the extended BBCH scale. Aust. J. Grape Wine Res. 1995, 1, 100–103. [Google Scholar] [CrossRef]
- Fedele, G.; González-Domínguez, E.; Caffi, T.; Mosetti, D.; Bigot, G.; Rossi, V. Valutazione di un modello matematico per la muffa grigia della vite. In Proceedings of the ATTI Giornate Fitopatologiche, Chianciano Terme, Italy, 6–9 March 2018. [Google Scholar]
- Elmer, P.A.G.; Michailides, T.J. Epidemiology of Botrytis cinerea in orchard and vine crops. In Botrytis: Biology, Pathology and Control; Elad, Y., Williamson, B., Tudzynski, P., Delen, N., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 2007; pp. 243–272. [Google Scholar]
- González-Domínguez, E.; Caffi, T.; Ciliberti, N.; Rossi, V. A mechanistic model of Botrytis cinerea on grapevines that includes weather, vine growth stage, and the main infection pathways. PLoS ONE 2015, 10, 1–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McClellan, W.D. Early Botrytis rot of grapes: Time of infection and latency of Botrytis cinerea Pers. in Vitis vinifera L. Phytopathology 1973, 63, 1151. [Google Scholar] [CrossRef]
- Nair, N.G.; Guilbaud-Oultorfi, S.; Barchia, I.; Emmett, R. Significance of carry over inoculum, flower infection and latency on the incidence of Botrytis cinerea in berries of grapevines at harvest in new south wales. Aust. J. Exp. Agric. 1995, 35, 1177–1180. [Google Scholar] [CrossRef]
- Pezet, R.; Viret, O.; Perret, C.; Tabacchi, R. Latency of Botrytis cinerea Pers.: Fr. and biochemical studies during growth and ripening of two grape berry cultivars, respectively susceptible and resistant to grey mould. J. Phytopathol. 2003, 151, 208–214. [Google Scholar] [CrossRef]
- Keller, M.; Viret, O.; Cole, F.M. Botrytis cinerea infection in grape flowers: Defense reaction, latency, and disease expression. Phytopathology 2003, 93, 316–322. [Google Scholar] [CrossRef] [Green Version]
- Ciliberti, N.; Fermaud, M.; Roudet, J.; Rossi, V. Environmental conditions affect Botrytis cinerea infection of mature grape berries more than the strain or transposon genotype. Phytopathology 2015, 105, 1090–1096. [Google Scholar] [CrossRef] [Green Version]
- Castoria, R.; De Curtis, F.; Lima, G.; Caputo, L.; Pacifico, S.; De Cicco, V. Aureobasidium pullulans (LS-30) an antagonist of postharvest pathogens of fruits: Study on its modes of action. Postharvest Biol. Technol. 2001, 22, 7–17. [Google Scholar] [CrossRef]
- Di Francesco, A.; Ugolini, L.; Lazzeri, L.; Mari, M. Production of volatile organic compounds by Aureobasidium pullulans as a potential mechanism of action against postharvest fruit pathogens. Biol. Control 2015, 81, 8–14. [Google Scholar] [CrossRef]
- Lewis, K.; Whipps, J.M.; Cooke, R.C. Mechanisms of biological disease control with special reference to the case study of Pythium oligandrum as an antagonist. Biotechnol. Fungi Improv. Plant Growth 1989, 16, 191. [Google Scholar]
- Carbó, A.; Torres, R.; Usall, J.; Solsona, C.; Teixidó, N. Fluidised-bed spray-drying formulations of Candida sake CPA-1 by adding biodegradable coatings to enhance their survival under stress conditions. Appl. Microbiol. Biotechnol. 2017, 101, 7865–7876. [Google Scholar] [CrossRef] [PubMed]
- Fu, N.; Chen, X.D. Towards a maximal cell survival in convective thermal drying processes. Food Res. Int. 2011, 44, 1127–1149. [Google Scholar] [CrossRef]
- Team R Core. R: A Language and Environment for Statistical Computing. 2019. Available online: https://www.r-project.org/ (accessed on 10 June 2019).
- Ciliberti, N.; Fermaud, M.; Languasco, L.; Rossi, V. Influence of fungal strain, temperature, and wetness duration on infection of grapevine inflorescences and young berry clusters by Botrytis cinerea. Phytopathology 2015, 105, 325–333. [Google Scholar] [CrossRef]
- Ciliberti, N.; Fermaud, M.; Roudet, J.; Languasco, L.; Rossi, V. Environmental effects on the production of Botrytis cinerea conidia on different media, grape bunch trash, and mature berries. Aust. J. Grape Wine Res. 2016, 22, 262–270. [Google Scholar] [CrossRef]
- Abbey, J.A.; Percival, D.; Abbey, L.; Asiedu, S.K.; Schilder, A. Biofungicides as alternative to synthetic fungicide control of grey mould (Botrytis cinerea)—Prospects and challenges. Biocontrol Sci. Technol. 2019, 29, 207–228. [Google Scholar] [CrossRef]
- Elad, Y.; Stewart, A. Microbial control of Botrytis spp. In Botrytis: Biology, Pathology and Control; Elad, Y., Williamson, B., Tudzynski, P., Delen, N., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 2007; pp. 223–241. [Google Scholar]
- Elmer, P.A.G.; Reglinski, T. Biosuppression of Botrytis cinerea in grapes. Plant Pathol. 2006, 55, 155–177. [Google Scholar] [CrossRef]
- Sharma, R.R.; Singh, D.; Singh, R. Biological control of postharvest diseases of fruits and vegetables by microbial antagonists: A review. Biol. Control 2009, 50, 205–221. [Google Scholar] [CrossRef]
- Jacometti, M.A.; Wratten, S.D.; Walter, M. Review: Alternatives to synthetic fungicides for Botrytis cinerea management in vineyards. Aust. J. Grape Wine Res. 2010, 16, 154–172. [Google Scholar] [CrossRef]
- Haidar, R.; Fermaud, M.; Calvo-Garrido, C.; Roudet, J.; Deschamps, A. Modes of action for biological control of Botrytis cinerea by antagonistic bacteria. Phytopathol. Mediterr. 2016, 33, 13–34. [Google Scholar]
- Shtienberg, D.; Elad, Y. Incorporation of weather forecasting in integrated, biological-chemical management of Botrytis cinerea. Phytopathology 1997, 87, 332–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fravel, D. Hurdles and bottlenecks on the road to biocontrol of plant pathogens. Australas. Plant Pathol. 1999, 28, 53–56. [Google Scholar] [CrossRef]
- Huang, H.C.; Bremer, E.; Hynes, R.K.; Erickson, R.S. Foliar application of fungal biocontrol agents for the control of white mold of dry bean caused by Sclerotinia sclerotiorum. Biol. Control 2000, 18, 270–276. [Google Scholar] [CrossRef]
- Stewart, A. Commercial biocontrol—Reality or fantasy? Australas. Plant Pathol. 2001, 30, 127–131. [Google Scholar] [CrossRef]
- Fedele, G.; González-Domínguez, E.; Caffi, T.; Rossi, V. Use of environmental niches to understand interactions among Botrytis cinerea, biocontrol agents, and the environment. In Proceedings of the IOBC Bulletin, Vila Real, Portugal, 5–8 November 2019. [Google Scholar]
- Calvo-Garrido, C.; Haidar, R.; Roudet, J.; Gautier, T.; Fermaud, M. Pre-selection in laboratory tests of survival and competition before field screening of antagonistic bacterial strains against Botrytis bunch rot of grapes. Biol. Control 2018, 124, 100–111. [Google Scholar] [CrossRef]
- Köhl, J.; Postma, J.; Nicot, P.; Ruocco, M.; Blum, B. Stepwise screening of microorganisms for commercial use in biological control of plant-pathogenic fungi and bacteria. Biol. Control 2011, 57, 1–12. [Google Scholar] [CrossRef]
- Magan, N. Ecophysiology of biocontrol agents for improved competence in the phyllosphere. Microbial Ecol. Aerial Plant Surfaces 2006. [Google Scholar] [CrossRef]
- Calvo-Garrido, C.; Viñas, I.; Usall, J.; Rodríguez-Romera, M.; Ramos, M.C.; Teixidó, N. Survival of the biological control agent Candida sake CPA-1 on grapes under the influence of abiotic factors. J. Appl. Microbiol. 2014, 117, 800–811. [Google Scholar] [CrossRef]
- Longa, C.M.O.; Pertot, I.; Tosi, S. Ecophysiological requirements and survival of a Trichoderma atroviride isolate with biocontrol potential. J. Basic Microbiol. 2008, 48, 269–277. [Google Scholar] [CrossRef]
- Calvo-Garrido, C.; Teixidó, N.; Roudet, J.; Viñas, I.; Usall, J.; Fermaud, M. Biological control of Botrytis bunch rot in Atlantic climate vineyards with Candida sake CPA-1 and its survival under limiting conditions of temperature and humidity. Biol. Control 2014, 79, 24–35. [Google Scholar] [CrossRef]
- Lahlali, R.; Jijakli, M.H. Enhancement of the biocontrol agent Candida oleophila (strain O) survival and control efficiency under extreme conditions of water activity and relative humidity. Biol. Control 2009, 51, 403–408. [Google Scholar] [CrossRef]
- Gent, D.H.; Mahaffee, W.F.; McRoberts, N.; Pfender, W.F. The use and role of predictive systems in disease management. Annu. Rev. Phytopathol. 2013, 51, 267–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Symbol | Meaning of Symbol | Dimension |
|---|---|---|
| K | Total surface area in the system | [1] |
| HS | Healthy-susceptible tissue | [N] |
| I | Affected by pathogen and infectious tissue | [N] |
| R | Affected by pathogen and removed tissue | [N] |
| Hr | Healthy and resistant tissue | [N] |
| Hb | Healthy and BCA colonized tissue | [N] |
| Ib | Infectious and BCA colonized tissue | [N] |
| Rb | Removed and BCA colonized tissue | [N] |
| BSUM | Total of BCA colonized tissue | [N] |
| RG | Rate of growth | [N.T−1] |
| RS | Rate of senescence | [N.T−1] |
| STARTP | Initial inflow of the pathogen into the system | [N.T−1] |
| RI | Rate of infection | [N.T−1] |
| RR | Rate of removal | [N.T−1] |
| STARTB | Initial inflow of the BCA into the system | [N.T−1] |
| RPIN | Rate of daily pathogen inflow | [N.T−1] |
| RBIN | Rate of daily BCA inflow | [N.T−1] |
| RRES | Rate of induction of resistance by BCA | [N.T−1] |
| RSUS | Rate of change from Hr to HS tissue | [N.T−1] |
| RCOLHr | Rate of BCA colonization for Hr | [N.T−1] |
| RCOLH | Rate of BCA colonization for HS | [N.T−1] |
| RCOLI | Rate of BCA colonization for I tissue | [N.T−1] |
| RCOLR | Rate of BCA colonization for R tissue | [N.T−1] |
| GRO | Rate of BCA growth under fluctuating temperature and moisture | [N.T−1] |
| BMORH | Rate of BCA mortality for the Hb tissue | [N.T−1] |
| BMORI | Rate of BCA mortality for the Ib tissue | [N.T−1] |
| BMORR | Rate of BCA mortality for the Rb tissue | [N.T−1] |
| RAUDPC | Rate of AUDPC calculation | [N.T−1] |
| RRG | Relative rate of growth | [N.N−1.T−1] |
| RRS | Relative rate of senescence | [N.N−1.T−1] |
| b | Relative rate of infection | [N.N−1.T−1] |
| h | Relative rate of change from I to R tissue | [N.N−1.T−1] |
| c0 | Relative rate of change from HS to Hr tissue | [N.N−1.T−1] |
| e | Relative rate of change from Hr to HS tissue | [N.N−1.T−1] |
| c1 | Relative rate of change from HS to Hb tissue | [N.N−1.T−1] |
| c2 | Relative rate of change from I to Ib and from R to Rb tissue | [N.N−1.T−1] |
| f | Relative rate of BCA mortality | [N.N−1.T−1] |
| COFR | Correction factor for occupied tissue | [1] |
| COFRHr | Correction factor for Hr tissue | [1] |
| COFRI | Correction factor for I tissue | [1] |
| COFRR | Correction factor for R tissue | [1] |
| PDUR | Duration of mobilization of pathogen inoculum | [T] |
| PIN | Day of the first seasonal infection | [T] |
| BDUR | Duration of mobilization of BCA inoculum BDUR | [T] |
| BIN | Day of the BCA application | [T] |
| T | Driving function for daily temperature | [1] |
| MD | Driving function for daily moisture duration | [1] |
| RH | Driving function for daily relative humidity | [1] |
| AUDPC | Area under disease progress curve | [1] |
| POCC | Total K units occupied by the pathogen | [N] |
| EFF | Overall BCA efficacy | [1] |
| C | Relative contribution of competition | [1] |
| A | Relative contribution of antibiosis | [1] |
| IR | Relative contribution of induced resistance | [1] |
| P | Relative contribution of mycoparasitism | [1] |
| Relative Rate | Parameter | |||||||
|---|---|---|---|---|---|---|---|---|
| ba | γ | ζ | ν | ϱ | ψ | Tmin | Tmax | |
| Botrytis cinerea | 7.750 | 2.140 | 0.469 | 35.360 | 40.260 | 0 | 30 | |
| GRO b | BCA strain | χ | δ | ε | ω | η | Tmin | Tmax |
| S1 | 6.416 | 1.292 | 0.469 | 2.300 | 0.048 | 0 | 35 | |
| S2 | 12.000 | 4.000 | 0.469 | 2.300 | 0.048 | 5 | 37 | |
| S3 | 4.000 | 0.600 | 0.469 | 2.300 | 0.048 | 0 | 30 | |
| S4 | 6.416 | 1.292 | 0.469 | 4.000 | 0.500 | 0 | 35 | |
| S5 | 12.000 | 4.000 | 0.469 | 4.000 | 0.500 | 5 | 37 | |
| S6 | 4.000 | 0.600 | 0.469 | 4.000 | 0.500 | 0 | 30 | |
| S7 | 6.416 | 1.292 | 0.469 | 2.300 | 0.010 | 0 | 35 | |
| S8 | 12.000 | 4.000 | 0.469 | 2.300 | 0.010 | 5 | 37 | |
| S9 | 4.000 | 0.600 | 0.469 | 2.300 | 0.010 | 0 | 30 | |
| fc | Survival capability | Tmin | Tmax | Topt | RHmin | RHmax | RHopt | |
| low | 0 | 35 | 10 | 0 | 100 | 30 | ||
| medium | 5 | 35 | 15 | 0 | 100 | 40 | ||
| high | 5 | 40 | 20 | 0 | 100 | 50 | ||
| Climate Type | Scenario | Average Temperature (°C) a | Average Relative Humidity (%) b | Total Wetness Duration (h) c |
|---|---|---|---|---|
| Warm and dry | 1 | 25.74 | 68.80 | 56 |
| 2 | 25.40 | 74.85 | 70 | |
| 3 | 25.88 | 69.75 | 61 | |
| Mild and semi-arid | 1 | 19.15 | 79.27 | 81 |
| 2 | 20.08 | 79.35 | 91 | |
| 3 | 18.74 | 80.97 | 112 | |
| Cold and wet | 1 | 17.08 | 87.32 | 588 |
| 2 | 15.37 | 87.55 | 287 | |
| 3 | 16.16 | 88.30 | 446 |
| Warm and Dry a | Mild and Semi-Arid | Cold and Wet | |||||
|---|---|---|---|---|---|---|---|
| Source of Variation | df b | Variance c | P (>F) | Variance | P (>F) | Variance | P (>F) |
| MOA | 1 | 0.05 | 0.724 | 0.763 | 0.017 | 2.59 | <0.001 |
| Application time | 1 | 0.03 | 0.791 | 0.155 | 0.279 | 1.66 | <0.001 |
| Strain | 8 | 0.03 | 1.000 | 0.412 | 0.002 | 4.96 | <0.001 |
| Survival capability | 2 | 97.34 | <0.001 | 97.884 | <0.001 | 86.02 | <0.001 |
| MOA × Application time | 1 | 0.14 | 0.562 | 0.009 | 0.788 | 1.31 | 0.001 |
| MOA × Strain | 8 | 0.02 | 1.000 | 0.015 | 0.998 | 0.10 | 0.553 |
| MOA × Survival capability | 2 | 0.05 | 0.879 | 0.264 | 0.137 | 0.06 | 0.626 |
| Application time × Strain | 8 | 0.03 | 1.000 | 0.011 | 1.000 | 1.04 | <0.001 |
| Application time × Survival capability | 2 | 2.15 | 0.007 | 0.311 | 0.097 | 1.68 | <0.001 |
| Strain × Survival capability | 16 | 0.01 | 1.000 | 0.137 | 0.417 | 0.25 | 0.010 |
| MOA × Application time × Strain | 8 | 0.02 | 1.000 | 0.002 | 1.000 | 0.10 | 0.547 |
| MOA × Strain × Survival capability | 16 | 0.01 | 1.000 | 0.017 | 1.000 | 0.04 | 0.991 |
| MOA × Application time × Survival capability | 2 | 0.08 | 0.823 | 0.008 | 0.949 | 0.03 | 0.799 |
| Application time × Strain × Survival capability | 16 | 0.01 | 1.000 | 0.012 | 1.000 | 0.12 | 0.446 |
| MOA × Application time × Strain × Survival capability | 16 | 0.01 | 1.000 | 0.000 | 1.000 | 0.03 | 0.999 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fedele, G.; Bove, F.; González-Domínguez, E.; Rossi, V. A Generic Model Accounting for the Interactions among Pathogens, Host Plants, Biocontrol Agents, and the Environment, with Parametrization for Botrytis cinerea on Grapevines. Agronomy 2020, 10, 222. https://doi.org/10.3390/agronomy10020222
Fedele G, Bove F, González-Domínguez E, Rossi V. A Generic Model Accounting for the Interactions among Pathogens, Host Plants, Biocontrol Agents, and the Environment, with Parametrization for Botrytis cinerea on Grapevines. Agronomy. 2020; 10(2):222. https://doi.org/10.3390/agronomy10020222
Chicago/Turabian StyleFedele, Giorgia, Federica Bove, Elisa González-Domínguez, and Vittorio Rossi. 2020. "A Generic Model Accounting for the Interactions among Pathogens, Host Plants, Biocontrol Agents, and the Environment, with Parametrization for Botrytis cinerea on Grapevines" Agronomy 10, no. 2: 222. https://doi.org/10.3390/agronomy10020222
APA StyleFedele, G., Bove, F., González-Domínguez, E., & Rossi, V. (2020). A Generic Model Accounting for the Interactions among Pathogens, Host Plants, Biocontrol Agents, and the Environment, with Parametrization for Botrytis cinerea on Grapevines. Agronomy, 10(2), 222. https://doi.org/10.3390/agronomy10020222






