Effect of Magnesium Supply and Storage Time on Anti-Nutritive Compounds in Potato Tubers
Abstract
1. Introduction
2. Materials and Methods
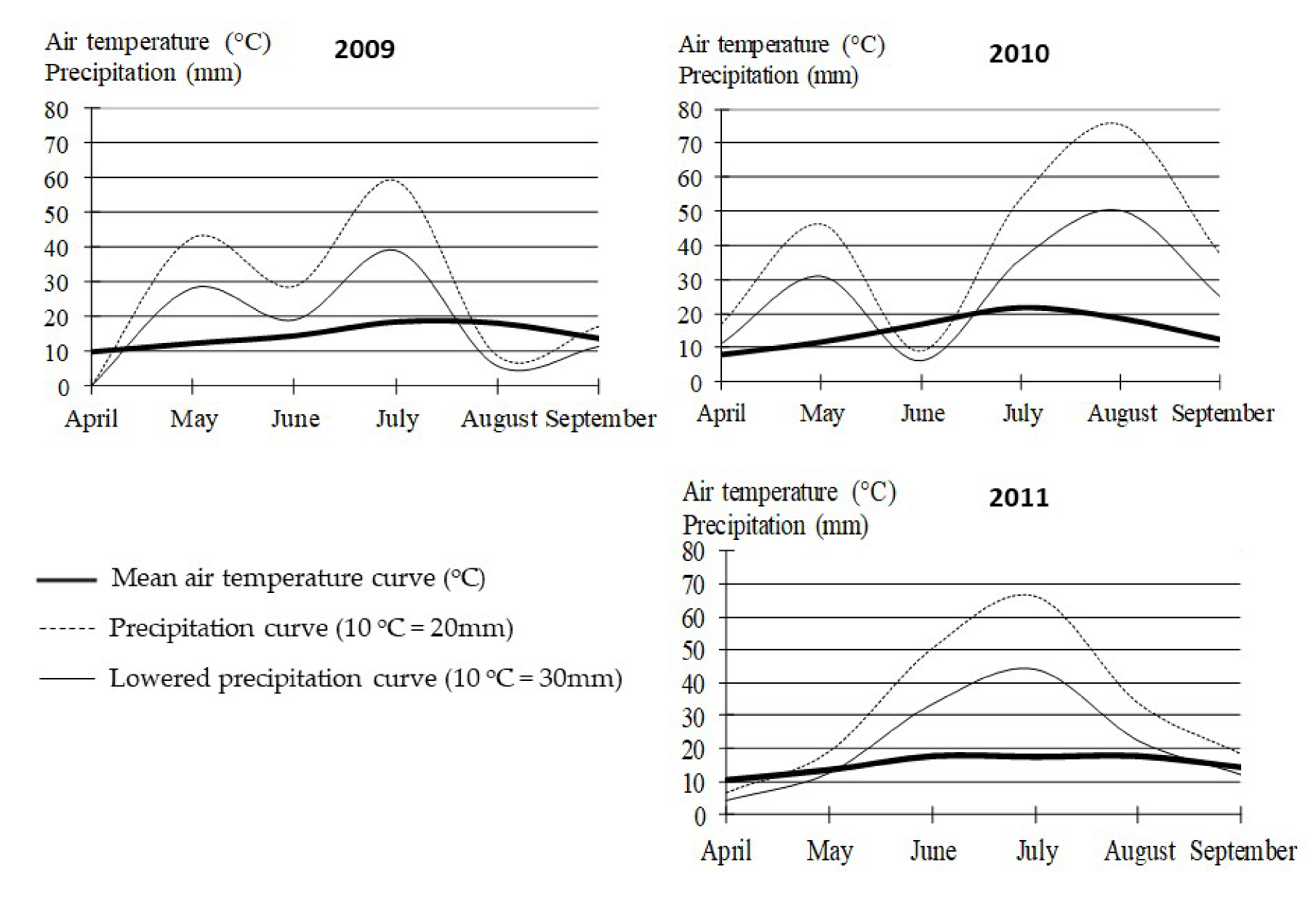
2.1. Field Experiment
2.2. Storage Conditions
2.3. Laboratory Analysis Procedure
2.4. Statistical Analysis of the Results
3. Results and Discussion
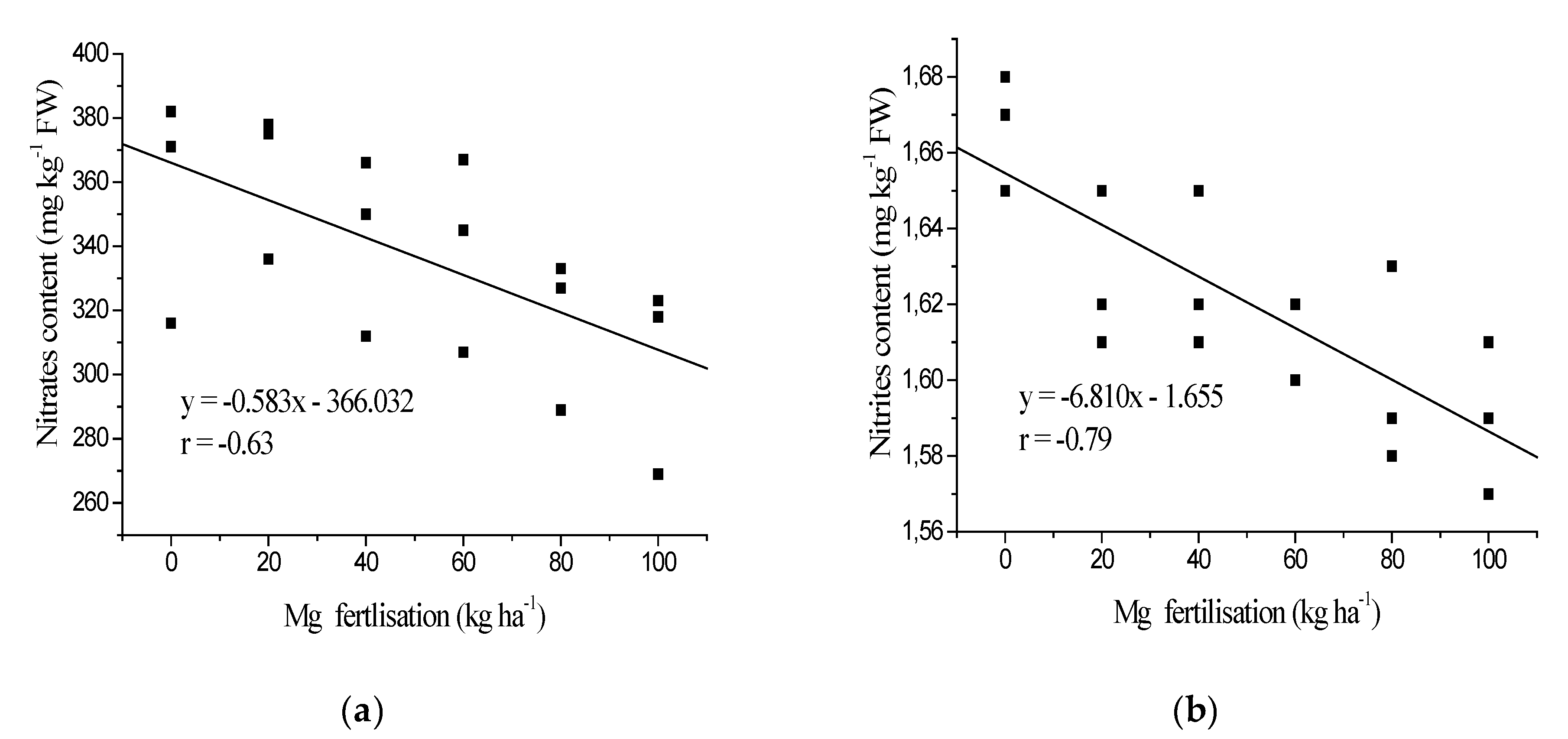
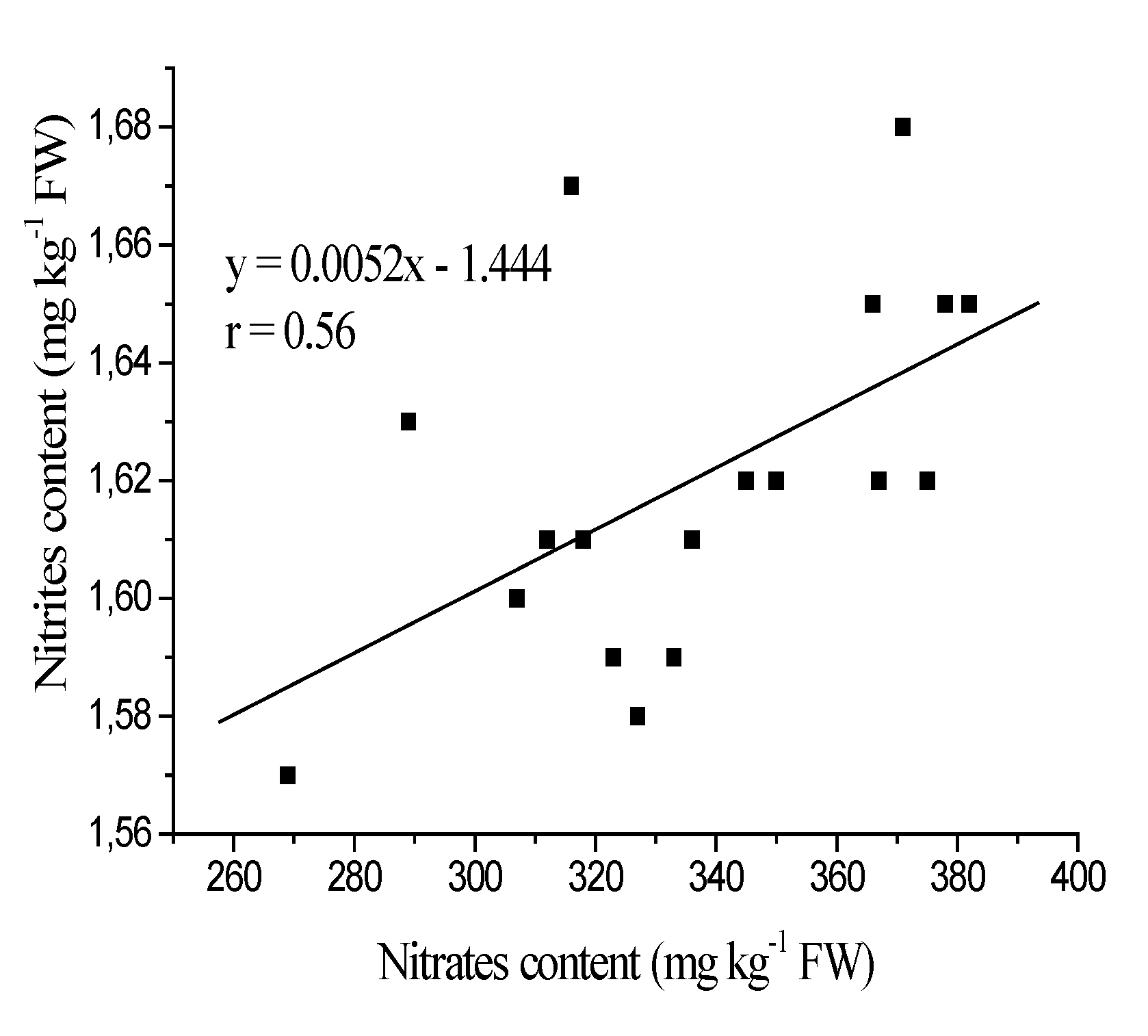
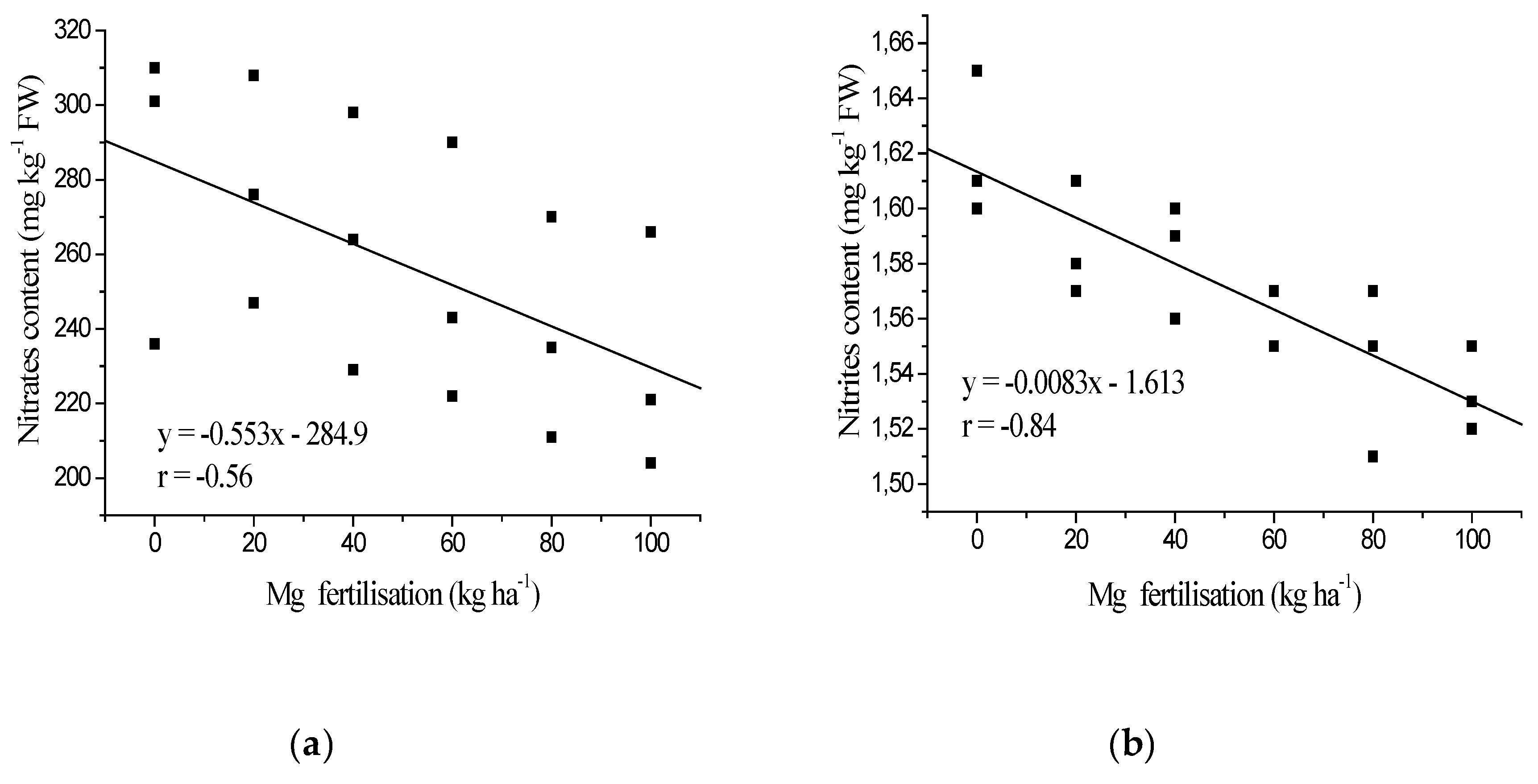
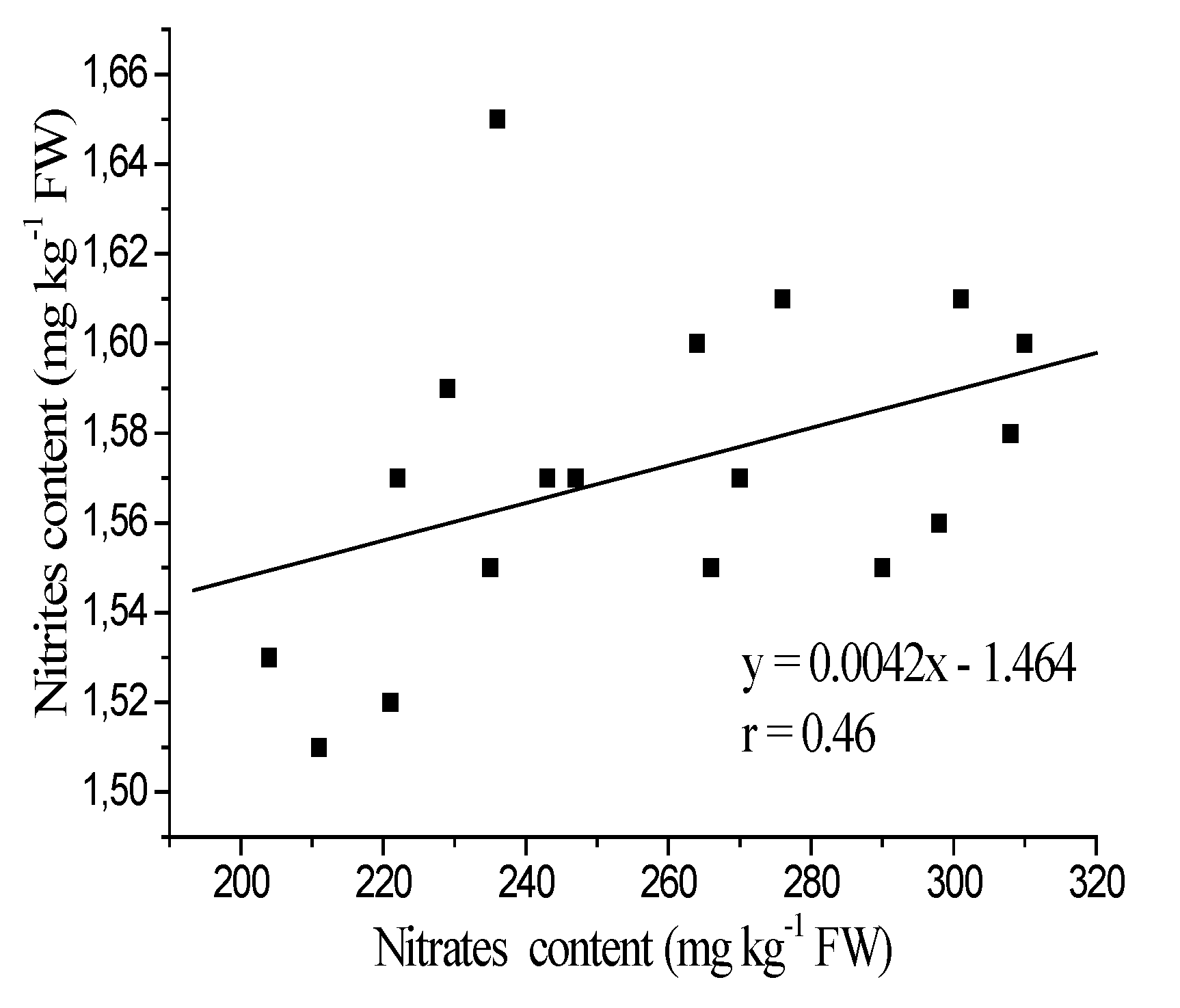
3.1. Nitrates and Nitrites Content
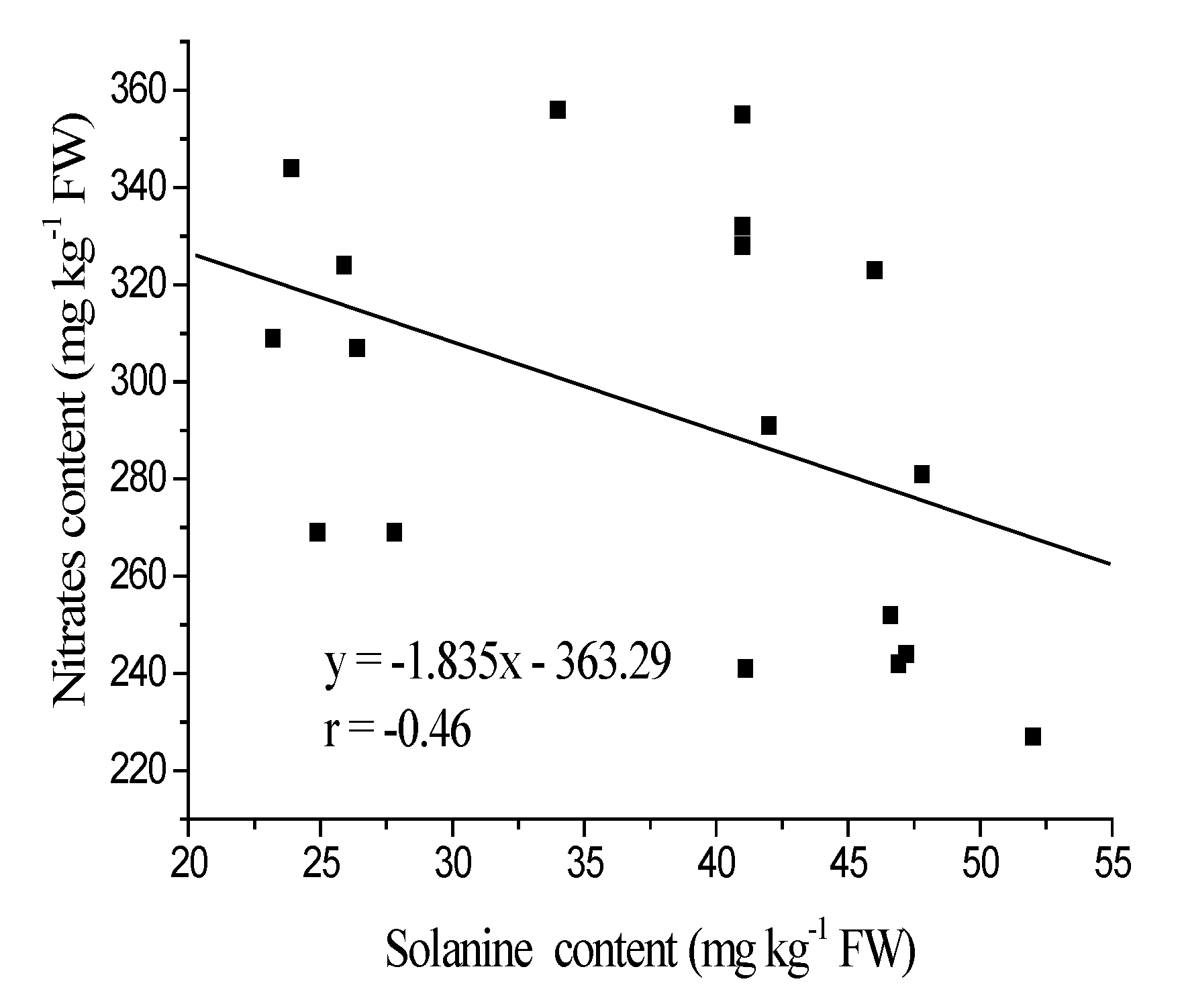
3.2. TGA Content
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Leszczyński, W. The quality of table potato. Żywność Suppl. 2000, 4, 5–27. (In Polish) [Google Scholar]
- Hamouz, K.; Lachman, J.; Dvorak, P.; Pivec, V. The effect of ecological growing on the potatoes yield and quality. Plant Soil Environ. 2005, 51, 397–402. [Google Scholar] [CrossRef]
- Wroniak, J. Nutrition qualities of edible potato. Ziemn. Pol. 2006, 6, 17–20. (In Polish) [Google Scholar]
- Ciećko, Z.; Żołnowski, A.; Mierzejewska, A. Effect of foliar nitrogen and magnesium fertilization on the total, protein nitrogen and nitrates(V) content in potato tubers. Ecol. Chem. Eng. 2010, 17, 593–600. [Google Scholar]
- Santamaria, P. Nitrate in vegetables: Toxicity, content, intake and EC regulation. J. Sci. Food Agric. 2006, 86, 10–17. [Google Scholar] [CrossRef]
- Hmelak, G.A.; Urih, G.; Langerholc, T.; Kristil, J. Nitrate content in potatoes cultivated in contaminated groundwater areas. J. Food Res. 2014, 3, 18–27. [Google Scholar] [CrossRef]
- Bottex, B.; Lou, J.; Dorne, C.M.; Carlander, D.; Bedford, D.; Przyrembel, H.; Heppner, C.; Kleiner, J.; Cockburn, A. Risk—Benefit health assessment of food—Food fortification and nitrate in vegetables. Trends Food Sci. Tech. 2008, 19, 109–115. [Google Scholar] [CrossRef]
- Zgórska, K.; Sowa-Niedziałkowska, G. Effect of the thermal and cultivar—Specific factors on qualitative changes in potato tubers during their long-term storage. Pam. Puł. 2005, 139, 327–336. [Google Scholar]
- Haddadin, M.S.Y.; Humeid, M.A.; Quaroot, F.A.; Robinson, R.K. Effect of exposure to light on the solanine content of two varieties of potato (Solanum tuberosum) popular in Jordan. Food Chem. 2001, 73, 205–208. [Google Scholar] [CrossRef]
- Machado, R.M.D.; Toledo, M.C.F.; Garcia, L.C. Effect of light and temperature on the formation of glycoalkaloids in potato tubers. Food Control 2007, 18, 503–508. [Google Scholar] [CrossRef]
- Şengül, M.; Keleş, F.; Keleş, M.S. The effect of storage conditions (temperature, light, time) and variety on the glycoalkaloid content of potato tubers and sprouts. Food Control 2004, 15, 281–286. [Google Scholar] [CrossRef]
- Tajner-Czopek, A.; Jarych-Szyszka, M.; Lisińska, G. Changes in glycoalkaloids content of potatoes destined for consumption. Food Chem. 2008, 106, 706–711. [Google Scholar] [CrossRef]
- Chung, J.C.; Chou, S.S.; Hwang, D.F. Changes in nitrate and nitrite content of four vegetables during storage at refrigerated and ambient temperatures. Food Addit. Contam. 2004, 21, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Rezaee, M.; Almasi, M.; Majdabadi, F.A.; Minaei, S.; Khodadadi, M. Potato sprout inhibition and tuber quality after post-harvest treatment with gamma irradiation on different dates. J. Agric. Sci. Tech. 2011, 13, 829–842. Available online: http://journals.modares.ac.ir/article-23-2714-en.html (accessed on 24 February 2020).
- Ginzberg, I.; Tokuhisa, J.G.; Veilleux, R.E. Potato steroidal glycoalkaloids: Biosynthesis and genetic manipulation. Potato Res. 2009, 52, 1–15. [Google Scholar] [CrossRef]
- Haase, N.U. Glycoalkaloid concentration in potato tubers related to storage and consumer offer. Potato Res. 2010, 53, 297–307. [Google Scholar] [CrossRef]
- Rytel, E. Changes in the levels of glycoalkaloids and nitrates after the dehydration of cooked potatoes. Am. J. Potato Res. 2012, 89, 501–507. [Google Scholar] [CrossRef]
- Lachman, J.; Hamouz, K.; Orsak, M.; Pivec, V. Potato glycoalkaloids and their significance in plant protection and human nutrition—Review. Rostl. Vyroba UZPI 2001, 47, 181–191. [Google Scholar]
- Smith, D.B.; Roddick, J.G.; Jones, J.L. Synergism between the potato alkaloids α-chaconine and α-solanine in inhibition of snail feeding. Phytochemistry 2001, 57, 229–234. [Google Scholar] [CrossRef]
- Friedman, M.; McDonald, G.M. Potato glycoalkaloids: Chemistry, analysis, safety and plant physiology. Crit. Rev. Plant Sci. 1997, 16, 55–132. [Google Scholar] [CrossRef]
- Muthumani, P.; Meera, R.; Sweetlin, D.P. Phyto Chemical Investigation and Determination of Crude Alkaloidal Content (Solasodine) in Solanum Leave Dunal (Dry and Fresh Berries). Int. J. Pharm. Biol. Arch. 2010, 1, 350–354. [Google Scholar]
- Gerendás, J.; Führs, H. The significance of magnesium for crop quality. Plant Soil. 2013, 368, 101–128. [Google Scholar] [CrossRef]
- Pasternak, K.; Kocot, J.; Horecka, A. Biochemistry of magnesium. J. Elem. 2010, 15, 601–616. [Google Scholar] [CrossRef]
- Pobereżny, J.; Wszelaczyńska, E. Effect of bioelements (N, K, Mg) and long-term storage of potato tubers on quantitative and qualitative losses Part II. Content of dry matter and starch. J. Elem. 2011, 16, 237–246. [Google Scholar] [CrossRef]
- Cakmak, I.; Yazici, A.M. Magnesium: A forgotten element in crop production. Better Crops 2010, 94, 23–25. [Google Scholar]
- Pawelzik, E.; Möller, K. Sustainable potato production worldwide: The challenge to assess conventional and organic production systems. Potato Res. 2014, 57, 273–290. [Google Scholar] [CrossRef]
- Senbayram, M.; Gransee, A.; Wahle, V.; Thiel, H. Role of magnesium fertilisers in agriculture: Plant–soil continuum. Crop Pasture Sci. 2015, 66, 1219–1229. [Google Scholar] [CrossRef]
- Cakmak, I.; Kirkby, E. Role of magnesium in carbon partitioning and alleviating photo-oxidative damage. Physiol. Plant. 2008, 133, 692–704. [Google Scholar] [CrossRef]
- Shaul, O. Magnesium transport and function in plants: The tip of the iceberg. Biometals 2002, 15, 309–323. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2006, First Update 2007; World Soil Resources Reports No. 103; FAO: Rome, Italy, 2007; p. 116. [Google Scholar]
- Gregorczyk, A.; Wróbel, J.; Mikiciuk, M. The modification of Walter and Lieth climatic diagrams. Zesz. Nauk. AR Szczec. 2005, 1, 33–40. (In Polish) [Google Scholar]
- Baker, W.H.; Thompson, T.L. Determination of nitrate nitrogen in plant samples by selective ion electrode. Plant Anal. Ref. Proc. S. US 1992, 368, 13–16. [Google Scholar]
- Bergers, W.W.A. A rapid quantitative assay for solanidine glycoalkaloids in potatoes and industrial potato protein. Potato Res. 1980, 23, 105–110. [Google Scholar] [CrossRef]
- Walker, T.; Thiele, G.; Suarez, V.; Crissman, C. Hindsight and Foresight about Potato Production and Consumption; Social Sciences Working Paper No. 2011–5; International Potato Center (CIP): Lima, Peru, 2011. [Google Scholar]
- Szulc, W.; Rutkowska, B. Diagnostics of boron deficiency for plants in reference to boron concentration in the soil solution. Plant Soil Environ. 2013, 59, 372–377. [Google Scholar] [CrossRef]
- GUS 2019. Statistical Yearbook of Agriculture 2018; GUS: Warsaw, Poland, 2018; p. 149. ISSN 2080-8798. (Publication available on website: stat.gov.pl). [Google Scholar]
- Kołodziejczyk, M. Influence of rainfall and thermal conditions on the yielding of medium late and late cultivars of edible potato. Ann. Univ. Mariae Curie Skłodowska Sect. E Agric. 2013, 68, 1–10. (In Polish) [Google Scholar]
- Camargo, D.C.; Montoya, F.; Córcoles, J.I.; Ortega, J.F. Modeling the impacts of irrigation treatments on potato growth and development. Agric. Water Manag. 2015, 150, 119–128. [Google Scholar] [CrossRef]
- Orlovius, K.; McHoul, J. Effect of two magnesium fertilizers on leaf magnesium concentration, yield, and quality of potato and sugar beet. J. Plant Nutr. 2015, 38, 2044–2054. [Google Scholar] [CrossRef]
- Zengin, M.; Gökmen, F.; Gezgin, S.; Cakmak, I. Effects of different fertilizers with potassium and magnesium on the yield and quality of potato. Asian J. Chem. 2008, 20, 663–676. [Google Scholar]
- Allison, M.F.; Fowler, J.H.; Allen, E.J. Factors affecting the magnesium nutrition of potatoes (Solanum tuberosum). J. Agric. Sci. 2001, 137, 397–409. [Google Scholar] [CrossRef]
- Ierna, A. Influence of harvest date on nitrate contents of three potato varieties for off-season production. J. Food Compos. Anal. 2009, 22, 551–555. [Google Scholar] [CrossRef]
- Wadas, W.; Łęczycka, T.; Borsiak-Marciniak, I. Effect of fertilization with multinutrient complex fertilizers on tuber quality of very early potato cultivars. Acta Sci. Pol. Hortorum Cultus 2012, 11, 27–41. [Google Scholar]
- Rogozińska, I.; Pawelzik, E.; Poberezny, J.; Delgado, E. The effect of different factors on the content of nitrate in some potato varieties. Potato Res. 2005, 48, 167–180. [Google Scholar] [CrossRef]
- Lachman, J.; Hamouz, K. The effect of selected factors on the content of protein and nitrates in potato tubers. Plant Soil Environ. 2005, 51, 431–438. [Google Scholar] [CrossRef]
- Amr, A.; Hadidi, N. Effect of cultivar and harvest date on nitrate and nitrite content of selected vegetables grown under open field and greenhouse conditions in Jordan. J. Food Compos. Anal. 2001, 14, 59–67. [Google Scholar] [CrossRef]
- Sušin, J.; Kmeckl, V.; Gregorčič, A. A survey of nitrate and nitrite content of fruit and vegetables grown in Slovenia during 1996–2002. Food Addit. Contam. 2006, 23, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Zarzecka, K.; Gugała, M.; Mystkowska, I. Herbicide Residues and Nitrate Concentration in Tubers of Table Potatoes. J. Toxicol. Environ. Health Part A 2010, 73, 1244–1249. [Google Scholar] [CrossRef] [PubMed]
- Murawa, D.; Banaszkiewicz, T.; Majewska, E.; Błaszczuk, B.; Wiśniewska, J. Nitrate and nitrite content in selected vegetables and potatoes commercially available in Olsztyn. Bromatol. Chem. Toksykol. 2008, 41, 67. [Google Scholar]
- Tamme, T.; Reinik, M.; Roasto, M.; Juhkam, K.; Tenno, T.; Kiis, A. Nitrates and nitrites in vegetables and vegetable-based products and their intakes by the Estonian population. Food Addit. Contam. 2006, 23, 355–361. [Google Scholar] [CrossRef]
- Walters, C.L. Nitrate and nitrate in foods. In Series in Food Science, Technology and Nutrition, 2nd ed.; Hill, M., Ed.; Woodhead Publishing: Cambridge, UK, 1996; pp. 93–112. [Google Scholar] [CrossRef]
- Hord, N.G.; Tang, Y.; Bryan, N.S. Food sources of nitrates and nitrites: The physiological context for potential health benefits. Am. J. Clin. Nutr. 2009, 90, 1–10. [Google Scholar] [CrossRef]
- Friedman, M. Potato glycoalkaloids and metabolites: Roles in the plant and in the diet. J. Agric. Food Chem. 2006, 54, 8655–8681. [Google Scholar] [CrossRef]
- Zarzyńska, K. Chemical composition of potato tubers in relation to crop production system and environmental conditions. J. Agric. Sci. Technol. B 2013, 3, 689–695. [Google Scholar]
- Bejarano, L.; Mignolet, E.; Devaux, A.; Espinola, N.; Carrasco, E.; Larondelle, Y. Glycoalkaloids in potato tubers: The effect of variety and drought stress on the α-solanine and α-chaconine contents of potatoes. J. Sci. Food Agric. 2000, 80, 2096–2100. [Google Scholar] [CrossRef]
- Mystkowska, I. Reduction of glycoalkaloids in potato under the influence of biostimulators. Appl. Ecol. Environ. Res. 2019, 17, 3567–3574. [Google Scholar] [CrossRef]
- Nahar, N.; Westerberg, E.; Arif, U.; Huchelmann, A.; Olarte Guasca, A.; Beste, L.; Dalman, K.; Dutta, P.C.; Jonsson, L.; Sitbon, F. Transcript profiling of two potato cultivars during glycoalkaloid-inducing treatments shows differential expression of genes in sterol and glycoalkaloid metabolism. Sci. Rep. 2017, 7, 43268. [Google Scholar] [CrossRef] [PubMed]
- Zrúst, J.; Haráćková, V.; Přichystalová, V.; Rejlková, M. Content of alpha-chaconine and alpha-solanine in groups of potato varieties listed in the National Book of Varieties of the Czech Republic. Rostl. Vyroba UZPI 2000, 46, 481–486. [Google Scholar]
- Zarzecka, K.; Gugała, M. Changes in the content of glycoalkaloids in potato tubers according to soil tillage and weed control methods. Plant Soil Environ. 2007, 53, 247–251. [Google Scholar] [CrossRef]
- Ostry, V.; Ruprich, J.; Skarkova, J. Glycoalkaloids in potato tubers: The effect of peeling and cooking in salted water. Acta Aliment. 2010, 39, 130–135. [Google Scholar] [CrossRef]
- Mensinga, T.T.G.; Speijers, J.A.; Meulenbelt, J. Health implications of exposure to environmental nitrogenous compounds. Toxicol. Rev. 2003, 22, 41–51. [Google Scholar] [CrossRef]
- Ysart, G.; Miller, P.; Barrett, G.; Farrington, D.; Lawrance, P.; Harrison, N. Dietary exposures to nitrate in UK. Food Addit. Contam. 1999, 16, 521–532. [Google Scholar] [CrossRef]
- Edwards, E.J.; Cobb, A.H. Effect of Temperature on Glycoalkaloid and Chlorophyll Accumulation in Potatoes (Solanum tuberosum L. Cv. King Edward) Stored at Low Photon Flux Density, Including Preliminary Modeling Using an Artificial Neural Network. J. Agric. Food Chem. 1997, 45, 1032–1038. [Google Scholar] [CrossRef]
- Väänänen, T. Glycoalkaloid Content and Starch Structure in Solanum Species and Interspecific Somatic Potato Hybrids. Ph.D. Thesis, University of Helsinki, Helsinki, Finland, 2007. [Google Scholar]



| Total glycoalkaloids (TGA) * |
|---|
| α-Solanine |
| β-Solanine |
| γ-Solanine |
| α-Chaconine |
| β-Chaconine |
| γ-Chaconine |
| Leptine |
| Solacauline |
| Tetroside from S. acaulia |
| Demissine |
| Solasonine |
| Solamargine |
| Solasodamine |
| Tomatine |
| Trioside from S. polyadenium |
| α, β and γ-Soladulcine |
| Fertilisation Dose MgO (kg ha−1) | 2009 | 2010 | 2011 | Mean |
|---|---|---|---|---|
| 0 | 32.05 | 31.45 | 35.91 | 33.14 ± 2.94 * |
| 20 | 32.28 | 33.03 | 38.08 | 34.46 ± 3.03 |
| 40 | 34.30 | 33.59 | 38.91 | 35.60 ± 3.46 |
| 60 | 35.06 | 33.93 | 39.59 | 36.19 ± 3.82 |
| 80 | 35.79 | 35.15 | 40.69 | 37.21 ± 3.78 |
| 100 | 36.75 | 36.20 | 41.45 | 38.14 ± 3.64 |
| mean | 34.37 | 33.89 | 39.11 | 35.79 ± 4.07 |
| LSD0.05 | Rates MgO—3.34 | |||
| Fertilisation Dose MgO (kg ha−1) (B) | Date Determination (A) | Mean ** | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| After Harvest (Control) | After Storage | ||||||||||||
| Three Months | Six Months | ||||||||||||
| 2009 | 2010 | 2011 | Mean | 2009 | 2010 | 2011 | Mean | 2009 | 2010 | 2011 | Mean | ||
| 0 | 371 | 382 | 316 | 356 ± 35.4 * | 309 | 356 | 241 | 302 ± 57.8 | 301 | 310 | 236 | 282 ± 40.4 | 314 ± 51.6 |
| 20 | 375 | 378 | 336 | 363 ± 23.4 | 344 | 355 | 281 | 327 ± 39.9 | 276 | 308 | 247 | 277 ± 30.5 | 322 ± 46.5 |
| 40 | 350 | 366 | 312 | 343 ± 27.7 | 324 | 328 | 242 | 298 ± 48.5 | 264 | 298 | 229 | 264 ± 34.5 | 301 ± 47.5 |
| 60 | 345 | 367 | 307 | 340 ± 30.4 | 307 | 332 | 252 | 297 ± 40.9 | 243 | 290 | 222 | 252 ± 34.8 | 296 ± 49.0 |
| 80 | 333 | 327 | 289 | 316 ± 23.9 | 269 | 323 | 244 | 279 ± 40.4 | 235 | 270 | 211 | 239 ± 29.7 | 278 ± 43.6 |
| 100 | 323 | 318 | 269 | 303 ± 29.8 | 269 | 291 | 227 | 262 ± 32.5 | 221 | 266 | 204 | 230 ± 32.0 | 265 ± 41.8 |
| mean | 350 | 356 | 305 | 337 ± 28.0 | 304 | 331 | 248 | 294 ± 42.3 | 257 | 290 | 225 | 257 ± 32.8 | 296 ± 45.8 |
| LSD0.05 NO3−1: A—27.9; B—31.1; A × B—n.s. | |||||||||||||
| Fertilization Dose MgO (kg ha−1) (B) | Date Determination (A) | Mean ** | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| After Harvest (Control) | After Storage | ||||||||||||
| Three Months | Six Months | ||||||||||||
| 2009 | 2010 | 2011 | Mean | 2009 | 2010 | 2011 | Mean | 2009 | 2010 | 2011 | Mean | ||
| 0 | 1.68 | 1.65 | 1.67 | 1.67 ± 0.02 * | 1.61 | 1.65 | 1.62 | 1.63 ± 0.02 | 1.61 | 1.60 | 1.65 | 1.62 ± 0.03 | 1.64 ± 0.03 |
| 20 | 1.62 | 1.65 | 1.61 | 1.63 ± 0.02 | 1.60 | 1.62 | 1.61 | 1.61 ± 0.01 | 1.61 | 1.58 | 1.57 | 1.59 ± 0.02 | 1.61 ± 0.02 |
| 40 | 1.62 | 1.65 | 1.61 | 1.63 ± 0.02 | 1.58 | 1.59 | 1.56 | 1.58 ± 0.02 | 1.60 | 1.56 | 1.59 | 1.58 ± 0.02 | 1.60 ± 0.03 |
| 60 | 1.62 | 1.62 | 1.60 | 1.61 ± 0.01 | 1.56 | 1.55 | 1.60 | 1.57 ± 0.03 | 1.57 | 1.55 | 1.57 | 1.56 ± 0.01 | 1.58 ± 0.03 |
| 80 | 1.59 | 1.58 | 1.63 | 1.60 ± 0.03 | 1.55 | 1.56 | 1.54 | 1.55 ± 0.01 | 1.55 | 1.57 | 1.51 | 1.54 ± 0.03 | 1.56 ± 0.03 |
| 100 | 1.59 | 1.61 | 1.57 | 1.59 ± 0.02 | 1.52 | 1.54 | 1.56 | 1.54 ± 0.02 | 1.52 | 1.55 | 1.53 | 1.53 ± 0.02 | 1.55 ± 0.03 |
| mean | 1.62 | 1.63 | 1.62 | 1.62 ± 0.01 | 1.57 | 1.59 | 1.58 | 1.58 ± 0.01 | 1.58 | 1.57 | 1.57 | 1.57 ± 0.04 | 1.59 ± 0.02 |
| LSD0.05 NO3−1: A—0.021; B—0.030; A × B—n.s. | |||||||||||||
| Fertilization Dose MgO (kg ha−1) (B) | Date Determination (A) | Mean ** | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| After Harvest (Control) | After Storage | ||||||||||||
| Three Months | Six Months | ||||||||||||
| 2009 | 2010 | 2011 | Mean | 2009 | 2010 | 2011 | Mean | 2009 | 2010 | 2011 | Mean | ||
| 0 | 25.0 | 33.0 | 28.8 | 28.9 ± 4.0 * | 23.2 | 41.1 | 34.0 | 32.8 ± 9.0 | 34.5 | 41.1 | 44.0 | 39.9 ± 4.9 | 33.9 ± 7.3 |
| 20 | 25.8 | 37.0 | 29.3 | 30.7 ± 5.7 | 23.9 | 47.8 | 41.0 | 37.6 ± 12.3 | 39.4 | 41.9 | 43.0 | 41.4 ± 1.8 | 36.6 ± 8.3 |
| 40 | 26.2 | 36.0 | 28.0 | 30.1 ± 5.2 | 25.9 | 46.9 | 41.0 | 37.9 ± 10.8 | 35.2 | 46.6 | 45.0 | 42.2 ± 6.2 | 36.8 ± 8.6 |
| 60 | 27.2 | 34.0 | 33.5 | 31.6 ± 3.8 | 26.4 | 46.6 | 41.0 | 38.0 ± 10.4 | 33.7 | 48.5 | 49.0 | 43.7 ± 8.7 | 37.8 ± 8.8 |
| 80 | 29.2 | 35.0 | 27.9 | 30.7 ± 3.8 | 24.9 | 47.2 | 46.0 | 39.4 ± 12.5 | 35.3 | 50.0 | 45.0 | 43.4 ± 7.5 | 37.8 ± 9.4 |
| 100 | 30.6 | 36.0 | 31.9 | 32.8 ± 2.8 | 27.8 | 52.0 | 42.0 | 40.6 ± 12.2 | 35.8 | 51.5 | 46.0 | 44.4 ± 8.0 | 39.3 ± 9.0 |
| mean | 27.3 | 35.2 | 29.9 | 30.8 ± 4.0 | 25.4 | 46.9 | 40.8 | 37.7 ± 11.1 | 35.7 | 46.6 | 45.3 | 42.5 ± 6.0 | 37.0 ± 8.4 |
| LSD0.05 NO3−1: A—27.9; B—31.1; A × B—n.s. | |||||||||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wszelaczyńska, E.; Pobereżny, J.; Kozera, W.; Knapowski, T.; Pawelzik, E.; Spychaj-Fabisiak, E. Effect of Magnesium Supply and Storage Time on Anti-Nutritive Compounds in Potato Tubers. Agronomy 2020, 10, 339. https://doi.org/10.3390/agronomy10030339
Wszelaczyńska E, Pobereżny J, Kozera W, Knapowski T, Pawelzik E, Spychaj-Fabisiak E. Effect of Magnesium Supply and Storage Time on Anti-Nutritive Compounds in Potato Tubers. Agronomy. 2020; 10(3):339. https://doi.org/10.3390/agronomy10030339
Chicago/Turabian StyleWszelaczyńska, Elżbieta, Jarosław Pobereżny, Wojciech Kozera, Tomasz Knapowski, Elke Pawelzik, and Ewa Spychaj-Fabisiak. 2020. "Effect of Magnesium Supply and Storage Time on Anti-Nutritive Compounds in Potato Tubers" Agronomy 10, no. 3: 339. https://doi.org/10.3390/agronomy10030339
APA StyleWszelaczyńska, E., Pobereżny, J., Kozera, W., Knapowski, T., Pawelzik, E., & Spychaj-Fabisiak, E. (2020). Effect of Magnesium Supply and Storage Time on Anti-Nutritive Compounds in Potato Tubers. Agronomy, 10(3), 339. https://doi.org/10.3390/agronomy10030339






