Nitrogen and Phosphorus Absorption and Yield of Tomato Increased by Regulating the Bacterial Community under Greenhouse Conditions via the Alternate Drip Irrigation Method
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Field and Experimental Design
2.2. Sampling
2.3. Soil Temperature, pH, Enzyme Activity, Carbon Dioxide (CO2) Flux and Porosity
2.4. Analysis of the Dry Matter, Nutrient Composition, and Roots of Tomato Plants
2.5. High-Throughput Analysis of the Soil Bacterial Community
2.6. Data Processing and Statistical Analyses
3. Results
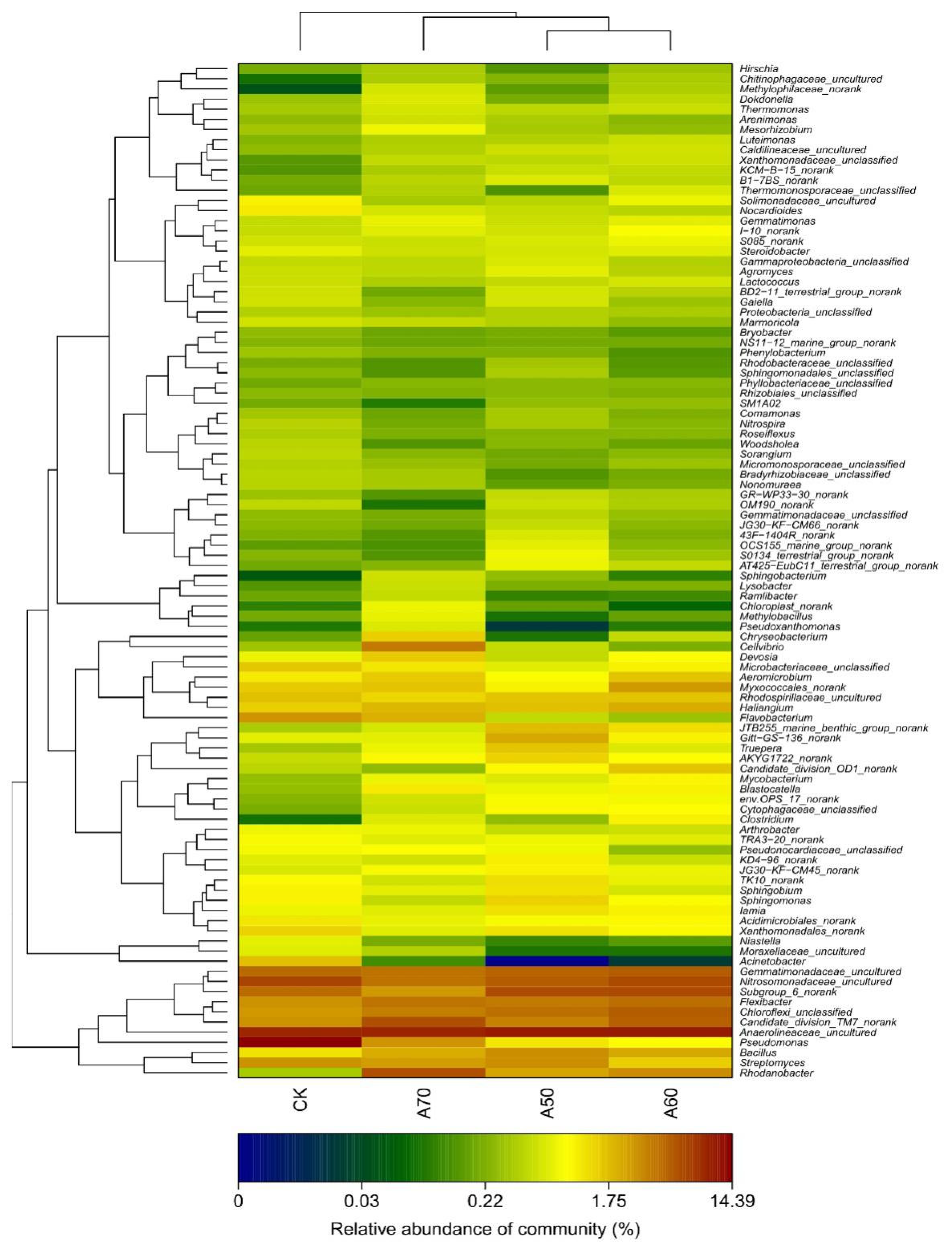
3.1. Structure of the Bacterial Communities in the Soils
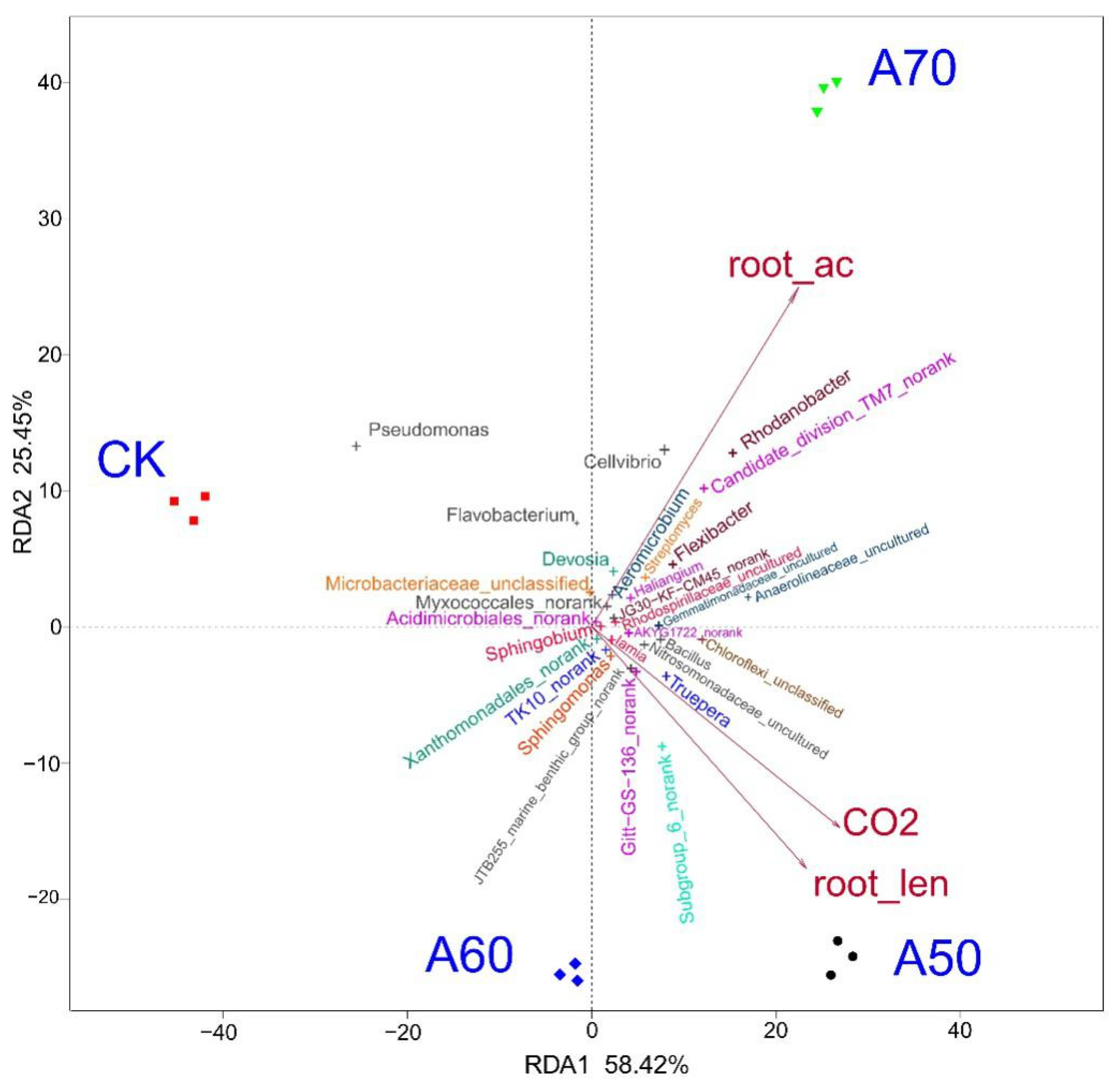
3.2. Factors Influencing the Bacterial Community Composition
3.3. Predicted Functional Metabolism of Bacterial Communities
3.4. Soil Nutrients and Tomato Growth Indices
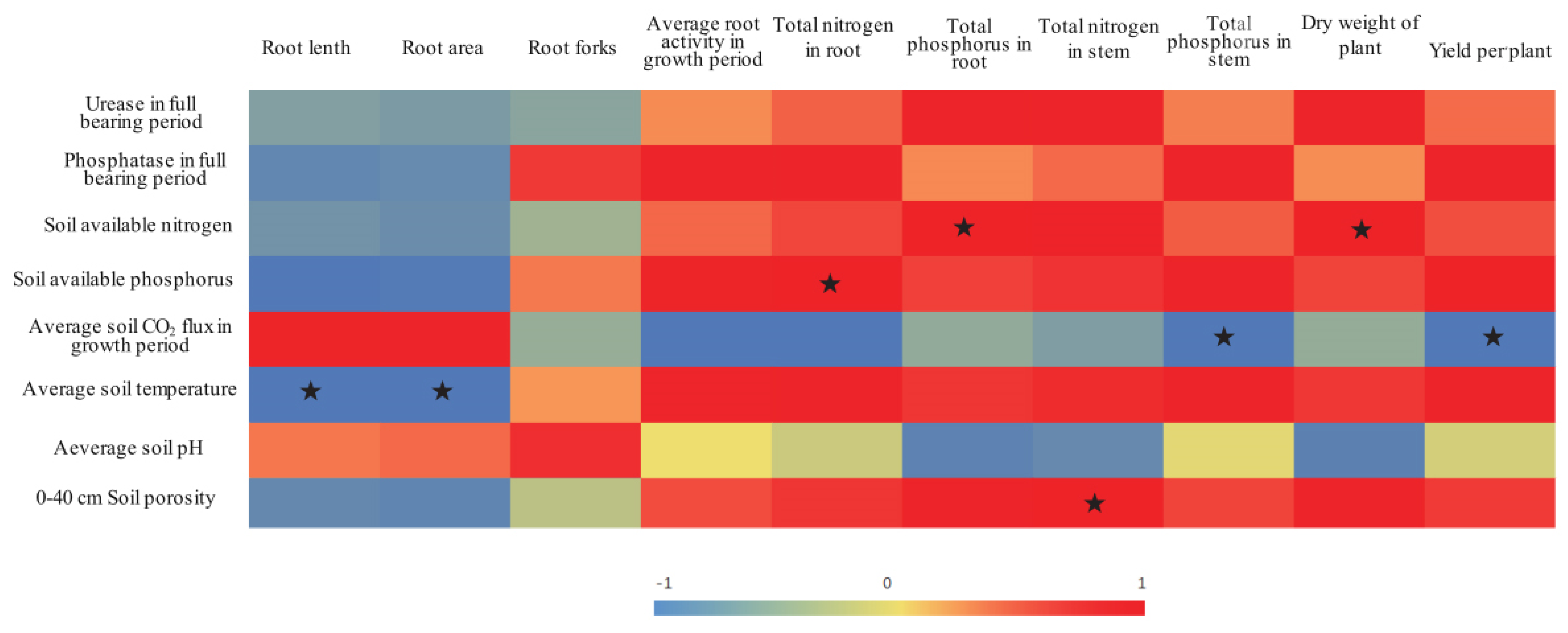
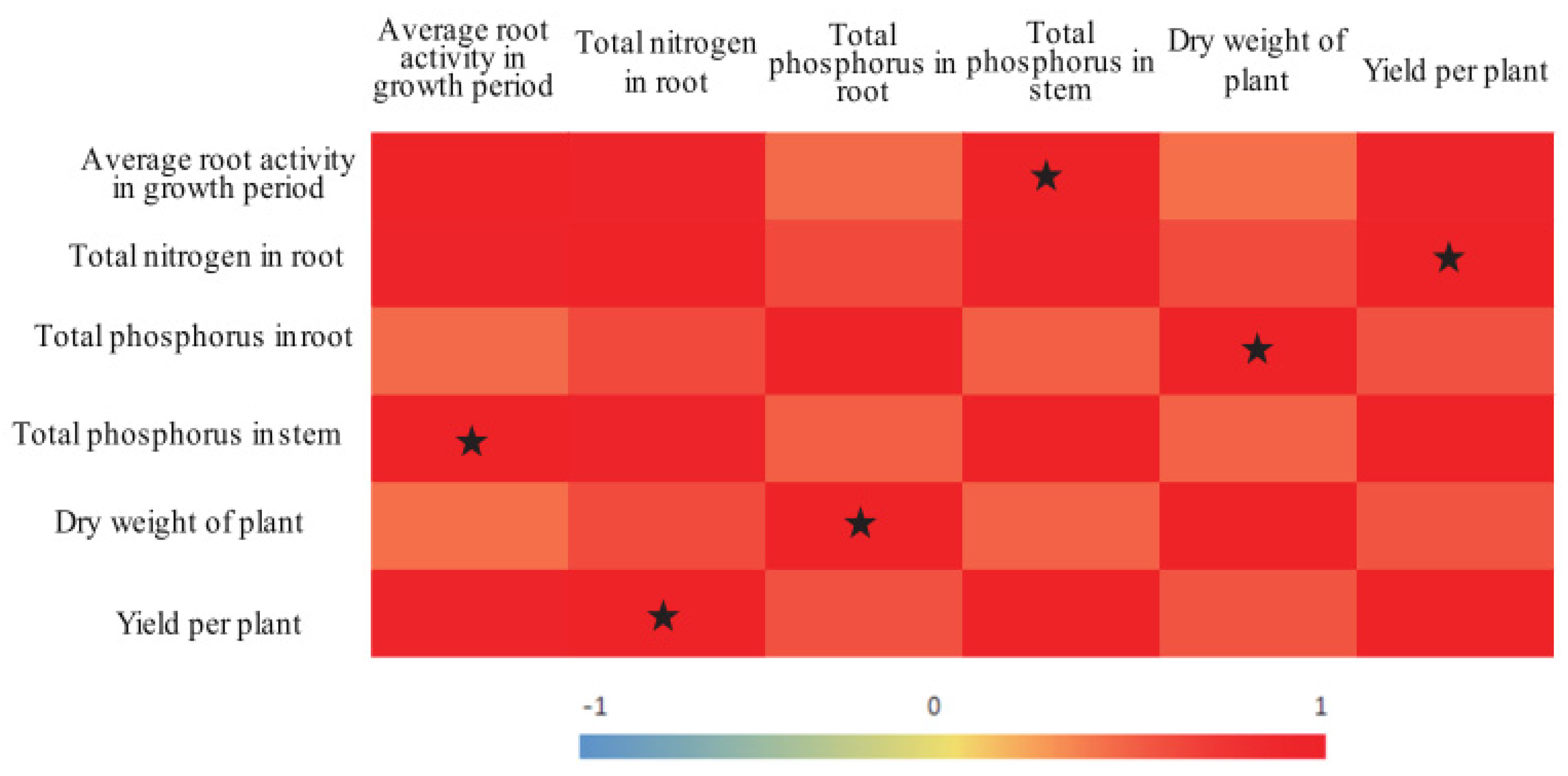
3.5. Correlation Analysis
3.6. Irrigation Amounts and Irrigation Water-Use Efficiency (IWUE)
4. Discussion
4.1. ADI Optimized the Bacterial Community and Improved Soil N and P Availability
4.2. The Relatively High Soil Wet/Dry Alternating Frequency by ADI Was not Conducive to Soil Nutrient Activation
4.3. The Relatively Low Soil Wet/Dry Alternating Frequency by ADI Increased Soil-Root-Bacterium Interactions
4.4. ADI with a Relatively Adequate Irrigation Amount Improved Soil Nutrient Absorption by Tomato Plants
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ju, X.T.; Kou, C.L.; Christie, P.; Dou, Z.X.; Zhang, F.S. Changes in the soil environment from excessive application of fertilizers and manures to two contrasting intensive cropping systems on the North China Plain. Environ. Pollut. 2007, 145, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Zhang, F.; Wei, Q.; Xu, Y.; Hu, J. Influence of land use change on soil nutrients in an intensive agricultural region of north china. Soil Till. Res. 2006, 88, 85–94. [Google Scholar] [CrossRef]
- Moreno, M.T.; Carmona, E.; de Santiago, A.; Ordovás, J.; Delgado, A. Olive husk compost improves the quality of intensively cultivated agricultural soils. Land Degrad. Dev. 2016, 27, 449–459. [Google Scholar] [CrossRef]
- Lal, R. Soil quality changes under continuous cropping for seventeen seasons of an alfisol in western Nigeria. Land Degrad. Dev. 1998, 9, 259–274. [Google Scholar] [CrossRef]
- Davies, W.J.; Zhang, J.; Yang, J.; Dodd, I.C. Novel crop science to improve yield and resource use efficiency in water-limited agriculture. J. Agric. Sci. 2011, 149, 123–131. [Google Scholar] [CrossRef]
- Ahmadi, S.H.; Andersen, M.N.; Plauborg, F.; Poulsen, R.T.; Jensen, C.R.; Sepaskhah, A.R.; Hansen, S. Effects of irrigation strategies and soils on field grown potatoes: Gas exchange and xylem [ABA]. Agric. Water Manag. 2010, 97, 1486–1494. [Google Scholar] [CrossRef]
- Wang, Z.; Kang, S.; Jensen, C.R.; Liu, F. Alternate partial root-zone irrigation reduces bundle-sheath cell leakage to CO2 and enhances photosynthetic capacity in maize leaves. J. Exp. Bot. 2012, 63, 1145–1153. [Google Scholar] [CrossRef]
- Du, S.; Kang, S.; Li, F.; Du, T. Water use efficiency is improved by alternate partial root-zone irrigation of apple in arid northwest China. Agric. Water Manag. 2017, 179, 184–192. [Google Scholar] [CrossRef]
- Liang, H.; Li, F.; Nong, M. Effects of alternate partial root-zone irrigation on yield and water use of sticky maize with fertigation. Agric. Water Manag. 2013, 116, 242–247. [Google Scholar] [CrossRef]
- Songsri, P.; Jogloy, S.; Vorasoot, N.; Akkasaeng, C.; Patanothai, A.; Holbrook, C.C. Root distributionof drought-resistant peanut genotypes in response to drought. J. Agron. Crop. Sci. 2008, 194, 92–103. [Google Scholar] [CrossRef]
- Chen, C.; Xu, F.; Zhu, J.R.; Xu, W.W. Nitrogen forms affect root growth, photosynthesis, and yield of tomato under alternate partial root-zone irrigation. J. Plant Nutr. Soil Sci. 2016, 179, 104–112. [Google Scholar] [CrossRef]
- Lima, R.S.N.D.; Assis-Figueiredo, F.A.M.M.; Martins, A.; Deus, B.C.D.S.D.; Ferraz, T.M.; Assis-Gomes, M.M.A.; Sousa, E.F.; Glenn, D.M.; Campostrini, E. Partial rootzone drying (PRD) and regulated deficit irrigation (RDI) effects on stomatal conductance, growth, photosynthetic capacity, and water-use efficiency of papaya. Sci. Hortic. 2015, 183, 13–22. [Google Scholar] [CrossRef]
- Shao, G.C.; Zhang, Z.Y.; Liu, N.; Yu, S.E.; Xing, W.G. Comparative effects of deficit irrigation (DI) and partial rootzone drying (PRD) on soil water distribution, water use, growth and yield in greenhouse grown hot Pepper. Sci. Hortic. 2008, 119, 11–16. [Google Scholar] [CrossRef]
- Wang, H.; Liu, F.; Andersen, M.N.; Jensen, C.R. Comparative effects of partial root-zone drying and deficitirrigation on nitrogen uptake in potatoes (Solanum tuberosum L.). Irrig. Sci. 2009, 27, 443–447. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, S.; Chen, C.; Shu, L.Z.; Zhou, X.J.; Zhu, S.N. Regulation of nitrogen forms on growth of eggplant under partial root-zone irrigation. Agric. Water Manag. 2014, 142, 56–65. [Google Scholar] [CrossRef]
- Doran, J.W.; Zeiss, M.R. Soil health and sustainability: Managing the biotic component of soil quality. Appl. Soil Ecol. 2000, 15, 3–11. [Google Scholar] [CrossRef]
- Santos, L.N.S.D.; Matsura, E.E.; Gonçalves, I.Z.; Barbosa, E.A.A.; Nazário, A.A.; Tuta, N.F.; Elaiuy, M.C.L.; Feitosa, D.R.C. Water storage in the soil profile under subsurface drip irrigation: Evaluating two installation depths of emitters and two water qualities. Agric. Water Manag. 2015, 170, 91–98. [Google Scholar] [CrossRef]
- Adesemoye, A.O.; Torbert, H.A.; Kloepper, J.W. Plant growth-promoting rhizobacteria allow reduced applicationrates of chem-ical fertilizers. Microb. Ecol. 2009, 58, 921–929. [Google Scholar] [CrossRef]
- Hernandez, M.; Chailloux, M. Las micorrizas arbusculares y lasbacterias rizosfericas como alternativa a la nutricion mineral deltomate. Cultiv. Trop. 2004, 25, 5–16. [Google Scholar]
- Cheung, M.K.; Au, C.H.; Chu, K.H.; Kwan, H.S.; Wong, C.K. Composition and genetic diversity of picoeukaryotes in subtropical coastal waters as revealed by 454 pyrosequencing. Int. Soc. Microb. Ecol. J. 2010, 4, 1053–1059. [Google Scholar] [CrossRef]
- Zhang, H.H.; Feng, J.; Chen, S.N.; Zhao, Z.F.; Li, B.Q.; Wang, Y.; Jia, J.Y.; Li, S.L.; Wang, Y.; Yan, M.M.; et al. Geographical patterns of nirS gene abundance and nirS-type denitrifying bacterial community associated with activated sludge from different wastewater treatment plants. Microb. Ecol. 2019, 77, 304–316. [Google Scholar] [CrossRef]
- Griffiths, R.I.; Thomson, B.C.; James, P.; Bell, T.; Bailey, M.; Whiteley, A.S. The bacterial biogeography of British soils. Environ. Microbiol. 2011, 13, 1642–1654. [Google Scholar] [CrossRef] [PubMed]
- Hayat, R.; Ali, S.; Amara, U.; Khalid, R.; Ahmed, I. Soil beneficial bacteria and their role in plant growth promotion: A review. Ann. Microbiol. 2010, 60, 579–598. [Google Scholar] [CrossRef]
- Tabatabai, M. Soil enzymes. In Methods of Soil Analyses, Part 2, Microbiological and Biochemical Properties; Weaver, R.W., Angle, J.S., Bottomly, P.S., Eds.; Soil Science Society of America: Madison, WI, USA, 1994; pp. 775–833. [Google Scholar]
- Franzluebbers, A.J.; Hons, F.M.; Zuberer, D.A. Tillage and crop effects on seasonal dynamics of soil CO2 evolution, water content, temperature, and bulk density. Appl. Soil Ecol. 1995, 2, 95–109. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Yan, M.M.; Chen, S.N.; Huang, T.L.; Li, B.Q.; Li, N.; Li, K.W.; Zong, R.R.; Miao, Y.T.; Huang, X. Community compositions of phytoplankton and eukaryotes during the mixing periods of a drinking water reservoir: Dynamics and interactions. Int. J. Environ. Res. Public Health 2020, 17, 1128. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Bittinger, K.; Bushman, F.D.; Desantis, T.Z.; Andersen, G.L.; Knight, R. Pynast: A flexible tool for aligning sequences to a template alignment. Bioinformatics 2010, 26, 266–267. [Google Scholar] [CrossRef]
- Price, M.N.; Dehal, P.S.; Arkin, A.P. Fasttree 2-approximately maximum-likelihood trees for large alignments. PLoS ONE 2010, 5, e9490. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microb. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The silva ribosomal rna gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, 590–596. [Google Scholar] [CrossRef]
- Langille, M.G.; Zaneveld, J.; Caporaso, J.G.; Mcdonald, D.; Knights, D.; Reyes, J.A.; Clemente, J.C.; Burkepile, D.E.; Thurber, R.L.V.; Knight, R.; et al. Predictive functional profiling of microbial communities using 16s rrna marker gene sequences. Nat. Biotechnol. 2013, 31, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Bray, J.R.; Curtis, J.T. An ordination of upland forest communities of southern Wisconsin. Ecol. Monogr. 1957, 27, 325–349. [Google Scholar] [CrossRef]
- R Core Team. R: A language and environment for statistical computing. R foundation for statistical computing, vienna, austria. Computing 2013, 14, 12–21. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; Hara, R.B.O.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H.H. Vegan: Community ecology package. R package version. 2.0–10. J. Stat. Softw. 2013, 48, 1–21. [Google Scholar]
- Jones, D.L.; Oburger, E. Solubilization of phosphorus by soil microorganisms. In Phosphorus in Action; Springer: Berlin/Heidelberg, Germany, 2010; pp. 169–198. [Google Scholar]
- Hutton, R.J.; Loveys, B.R. A partial root zone drying irrigation strategy for citrus-effects on water use efficiency and fruit characteristics. Agric. Water Manag. 2011, 98, 1485–1496. [Google Scholar] [CrossRef]
- Bronick, C.J.; Lal, R. Soil structure and management: A review. Geoderma 2005, 124, 3–22. [Google Scholar] [CrossRef]
- Taylor, A.E.; Zeglin, L.H.; Dooley, S.; Myrold, D.D.; Bottomley, P.J. Evidence for different contributions of archaeaand bacteria to the ammonia-oxidizing potential ofdiverse Oregon soils. Appl. Environ. Microb. 2010, 76, 7691–7698. [Google Scholar] [CrossRef]
- Shahnazari, A.; Ahmadi, S.H.; Laerke, P.E.; Liu, F.L.; Plauborg, F.; Jacobsen, S.E.; Jensen, C.R.; Andersen, M.N. Nitrogen dynamics in the soil-plant system under deficit and partial root-zone drying irrigation strategies in potatoes. Eur. J. Agron. 2008, 28, 65–73. [Google Scholar] [CrossRef]
- Chaparro, J.M.; Sheflin, A.M.; Manter, D.K.; Vivanco, J.M. Manipulating the soil microbiome to increase soil health and plant fertility. Biol. Fert. Soils 2012, 48, 489–499. [Google Scholar] [CrossRef]
- Raine, S.R.; Meyer, W.S.; Rassam, D.W.; Hutson, J.L.; Cook, F.J. Soil-water and solute movement under precision irrigation: Knowledge gaps for managing sustainable root zones. Irrig. Sci. 2007, 26, 91–100. [Google Scholar] [CrossRef]
- Williams, M.A.; Rice, C.W. Seven years of enhanced water availability influences the physiological, structural, and functional attributes of a soil microbial community. Appl. Soil Ecol. 2006, 35, 535–545. [Google Scholar] [CrossRef]
- Torsvik, V.; Øvreås, L. Microbial diversity and function in soil: From genes to ecosystems. Curr. Opin. Microbiol. 2002, 5, 240–245. [Google Scholar] [CrossRef]
- Adu, J.K.; Oades, J.M. Physical factors influencing decomposition of organic materials in soil aggregates. Soil Biol. Biochem. 1978, 10, 109–115. [Google Scholar] [CrossRef]
- Kemper, W.D.; Rosenau, R.; Nelson, S. Gas displacement and aggregate stability of soil. Soil. Sci. Soc. Am. J. 1985, 49, 25–28. [Google Scholar] [CrossRef]
- Wu, J.; Brookes, P.C. The proportional mineralisation of microbial biomass and organic matter caused by air-drying and rewetting of a grassland soil. Soil Biol. Biochem. 2005, 37, 507–515. [Google Scholar] [CrossRef]
- González, E.M.; Larrainzar, E.; Marino, D.; Wienkoop, S.; Gil-Quintana, E.; Arrese-lgor, C. Physiological responses of N2-fixing legumes to water limitation. In Legume Nitrogen Fixation in a Changing Environment; Springer International Publishing: Cham, Switzerland, 2015; pp. 5–33. [Google Scholar]
- Hinsinger, P.; Herrmann, L.; Lesueur, D.; Robin, A.; Trap, J.; Waithaisong, K.; Plassard, C. Impact of roots, microorganisms and microfauna on the fate of soil phosphorus in the rhizosphere. Annual Plant Rev. Online 2018, 48, 377–407. [Google Scholar]
- Nardi, S.; Pizzeghello, D.; Schiavon, M.; Ertani, A. Plant biostimulants: Physiological responses induced by protein hydrolyzed-based products and humic substances in plant metabolism. Sci. Agric. 2016, 73, 18–23. [Google Scholar] [CrossRef]
- Pugliese, M.; Liu, B.P.; Gullino, M.L.; Garibaldi, A. Microbial enrichment of compost with biological control agents to enhance suppressiveness to four soil-borne diseases in greenhouse. J. Plant Dis. Protect. 2011, 118, 45–50. [Google Scholar] [CrossRef]
- North, G.B.; Nobel, P.S. Changes in hydraulic conductivity and anatomy caused by drying and rewetting roots of Agave desert (Agavaceae). Am. J. Bot. 1991, 78, 906–915. [Google Scholar] [CrossRef]
- Gregory, P.J. Roots, rhizosphere and soil: The route to a better understanding of soil science? Eur. J. Soil Sci. 2006, 57, 2–12. [Google Scholar] [CrossRef]
- Azcón, R.; Rodríguez, R.; Amora-Lazcano, E.; Ambrosano, E. Uptake and metabolism of nitrate in mycorrhizal plants as affected by water availability and N concentration in soil. Eur. J. Soil Sci. 2008, 59, 131–138. [Google Scholar] [CrossRef]
- Güsewell, S. N: P ratios in terrestrial plants: Variation and functional significance. New Phytol. 2004, 164, 243–266. [Google Scholar] [CrossRef]
| Metabolism | CK | A50 | A60 | A70 | Related Genes | |
|---|---|---|---|---|---|---|
| Nitrogen metabolism | Nitrogen fixation | 2793 c | 3493 b | 3881 a | 3783 a | K02586 nifD; K02591 nifK; K02588 nifH; K00531 anfG |
| Nitrification | 3521 a | 3211 b | 3339 c | 3056 c | K10944 pmoA-amoA; K10945 pmoB-amoB; K10946 pmoC-amoC; K10535 hao; K00370 narG, narZ, nxrA; K00371 narH, narY, nxrB | |
| Denitrification | 9229 c | 9841 b | 10,829 a | 11,285 a | K00370 narG, narZ, nxrA; K00371 narH, narY, nxrB; K00374 narI, narV; K02567 napA; K02568 napB; K00368 nirK; K04561 norB; K02305 norC; K00376 nosZ | |
| Total | 15,543 c | 16,545 b | 18,049 a | 18,124 a | ||
| Phosphorus metabolism | 112,683 b | 117,388 a | 117,897 a | 118,024 a | K00325 pntB; K05946 phoU; K00655 plsC; K01514 ppX1; K06189 corC; K09459 EC4.1.1.82; K02043 phnF; K06162 phnM; K06163 phnJ; K06164 phnI; K02037 pstC; K02038 pstA; K06165 phnH; K01077 phoA; K07042 ybeY; K05781 phnK; K05306 phnX; K06080 RcsF; K07660 phoP; K03820 Lnt; K06217 phoH; K09994 phnO; K06193 phnA; K03430 phnW; K02221 yggT; K02036 pstB; K03306 Pit; K07221 oprO_P; K01507 ppa; K04750 phnB; K00937 ppk; K06019 ppaX; K07657 phoB; K07637 phoQ; K07636 phoR | |
| Treatment | Urease during the Peak Fruiting Period (µmol NH3·g−1·h−1) | Phosphatase during the Peak Fruiting Period (µmol pNP·g−1·h−1) | Soil Available Nitrogen (mg·kg−1) | Soil Available Phosphorus (mg·kg−1) | Total Nitrogen in the Roots (%) | Total Phosphorus in the Roots (%) | Total Nitrogen in the Stems (%) | Total Phosphorus in the Stems (%) | Yield Per Plant (kg) |
|---|---|---|---|---|---|---|---|---|---|
| CK | 8.35 a | 27.01 a | 46.91 d | 94.43 c | 1.688 c | 0.186 c | 1.580 c | 0.089 d | 2.27 b |
| A50 | 6.24 b | 3.36 c | 69.27 c | 140.75 b | 1.676 c | 0.198 b | 1.377 d | 0.092 c | 2.33 b |
| A60 | 9.34 a | 8.33 b | 102.55 a | 155.71 b | 1.804 b | 0.361 a | 1.913 a | 0.156 b | 2.56 b |
| A70 | 8.41 a | 24.95 a | 89.61 a | 274.54 a | 1.923 a | 0.295 a | 1.767 b | 0.253 a | 2.82 a |
| Nitrogen Fixation-Related Gene Copy Number | Nitrification-Related Gene Copy Number | Denitrification-Related Gene Copy Number | Total Nitrogen Metabolism-Related Gene Copy Number | Total phoSphorus Metabolism-Related Gene Copy Number | Root Activity | Root Forks | Soil Available Nitrogen | Soil Available Phosphorus | Yield | Total Dry Matter | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Nitrogen Fixation-related gene copy number | 1 | −0.73 | 0.90 | 0.95 * | 0.97 * | 0.99 * | 0.87 | 0.97 * | 0.45 | 0.80 | 0.98 * |
| Nitrification-related gene copy number | −0.72 | 1 | −0.75 | −0.69 | −0.82 | −0.79 | −0.95 | −0.56 | −0.45 | −0.53 | −0.60 |
| Denitrification-related gene copy number | 0.90 | −0.75 | 1 | 0.98 * | 0.83 | 0.95 | 0.77 | 0.91 | 0.79 | 0.85 | 0.89 |
| Total nitrogen metabolism-related gene copy number | 0.95 * | −0.69 | 0.98 * | 1 | 0.87 | 0.97 * | 0.77 | 0.97 * | 0.67 | 0.95 * | 0.96 * |
| Total phosphorus metabolism-related gene copy number | 0.97 * | −0.82 | 0.83 | 0.87 | 1 | 0.96 * | 0.95 * | 0.87 | 0.31 | 0.66 | 0.91 |
| Treatment | Irrigation Amount/mm | IWUE/(kg·m−3) |
|---|---|---|
| CK | 291.17 ab | 44.16 c |
| A50 | 204.78 c | 64.38 a |
| A60 | 274.42 b | 52.79 b |
| A70 | 309.89 a | 51.69 b |
| Treatment | Soil pH | Average Soil Temperature T (°C) | 0–40-cm Soil Porosity (%) | CO2 Flux (mg·m−2·min−1) | Root Activity (mg TTC·g−1·h−1) | Root Length (cm) | Root Area (cm2) | Root Forks |
|---|---|---|---|---|---|---|---|---|
| CK | 7.835 a | 16.20 ab | 39.90 b | 3.36 a | 12.53 c | 1473.69 d | 720.99 b | 3969.00 c |
| A50 | 8.219 a | 15.44 b | 40.27 b | 4.56 a | 16.22 b | 2524.88 a | 1044.55 a | 10307 ab |
| A60 | 7.697 a | 16.61 ab | 49.04 a | 4.36 a | 18.27 a | 2080.02 b | 959.31 a | 8979 b |
| A70 | 8.115 a | 17.18 a | 46.91 a | 4.11 a | 18.62 a | 1869.25 c | 929.81 a | 11371 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Niu, W.; Li, Y. Nitrogen and Phosphorus Absorption and Yield of Tomato Increased by Regulating the Bacterial Community under Greenhouse Conditions via the Alternate Drip Irrigation Method. Agronomy 2020, 10, 315. https://doi.org/10.3390/agronomy10030315
Wang J, Niu W, Li Y. Nitrogen and Phosphorus Absorption and Yield of Tomato Increased by Regulating the Bacterial Community under Greenhouse Conditions via the Alternate Drip Irrigation Method. Agronomy. 2020; 10(3):315. https://doi.org/10.3390/agronomy10030315
Chicago/Turabian StyleWang, Jingwei, Wenquan Niu, and Yuan Li. 2020. "Nitrogen and Phosphorus Absorption and Yield of Tomato Increased by Regulating the Bacterial Community under Greenhouse Conditions via the Alternate Drip Irrigation Method" Agronomy 10, no. 3: 315. https://doi.org/10.3390/agronomy10030315
APA StyleWang, J., Niu, W., & Li, Y. (2020). Nitrogen and Phosphorus Absorption and Yield of Tomato Increased by Regulating the Bacterial Community under Greenhouse Conditions via the Alternate Drip Irrigation Method. Agronomy, 10(3), 315. https://doi.org/10.3390/agronomy10030315






