Phenotypic Variation and Relationships between Fatty Acid Concentrations and Feed Value of Perennial Ryegrass Genotypes from a Breeding Population
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Forage Chemical Composition
2.2.1. Crude Protein, Water-Soluble Carbohydrate and Fibre
2.2.2. Fatty Acids
2.3. Statistical Analysis
3. Results
3.1. Fatty Acid and Chemical Composition
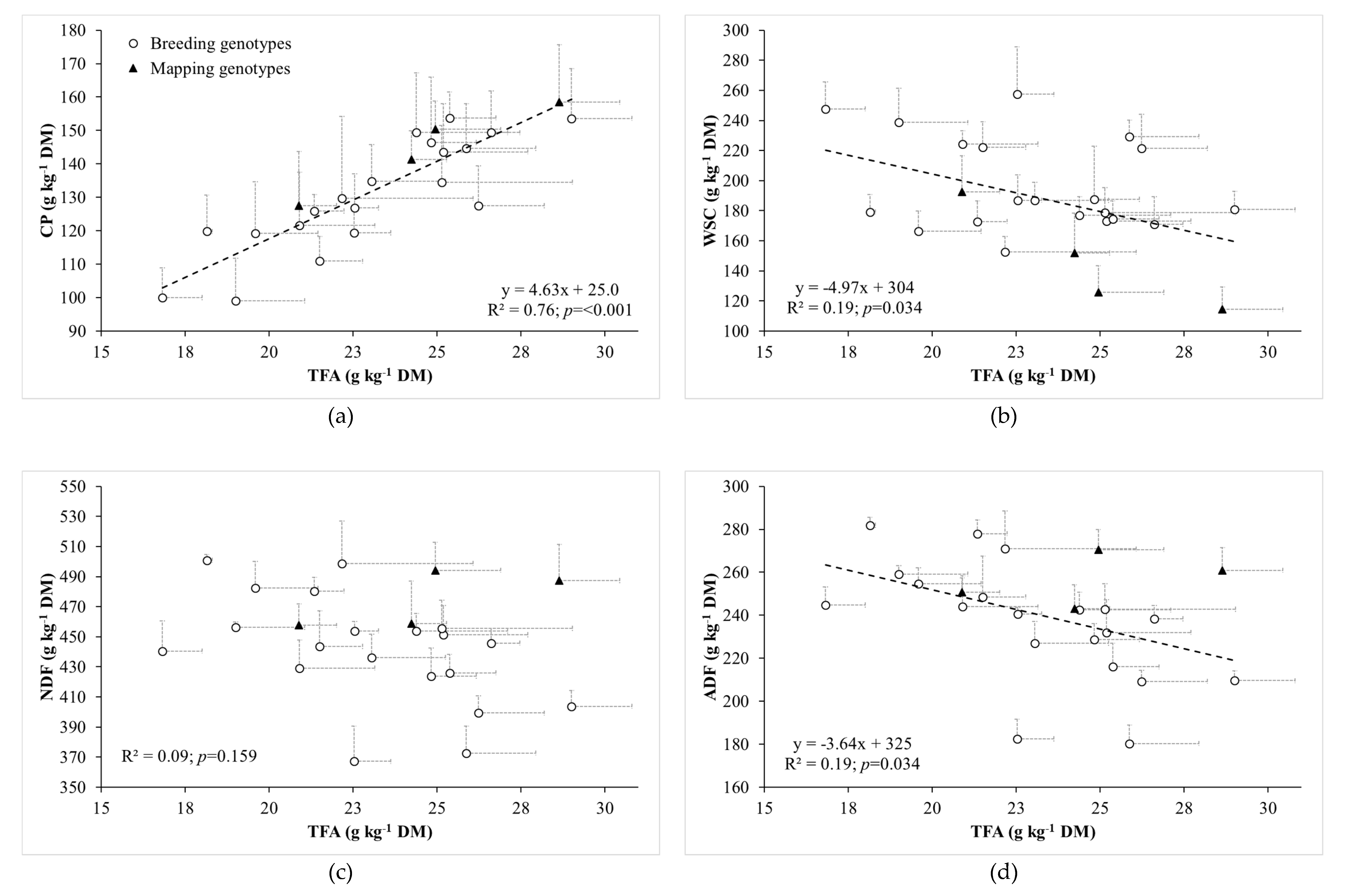
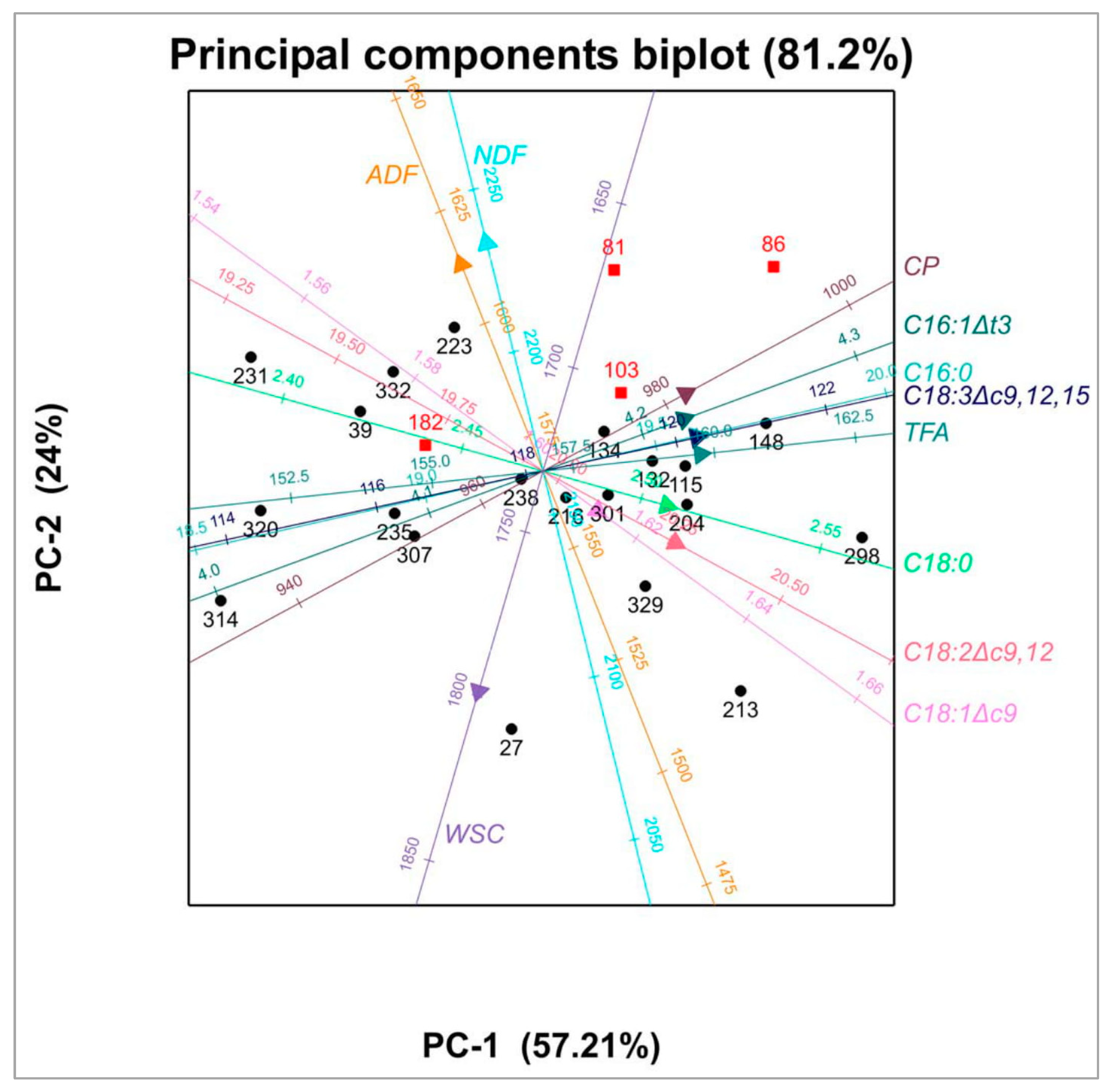
3.2. Relationships between Fatty Acid Concentrations and Herbage Chemical Composition
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Smit, H.J.; Tas, B.M.; Taweel, H.Z.; Elgersma, A. Sward characteristics important for intake in six Lolium perenne varieties. Grass Forage Sci. 2005, 60, 128–135. [Google Scholar] [CrossRef]
- Ullmann, I.; Herrmann, A.; Hasler, M.; Taube, F. Influence of the critical phase of stem elongation on yield and forage quality of perennial ryegrass genotypes in the first reproductive growth. Field Crops Res. 2017, 205, 23–33. [Google Scholar] [CrossRef]
- Moorby, J.M.; Kingston-Smith, A.H.; Abberton, M.T.; Humphreys, M.O.; Theodorou, M.K. Improvement of forages to increase the efficiency of nitrogen and energy use by ruminants. Recent Adv. Anim. Nutr. 2009, 2008, 39–65. [Google Scholar] [CrossRef]
- Wilkins, P.W.; Humphreys, M.O. Progress in breeding perennial forage grasses for temperate agriculture. J. Agric. Sci. 2003, 140, 129–150. [Google Scholar] [CrossRef]
- Humphreys, M.O. Genetic improvement of forage crops—Past, present and future. J. Agric. Sci. 2005, 143, 441–448. [Google Scholar] [CrossRef]
- Humphreys, M.O. Water-soluble carbohydrates in perennial ryegrass breeding II. Cultivar and hybrid progeny performance in cut plots. Grass Forage Sci. 1989, 44, 237–244. [Google Scholar] [CrossRef]
- Kingston-Smith, A.H.; Thomas, H.M. Strategies of plant breeding for improved rumen function. Ann. Appl. Biol. 2003, 142, 13–24. [Google Scholar] [CrossRef]
- Benbrook, C.M.; Davis, D.R.; Heins, B.J.; Latif, M.A.; Leifert, C.; Peterman, L.; Butler, G.; Faergeman, O.; Abel-Caines, S.; Baranski, M. Enhancing the fatty acid profile of milk through forage-based rations, with nutrition modeling of diet outcomes. Food Sci. Nutr. 2018, 6, 681–700. [Google Scholar] [CrossRef]
- Prache, S.; Martin, B.; Coppa, M. Review: Authentication of grass-fed meat and dairy products from cattle and sheep. Animal 2019, 14, 854–863. [Google Scholar] [CrossRef]
- Elgersma, A. Grazing increases the unsaturated fatty acid concentration of milk from grass-fed cows: A review of the contributing factors, challenges and future perspectives. Eur. J. Lipid Sci. Technol. 2015, 117, 1345–1369. [Google Scholar] [CrossRef]
- Morgan, S.; Huws, S.A.; Scollan, N.D. Progress in forage-based strategies to improve the fatty acid composition of beef. In Proceedings of the 24th General Meeting of the European Grassland Federation (Grassland—A European Resource?), Lublin, Poland, 3–7 June 2012; Volume 17, pp. 295–307. [Google Scholar]
- Scollan, N.D.; Price, E.M.; Morgan, S.A.; Huws, S.A.; Shingfield, K.J. Can we improve the nutritional quality of meat? Proc. Nutr. Soc. 2017, 76, 603–618. [Google Scholar] [CrossRef] [PubMed]
- Barret, B.A.; Faville, M.J.; Nichols, S.N.; Simpson, W.R.; Bryan, G.T.; Conner, A.J. Breaking through the feed barrier: Options for improving forage genetics. Anim. Prod. Sci. 2015, 55, 883–892. [Google Scholar] [CrossRef]
- Ellis, J.L.; Dijkstra, J.; France, J.; Parsons, A.J.; Edwards, G.R.; Rasmussen, S.; Kebreab, E.; Bannink, A. Effect of high-sugar grasses on methane emissions simulated using a dynamic model. J. Dairy Sci. 2012, 95, 272–285. [Google Scholar] [CrossRef]
- Foskolos, A.; Moorby, J. The use of high sugar grasses as a strategy to improve nitrogen utilization efficiency: A meta-analysis. In Proceedings of the British Society of Animal Science (Advances in Animal Biosciences), Chester, UK, 26–27 April 2017; Volume 8, p. 72. [Google Scholar]
- Staerfl, S.M.; Zeitz, J.O.; Amelchanka, S.L.; Kälber, T.; Kreuzer, M.; Leiber, F. Comparison of the milk fatty acid composition from dairy cows fed high-sugar ryegrass, low-sugar ryegrass, or maize. Dairy Sci. Technol. 2013, 93, 201–210. [Google Scholar] [CrossRef][Green Version]
- Vanhercke, T.; El Tahchy, A.; Liu, Q.; Zhou, X.-R.; Shrestha, P.; Divi, U.K.; Ral, J.-P.; Mansour, M.P.; Nichols, P.D.; James, C.N.; et al. Metabolic engineering of biomass for high energy density: Oilseed-like triacylglycerol yields from plant leaves. Plant Biotechnol. J. 2014, 12, 231–239. [Google Scholar] [CrossRef]
- Winichayakul, S.; Scott, R.W.; Roldan, M.; Hatier, J.-H.B.; Livingston, S.; Cookson, R.; Curran, A.C.; Roberts, N.J. In vivo packaging of triacylglycerols enhances arabidopsis leaf biomass and energy density. Plant Physiol. 2013, 162, 626–639. [Google Scholar] [CrossRef]
- Glasser, F.; Doreau, M.; Maxin, G.; Baumont, R. Fat and fatty acid content and composition of forages: A meta-analysis. Anim. Feed Sci. Technol. 2013, 185, 19–34. [Google Scholar] [CrossRef]
- Dewhurst, R.J.; Scollan, N.D.; Youell, S.J.; Tweed, J.K.S.; Humphreys, M.O. Influence of species, cutting date and cutting interval on the fatty acid composition of grasses. Grass Forage Sci. 2001, 56, 68–74. [Google Scholar] [CrossRef]
- Palladino, R.A.; O’Donovan, M.; Kennedy, E.; Murphy, J.J.; Boland, T.M.; Kenny, D.A. Fatty acid composition and nutritive value of twelve cultivars of perennial ryegrass. Grass Forage Sci. 2009, 64, 219–226. [Google Scholar] [CrossRef]
- Hegarty, M.; Yadav, R.; Lee, M.; Armstead, I.; Sanderson, R.; Scollan, N.; Powell, W.; Skøt, L. Genotyping by RAD sequencing enables mapping of fatty acid composition traits in perennial ryegrass (Lolium perenne (L.)). Plant Biotechnol. J. 2013, 11, 572–581. [Google Scholar] [CrossRef]
- Barrett, B.; Griffiths, A.; Mercer, C.; Ellison, N.; Faville, M.; Easton, S.; Woodfield, D.R. Marker-assisted selection to accelerate forage improvement. N. Zeal. Grassl. Assoc. 2001, 63, 241–245. [Google Scholar]
- Nakaya, A.; Isobe, S.N. Will genomic selection be a practical method for plant breeding? Ann. Bot. 2012, 110, 1303–1316. [Google Scholar] [CrossRef]
- Roldán-Ruiz, I.; Kölliker, R. Marker-assisted selection in forage crops and turf: A review. In Sustainable Use of Genetic Diversity in Forage and Turf Breeding; Huyghe, C., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 383–390. ISBN 978-90-481-8706-5. [Google Scholar]
- Humphreys, M.; Feuerstein, U.; Vandewalle, M.; Baert, J. Ryegrasses. In Fodder Crops and Amenity Grasses; Boller, B., Posselt, U.K., Veronesi, F., Eds.; Handbook of Plant Breeding; Springer: New York, NY, USA, 2010; pp. 211–260. ISBN 978-1-4419-0760-8. [Google Scholar]
- Sukhija, P.S.; Palmquist, D.L. Rapid method for determination of total fatty acid content and composition of feedstuffs and feces. J. Agric. Food Chem. 1988, 36, 1202–1206. [Google Scholar] [CrossRef]
- Minson, D.J. Forage in Ruminant Nutrition; Animal Feeding and Nutrition; Academic Press: San Diego, CA, USA, 1990; ISBN 978-0-12-498310-6. [Google Scholar]
- MAFF. Feed Composition: UK Tables of Feed Composition and Nutritive Value for Ruminants, 2nd ed.; MAFF: Chalcombe, UK, 1992; ISBN 978-0-948617-24-9. [Google Scholar]
- Ewing, W.N. The Feeds Directory: Commodity Products Guide; Context: Packington, UK, 2002; ISBN 1-899043-01-2. [Google Scholar]
- McDonald, P.; Edwards, R.A.; Greenhalgh, J.F.D.; Morgan, C.A.; Sinclair, L.A.; Wilkinson, R.G. Animal Nutrition, 7th ed.; Prentice Hall/Pearson: Harlow, UK, 2011; ISBN 978-1-4082-0423-8. [Google Scholar]
- Gilliland, T.J.; Barrett, P.D.; Mann, R.L.; Agnew, R.E.; Fearon, A.M. Canopy morphology and nutritional quality traits as potential grazing value indicators for lolium perenne varieties. J. Agric. Sci. 2002, 139, 257–273. [Google Scholar] [CrossRef]
- Lee, M.A. A global comparison of the nutritive values of forage plants grown in contrasting environments. J. Plant Res. 2018, 131, 641–654. [Google Scholar] [CrossRef]
- Dewhurst, R.J.; Moorby, J.M.; Scollan, N.D.; Tweed, J.K.S.; Humphreys, M.O. Effects of a stay-green trait on the concentrations and stability of fatty acids in perennial ryegrass. Grass Forage Sci. 2002, 57, 360–366. [Google Scholar] [CrossRef]
- Elgersma, A.; Ellen, G.; Horst, H.; Muuse, B.G.; Boer, H.; Tamminga, S. Influence of cultivar and cutting date on the fatty acid composition of perennial ryegrass (Lolium perenne L.). Grass Forage Sci. 2003, 58, 323–331. [Google Scholar] [CrossRef]
- Van Ranst, G.; Fievez, V.; Vandewalle, M.; De Riek, J.; Van Bockstaele, E. Influence of herbage species, cultivar and cutting date on fatty acid composition of herbage and lipid metabolism during ensiling. Grass Forage Sci. 2009, 64, 196–207. [Google Scholar] [CrossRef]
- Boufaïed, H.; Chouinard, P.Y.; Tremblay, G.F.; Petit, H.V.; Michaud, R.; Bélanger, G. Fatty acids in forages. I. Factors affecting concentrations. Can. J. Anim. Sci. 2003, 83, 501–511. [Google Scholar] [CrossRef]
- Clapham, W.M.; Foster, J.G.; Neel, J.P.S.; Fedders, J.M. Fatty acid composition of traditional and novel forages. J. Agric. Food Chem. 2005, 53, 10068–10073. [Google Scholar] [CrossRef]
- Elgersma, A.; Ellen, G.; van der Horst, H.; Muuse, B.G.; Boer, H.; Tamminga, S. Comparison of the fatty acid composition of fresh and ensiled perennial ryegrass (Lolium perenne L.), affected by cultivar and regrowth interval. Anim. Feed Sci. Technol. 2003, 108, 191–205. [Google Scholar] [CrossRef]
- Elgersma, A.; Maudet, P.; Witkowska, I.M.; Wever, A.C. Effects of nitrogen fertilisation and regrowth period on fatty acid concentrations in perennial ryegrass (Lolium perenne L.). Ann. Appl. Biol. 2005, 147, 145–152. [Google Scholar] [CrossRef]
- Singh, S.P.; Tomar, B.S. Cell Biology, 9th ed.; Rastogi Publications: Meerut, India, 2007; ISBN 978-81-7133-909-9. [Google Scholar]
- Jafari, A.A. Environmental and Genetic Variation for Water Soluble Carbohydrate Content in Cool Season Forage Grasses. Available online: https://www.intechopen.com/books/carbohydrates-comprehensive-studies-on-glycobiology-and-glycotechnology/environmental-and-genetic-variation-for-water-soluble-carbohydrate-content-in-cool-season-forage-gra (accessed on 1 March 2020).
- Rezaeifard, M.; Jafari, A.A.; Assareh, M.H. Effects of phenological stages on forage yield quality traits in cocksfoot. J. Food Agric. Environ. 2010, 8, 365–369. [Google Scholar]
- Waite, R.; Boyd, J. The water-soluble carbohydrates of grasses. 2. Grasses cut at grazing height several times during the growing season. J. Sci. Food Agric. 1953, 4, 257–261. [Google Scholar] [CrossRef]
- Khan, N.A.; Cone, J.W.; Fievez, V.; Hendriks, W.H. Causes of variation in fatty acid content and composition in grass and maize silages. Anim. Feed Sci. Technol. 2012, 174, 36–45. [Google Scholar] [CrossRef]
- Parsons, A.J.; Edwards, G.R.; Newton, P.C.D.; Chapman, D.F.; Caradus, J.R.; Rasmussen, S.; Rowarth, J.S. Past lessons and future prospects: plant breeding for yield and persistence in cool-temperate pastures. Grass Forage Sci. 2011, 66, 153–172. [Google Scholar] [CrossRef]
- Alves, M.R.; Oliveira, M.B. Interpolative biplots applied to principal component analysis and canonical correlation analysis. J. Chemom. 2003, 17, 594–602. [Google Scholar] [CrossRef]
| Variable | DM% | CP | WSC | NDF | ADF |
|---|---|---|---|---|---|
| Population | |||||
| Mapping | 23.7 | 144 | 146 | 475 | 256 |
| Breeding | 24.8 | 131 | 196 | 441 | 237 |
| s.e.d. | 0.53 | 7.2 | 9.8 | 7.6 | 5.0 |
| p | 0.041 | 0.057 | <0.001 | <0.001 | <0.001 |
| Mapping Population Genotypes | |||||
| 81 | 22.8 | 150 | 126 | 494 | 271 |
| 86 | 22.2 | 158 | 114 | 487 | 261 |
| 103 | 25.2 | 141 | 152 | 459 | 243 |
| 182 | 24.6 | 128 | 193 | 458 | 251 |
| s.e.d. | 1.00 | 13.4 | 18.4 | 14.3 | 9.4 |
| p | 0.104 | 0.386 | 0.015 | 0.143 | 0.168 |
| Breeding Population Genotypes | |||||
| 27 | 28.3 | 119 | 257 | 367 | 183 |
| 39 | 26.3 | 119 | 166 | 482 | 255 |
| 115 | 23.8 | 144 | 173 | 451 | 232 |
| 132 | 24.8 | 135 | 179 | 456 | 243 |
| 134 | 24.5 | 149 | 177 | 454 | 243 |
| 148 | 24.2 | 149 | 171 | 446 | 238 |
| 204 | 24.4 | 154 | 175 | 426 | 216 |
| 213 | 24.0 | 145 | 229 | 373 | 180 |
| 216 | 25.7 | 135 | 187 | 436 | 227 |
| 223 | 25.5 | 130 | 153 | 499 | 271 |
| 231 | 25.3 | 120 | 179 | 501 | 282 |
| 235 | 24.4 | 111 | 222 | 444 | 249 |
| 238 | 24.8 | 127 | 187 | 454 | 241 |
| 298 | 20.8 | 154 | 181 | 404 | 210 |
| 301 | 24.0 | 146 | 188 | 424 | 229 |
| 307 | 24.9 | 122 | 224 | 429 | 244 |
| 314 | 29.0 | 100 | 248 | 441 | 245 |
| 320 | 25.0 | 99.1 | 239 | 456 | 259 |
| 329 | 23.4 | 128 | 222 | 399 | 209 |
| 332 | 23.0 | 126 | 173 | 480 | 278 |
| s.e.d. | 1.09 | 14.6 | 20.0 | 15.6 | 10.2 |
| p | <0.001 | 0.078 | <0.001 | <0.001 | <0.001 |
| Variable | C16:0 | C16:1Δt3 | C18:0 | C18:1Δc9 | C18:2Δc9,12 | C18:3Δc9,12,15 | TFA |
|---|---|---|---|---|---|---|---|
| Population | |||||||
| Mapping | 4.11 | 0.441 | 0.376 | 0.602 | 3.46 | 14.2 | 24.7 |
| Breeding | 3.90 | 0.352 | 0.383 | 0.579 | 3.68 | 12.5 | 23.0 |
| s.e.d. | 0.113 | 0.0235 | 0.0101 | 0.0192 | 0.124 | 0.70 | 0.96 |
| p | 0.061 | <0.001 | 0.497 | 0.227 | 0.078 | 0.023 | 0.087 |
| Mapping Population Genotypes | |||||||
| 81 | 4.18 | 0.451 | 0.345 | 0.559 | 3.60 | 14.3 | 24.9 |
| 86 | 4.56 | 0.467 | 0.438 | 0.619 | 3.53 | 17.4 | 28.6 |
| 103 | 4.01 | 0.465 | 0.423 | 0.588 | 3.49 | 13.7 | 24.2 |
| 182 | 3.70 | 0.381 | 0.297 | 0.644 | 3.20 | 11.3 | 20.9 |
| s.e.d. | 0.211 | 0.0439 | 0.0189 | 0.0360 | 0.232 | 1.30 | 1.79 |
| p | 0.034 | 0.441 | <0.001 | 0.354 | 0.620 | 0.012 | 0.025 |
| Breeding Population Genotypes | |||||||
| 27 | 3.85 | 0.298 | 0.407 | 0.529 | 3.62 | 12.0 | 22.5 |
| 39 | 3.53 | 0.289 | 0.315 | 0.564 | 3.51 | 9.95 | 19.6 |
| 115 | 4.38 | 0.384 | 0.425 | 0.651 | 4.09 | 13.8 | 25.2 |
| 132 | 4.14 | 0.391 | 0.448 | 0.633 | 4.04 | 14.0 | 25.1 |
| 134 | 4.17 | 0.351 | 0.378 | 0.557 | 3.80 | 13.7 | 24.4 |
| 148 | 4.50 | 0.500 | 0.533 | 0.562 | 3.67 | 15.4 | 26.6 |
| 204 | 4.24 | 0.388 | 0.342 | 0.674 | 4.11 | 14.1 | 25.4 |
| 213 | 4.32 | 0.387 | 0.416 | 0.713 | 4.21 | 14.2 | 25.9 |
| 216 | 3.88 | 0.416 | 0.390 | 0.639 | 3.54 | 12.4 | 23.1 |
| 223 | 3.86 | 0.336 | 0.362 | 0.496 | 3.50 | 11.9 | 22.2 |
| 231 | 3.39 | 0.239 | 0.298 | 0.536 | 3.03 | 9.19 | 18.1 |
| 235 | 3.76 | 0.293 | 0.347 | 0.527 | 3.38 | 11.6 | 21.5 |
| 238 | 3.96 | 0.324 | 0.409 | 0.567 | 3.80 | 11.8 | 22.6 |
| 298 | 4.29 | 0.523 | 0.467 | 0.593 | 4.56 | 16.9 | 29.0 |
| 301 | 4.04 | 0.341 | 0.401 | 0.533 | 3.71 | 14.1 | 24.8 |
| 307 | 3.55 | 0.341 | 0.336 | 0.553 | 3.53 | 11.1 | 20.9 |
| 314 | 3.12 | 0.196 | 0.339 | 0.664 | 3.18 | 7.76 | 16.8 |
| 320 | 3.33 | 0.275 | 0.353 | 0.465 | 3.07 | 9.98 | 19.0 |
| 329 | 4.03 | 0.448 | 0.393 | 0.518 | 3.84 | 15.2 | 26.2 |
| 332 | 3.60 | 0.311 | 0.292 | 0.608 | 3.38 | 11.7 | 21.4 |
| s.e.d. | 0.231 | 0.0479 | 0.0207 | 0.0392 | 0.253 | 1.42 | 1.96 |
| p | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| C16:0 | C16:1Δt3 | C18:0 | C18:1Δc9 | C18:2Δc9,12 | C18:3Δc9,12,15 | |
|---|---|---|---|---|---|---|
| CP | 0.90 c | 0.79 c | 0.53 b | 0.35 | 0.66 c | 0.87 c |
| WSC | −0.50 a | −0.51 a | −0.14 | −0.09 | −0.11 | −0.47 a |
| NDF | −0.20 | −0.15 | −0.36 | −0.20 | −0.51 a | −0.24 |
| ADF | −0.38 | −0.27 | −0.46 a | −0.31 | −0.65 c | −0.36 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morgan, S.A.; Huws, S.A.; Lister, S.J.; Sanderson, R.; Scollan, N.D. Phenotypic Variation and Relationships between Fatty Acid Concentrations and Feed Value of Perennial Ryegrass Genotypes from a Breeding Population. Agronomy 2020, 10, 343. https://doi.org/10.3390/agronomy10030343
Morgan SA, Huws SA, Lister SJ, Sanderson R, Scollan ND. Phenotypic Variation and Relationships between Fatty Acid Concentrations and Feed Value of Perennial Ryegrass Genotypes from a Breeding Population. Agronomy. 2020; 10(3):343. https://doi.org/10.3390/agronomy10030343
Chicago/Turabian StyleMorgan, Sarah A., Sharon A. Huws, Sue J. Lister, Ruth Sanderson, and Nigel D. Scollan. 2020. "Phenotypic Variation and Relationships between Fatty Acid Concentrations and Feed Value of Perennial Ryegrass Genotypes from a Breeding Population" Agronomy 10, no. 3: 343. https://doi.org/10.3390/agronomy10030343
APA StyleMorgan, S. A., Huws, S. A., Lister, S. J., Sanderson, R., & Scollan, N. D. (2020). Phenotypic Variation and Relationships between Fatty Acid Concentrations and Feed Value of Perennial Ryegrass Genotypes from a Breeding Population. Agronomy, 10(3), 343. https://doi.org/10.3390/agronomy10030343





