Efficiency of Marine Bacteria and Yeasts on the Biocontrol Activity of Pythium ultimum in Ancho-Type Pepper Seedlings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Marine Antagonistic Microorganisms
2.2. Pythium Ultimum
2.3. Pathogenicity Test
2.4. Antagonistic Test
2.5. Detection of Lytic Activity
2.5.1. Phytopathogen Cells and Culture of Marine Microorganisms
2.5.2. β-1,3-glucanase Activity
2.6. Inoculation of Ancho-Type Pepper Plants with Marine Bacteria and Yeasts
2.7. Scanning Electron Microscopy Micrographs
2.8. Root Colonization of Marine Microorganisms
2.9. Statistical Analyses
3. Results
3.1. Pathogenicity of Pythium ultimum
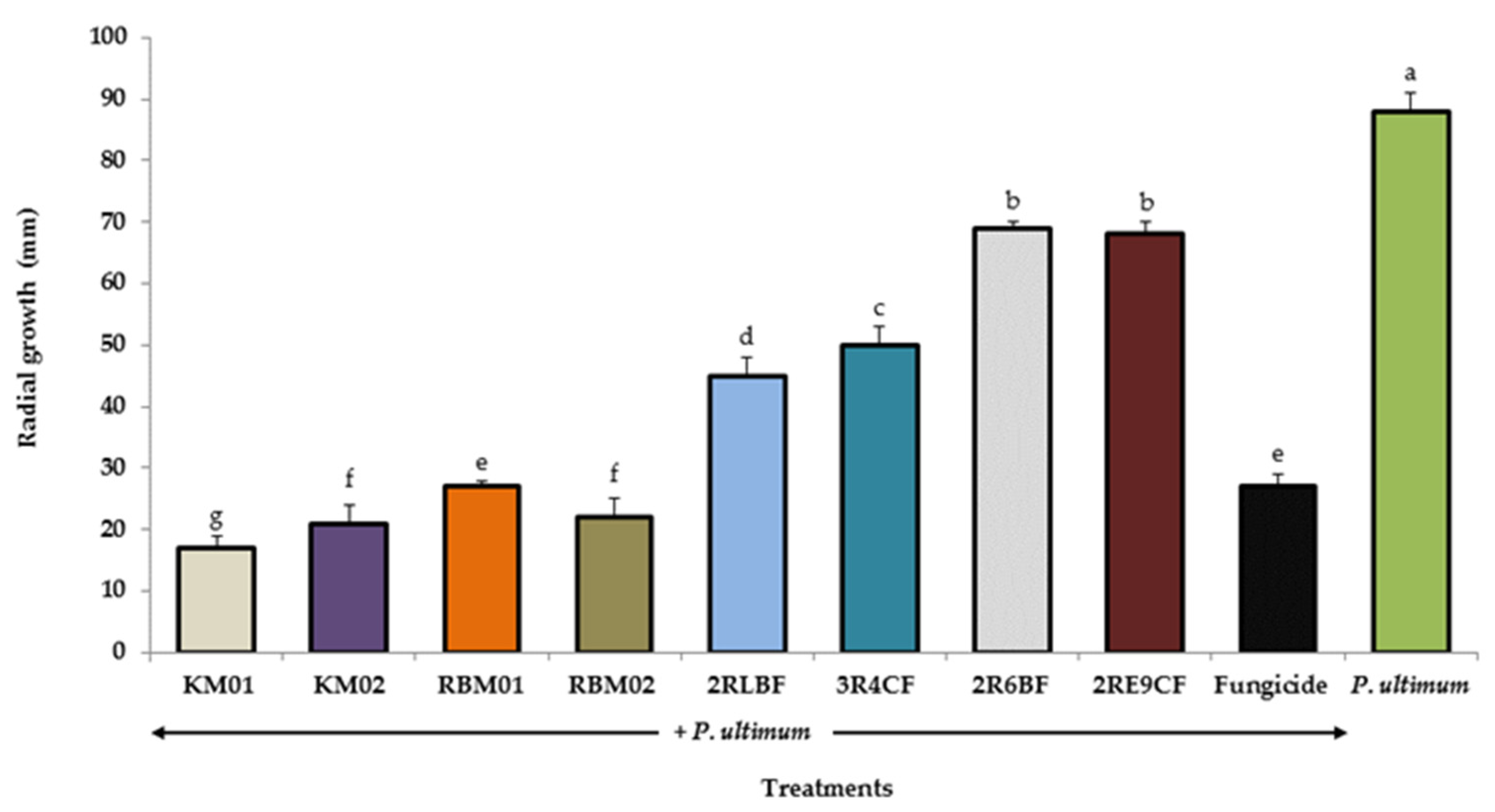
3.2. In Vitro Radial Growth of Pythium Ultimum
3.3. Lytic Activity
3.4. Protection of Ancho-Type Pepper Plants by Marine Bacteria and Yeasts
3.5. Roots Colonized by Marine Microorganisms
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hyder, S.; Naseem, S.; Azhar, S.; Ashfaq, M.; Ali, Z.; Khalid, A.; Inam-ul-Haq, M. Disease incidence and severity of Pythium spp. and Phytophthora spp. affecting chili pepper and tomato crops in Punjab, Pakistan. Philipp. Agric. Sci. 2018, 101, 36–44. [Google Scholar]
- Lema, A.A.; Mudansiru, A.; Alexander, B.A.; Sakinatu, M.J. Evaluation of fungal species isolated from three different varieties of pepper (Capsicum chinense, C. frutescens and C. annumm L.) in Dutsin-ma, Katsina State. Ann. Biol. Sci. 2018, 6, 13–17. [Google Scholar] [CrossRef]
- Zagade, S.N.; Deshpande, G.D.; Gawade, D.B.; Atnoorkar, A.A.; Pawar, S.V. Biocontrol agents and fungicides for management of damping off in chilli. World J. Agric. Sci. 2012, 8, 590–597. [Google Scholar]
- Halo, B.A.; Al-Yahyai, R.A.; Maharachchikumbura, S.S.; Al-Sadi, A.M. Talaromyces variabilis interferes with Pythium aphanidermatum growth and suppresses Pythium-induced damping-off of cucumbers and tomatoes. Sci. Rep. 2019, 9, 11255. [Google Scholar] [CrossRef]
- Lamichhane, J.R.; Dürr, C.; Schwanck, A.A.; Robin, M.H.; Sarthou, J.P.; Cellier, V.; Messéan, J.; Aubertot, J.N. Integrated management of damping-off diseases. A review. Agron. Sustain. Dev. 2017, 37, 10. [Google Scholar] [CrossRef]
- Pandey, M.; Ahmad, S.; Khan, K.Z. Efficacy of selected plant extracts and biocontrol agents against damping-off (Pythium aphanidermatum) of chilli. Res. Environ. Life Sci. 2017, 10, 87–90. [Google Scholar]
- Grijalba, P.E.; Ridao, A.C.; Steciow, M.; López, M.V. Relationship between Rps 1k gene and resistance to Pythium ultimum and P. irregulare in soybean. Summa Phytopathol. 2017, 43, 94–97. [Google Scholar] [CrossRef] [Green Version]
- Tekale, A.G.; Guldekar, D.D.; Kendre, V.P.; Deshmukh, A.P.; Potdukhe, S.R. Efficacy of fungicides and bioagents against damping off in chilli caused by Pythium aphanidermatum. Int. J. Curr. Microbiol. App. Sci. 2019, 8, 637–648. [Google Scholar] [CrossRef]
- Muthu, K.A. Occurrence and distribution of indigenous isolates of Pythium species in northern India. Adv. Plants Agric. Res. 2016, 4, 319–327. [Google Scholar]
- Pandey, M.; Ahmad, S.; John, S.A.; Khan, K.Z. Antifungal potential of native Trichoderma isolates against Pythium aphanidermatum causing chilli damping-off. Ann. Plant Prot. Sci. 2019, 27, 102–106. [Google Scholar] [CrossRef]
- Liu, K.; McInroy, J.A.; Hu, C.H.; Kloepper, J.W. Mixtures of Plant-Growth-Promoting rhizobacteria enhance biological control of multiple plant diseases and plant-growth promotion in the presence of pathogens. Plant Dis. 2018, 102, 67–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selim, H.M.; Gomaa, N.M.; Essa, A.M. Application of endophytic bacteria for the biocontrol of Rhizoctonia solani (Cantharellales: Ceratobasidiaceae) damping-off disease in cotton seedlings. Biocontrol Sci. Technol. 2017, 27, 81–95. [Google Scholar] [CrossRef]
- Le, C.N.; Hoang, T.K.; Thai, T.H.; Tran, T.L.; Phan, T.P.N.; Raaijmakers, J.M. Isolation, characterization and comparative analysis of plant-associated bacteria for suppression of soil-borne diseases of field-grown groundnut in Vietnam. Biol. Control 2018, 121, 256–262. [Google Scholar] [CrossRef]
- Haidar, R.; Fermaud, M.; Calvo-Garrido, C.; Roudet, J.; Deschamps, A. Modes of action for biological control of Botrytis cinerea by antagonistic bacteria. Phytopathol. Mediterr. 2016, 55, 301–322. [Google Scholar]
- Lin, M.; Zhang, Y.; Sun, C.; Huang, Y.; Zhang, J.; Zheng, X.; Yu, T. Characterization and overexpression of RHO1 from Cryptococcus laurentii ZJU10 activates CWI signaling pathway on enhancing the inhibition of blue mold on pears. Int. J. Food Microbiol. 2018, 278, 1–10. [Google Scholar] [CrossRef]
- Bodhankar, S.; Grover, M.; Hemanth, S.; Reddy, G.; Rasul, S.; Yadav, S.K.; Desai, S.; Mallappa, M.; Mandapaka, M.; Srinivasarao, C. Maize seed endophytic bacteria: Dominance of antagonistic, lytic enzyme-producing Bacillus spp. 3 Biotech 2017, 7, 232. [Google Scholar] [CrossRef]
- Zhang, S.B.; Zhang, W.J.; Zhai, H.C.; Lv, Y.Y.; Cai, J.P.; Jia, F.; Wang, J.S.; Hu, Y.S. Expression of a wheat β-1,3-glucanase in Pichia pastoris and its inhibitory effect on fungi commonly associated with wheat kernel. Protein Expr. Purif. 2019, 154, 134–139. [Google Scholar] [CrossRef]
- Gond, S.K.; Bergen, M.S.; Torres, M.S.; White, J.F., Jr. Endophytic Bacillus spp. produce antifungal lipopeptides and induce host defence gene expression in maize. Microbiol. Res. 2015, 172, 79–87. [Google Scholar] [CrossRef]
- Sun, C.; Fu, D.; Lu, H.; Zhang, J.; Zheng, X.; Yu, T. Autoclaved yeast enhances the resistance against Penicillium expansum in postharvest pear fruit and its possible mechanisms of action. Biol. Control 2018, 119, 51–58. [Google Scholar] [CrossRef]
- Rivas-Garcia, T.; Murillo-Amador, B.; Nieto-Garibay, A.; Chiquito-Contreras, R.; Rincon-Enriquez, G.; Hernandez-Montiel, L.G. Effect of ulvan on the biocontrol activity of Debaryomyces hansenii and Stenotrophomonas rhizophila against fruit rot of Cucumis melo L. Agronomy 2018, 8, 273. [Google Scholar] [CrossRef] [Green Version]
- Ünal, F.; Aşkın, A.; Koca, E.; Yıldırır, M.; Bingöl, M.Ü. Mycelial compatibility groups, pathogenic diversity and biological control of Sclerotium rolfsii on turfgrass. Egypt. J. Biol. Pest Control 2019, 29, 44. [Google Scholar] [CrossRef]
- Jaaffar, A.K.M.; Parejko, J.A.; Paulitz, T.C.; Weller, D.M.; Thomashow, L.S. Sensitivity of Rhizoctonia isolates to phenazine-1-carboxylic acid and biological control by phenazine-producing Pseudomonas spp. Phytopathology 2017, 107, 692–703. [Google Scholar] [CrossRef] [Green Version]
- Freitas, M.A.; Medeiros, F.H.; Melo, I.S.; Pereira, P.F.; Peñaflor, M.F.G.; Bento, J.M.; Paré, P.W. Stem inoculation with bacterial strains Bacillus amyloliquefaciens (GB03) and Microbacterium imperiale (MAIIF2a) mitigates fusarium root rot in cassava. Phytoparasitica 2019, 47, 135–142. [Google Scholar] [CrossRef]
- Czarnecka, M.; Żarowska, B.; Połomska, X.; Restuccia, C.; Cirvilleri, G. Role of biocontrol yeasts Debaryomyces hansenii and Wickerhamomyces anomalus in plants defence mechanisms against Monilinia fructicola in apple fruits. Food Microbiol. 2019, 83, 1–8. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, W.; Zeng, J.; Shao, Y. Mechanisms of action of the yeast Debaryomyces nepalensis for control of the pathogen Colletotrichum gloeosporioides in mango fruit. Biol. Control 2018, 123, 111–119. [Google Scholar] [CrossRef]
- Li, J.; Li, H.; Ji, S.; Chen, T.; Tian, S.; Qin, G. Enhancement of biocontrol efficacy of Cryptococcus laurentii by cinnamic acid against Penicillium italicum in citrus fruit. Postharvest Biol. Technol. 2019, 149, 42–49. [Google Scholar] [CrossRef]
- Hernandez-Montiel, L.G.; Zulueta-Rodriguez, R.; Angulo, C.; Rueda-Puente, E.O.; Quiñonez-Aguilar, E.E.; Galicia, R. Marine yeasts and bacteria as biological control agents against anthracnose on mango. J. Phytopathol. 2017, 165, 833–840. [Google Scholar] [CrossRef]
- Dionisi, H.M.; Lozada, M.; Olivera, N.L. Bioprospection of marine microorganisms: Biotechnological applications and methods. Rev. Argent. Microbiol. 2012, 44, 49–60. [Google Scholar]
- Chiquito-Contreras, R.G.; Solís-Palacios, R.; Reyes-Pérez, J.J.; Murillo-Amador, B.; Alejandre-Rosas, J.; Hernández-Montiel, L.G. Promoción del crecimiento de plantas de albahaca utilizando hongos micorrízicos arbusculares y una bacteria marina. Acta Universitaria 2018, 28, 68–76. [Google Scholar] [CrossRef]
- Hernandez-Montiel, L.G.; Gutierrez-Perez, E.; Murillo-Amador, B.; Vero, S.; Chiquito-Contreras, R.G.; Rincon-Enriquez, G. Mechanisms employed by Debaryomyces hansenii in biological control of anthracnose disease on papaya fruit. Postharvest Biol. Technol. 2018, 139, 31–37. [Google Scholar] [CrossRef]
- St-Arnaud, M.; Hamel, C.; Caron, M.; Fortin, J.A. Inhibition of Pythium ultimum in roots and growth substrate of mycorrhizal Tagetes patula colonized with Glomus intraradices. Can. J. Plant Pathol. 1994, 16, 187–194. [Google Scholar] [CrossRef]
- Akköpru, A.; Demir, S. Biological control of Fusarium wilt in tomato caused by Fusarium oxysporum f.sp. lycopersici by AMF Glomus intraradices and some rhizobacteria. J. Plant Physiol. 2005, 153, 544–550. [Google Scholar]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar]
- Usall, J.; Teixido, N.; Torres, R.; Ochoa, X.; Viñas, I. Pilot test of Candida sake (CPA) applications to control postharvest blue mold on apple fruit. Postharvest Biol. Technol. 2001, 21, 147–156. [Google Scholar] [CrossRef]
- Swanson, K.M.; Petran, R.L.; Hanlin, J.H. Culture methods for enumeration of microorganisms. In Compendium of Methods for the Microbiological Examination of Foods; Downs, F.P., Ito, K., Eds.; APHA: Washington, DC, USA, 2001; pp. 53–67. [Google Scholar]
- Koch, E.; Becker, J.O.; Berg, G.; Hauschild, R.; Jehle, J.; Köhl, J.; Smalla, K. Biocontrol of plant diseases is not an unsafe technology! J. Plant Dis. Protect. 2018, 125, 121–125. [Google Scholar] [CrossRef]
- Pretscher, J.; Fischkal, T.; Branscheidt, S.; Jäger, L.; Kahl, S.; Schlander, M.; Thinesm, E.; Claus, H. Yeasts from different habitats and their potential as biocontrol agents. Fermentation 2018, 4, 31. [Google Scholar] [CrossRef] [Green Version]
- Reddy, M.R.; Shivaprakash, M.K.; Adithya, S. In Vitro evaluation of bacterial endophytes for biocontrol of Pythium aphanidermatum and plant growth promotion in Setaria italica L. grown in seedling trays. Curr. J. Appl. Sci. Technol. 2019, 38, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Wang, Y.; Wang, Y.; Li, B.; Gu, X.; Zhang, X.; Boateng, N.A.S.; Zhang, H. Effect of β-glucan on the biocontrol efficacy of Cryptococcus podzolicus against postharvest decay of pears and the possible mechanisms involved. Postharvest Biol. Technol. 2020, 160, 111057. [Google Scholar] [CrossRef]
- Dewi, R.T.K.; Mubarik, N.R.; Suhartono, M.T. Medium optimization of β-glucanase production by Bacillus subtilis SAHA 32.6 used as biological control of oil palm pathogen. Emir. J. Food Agric. 2016, 28, 116–125. [Google Scholar] [CrossRef] [Green Version]
- Bibi, F.; Yasir, M.; Song, G.C.; Lee, S.Y.; Chung, Y.R. Diversity and characterization of endophytic bacteria associated with tidal flat plants and their antagonistic effects on oomycetous plant pathogens. Plant Pathol. J. 2012, 28, 20–31. [Google Scholar] [CrossRef] [Green Version]
- Reyes-Perez, J.J.; Hernandez-Montiel, L.G.; Vero, S.; Noa-Carrazana, J.C.; Quiñones-Aguilar, E.E.; Rincón-Enríquez, G. Postharvest biocontrol of Colletotrichum gloeosporioides on mango using the marine bacterium Stenotrophomonas rhizophila and its possible mechanisms of action. J. Food Sci. Technol. 2019, 56, 4992–4999. [Google Scholar] [CrossRef]
- Zeng, L.; Yu, C.; Fu, D.; Lu, H.; Zhu, R.; Lu, L.; Zheng, X.; Yu, T. Improvement in the effectiveness of Cryptococcus laurentii to control postharvest blue mold of pear by its culture in β-glucan amended nutrient broth. Postharvest Biol. Technol. 2015, 104, 26–32. [Google Scholar] [CrossRef]
- Schreiter, S.; Sandmann, M.; Smalla, K.; Grosch, R. Soil type dependent rhizosphere competence and biocontrol of two bacterial inoculant strains and their effects on the rhizosphere microbial community of field-grown lettuce. PLoS ONE 2014, 9, e103726. [Google Scholar] [CrossRef] [Green Version]
- Tan, C.; Wang, L.; Xue, Y.; Lin, S.; Yu, G.; Yang, S. Purification and molecular characterization of a Metschnikowia saccharicola killer toxin lethal to a crab pathogenic yeast. FEMS Microbiol. Lett. 2018, 365, fny038. [Google Scholar] [CrossRef]
- Pi, H.; Helmann, J.D. Sequential induction of Fur-regulated genes in response to iron limitation in Bacillus subtilis. Proc. Natl. Acad. Sci. USA 2017, 114, 12785–12790. [Google Scholar] [CrossRef] [Green Version]
- Romanazzi, G.; Sanzani, S.M.; Bi, Y.; Tian, S.; Martínez, P.G.; Alkan, N. Induced resistance to control postharvest decay of fruit and vegetables. Postharvest Biol. Technol. 2016, 122, 82–94. [Google Scholar] [CrossRef]
- Lai, J.; Cao, X.; Yu, T.; Wang, Q.; Zhang, Y.; Zheng, X.; Lu, H. Effect of Cryptococcus laurentii on inducing disease resistance in cherry tomato fruit with focus on the expression of defense-related genes. Food Chem. 2018, 254, 208–216. [Google Scholar] [CrossRef]
- Farace, G.; Fernandez, O.; Jacquens, L.; Coutte, F.; Krier, F.; Jacques, P.; Clément, C.; Ait Barka, E.; Jacquard, C.; Dore, S. Cyclic lipopeptides from Bacillus subtilis activate distinct patterns of defence responses in grapevine. Mol. Plant Pathol. 2015, 16, 177–187. [Google Scholar] [CrossRef]
- Elhalag, K.M.; Messiha, N.A.S.; Emara, H.M.; Abdallah, S.A. Evaluation of antibacterial activity of Stenotrophomonas maltophilia against Ralstonia solanacearum under different application conditions. J. Appl. Microbiol. 2016, 120, 1629–1645. [Google Scholar] [CrossRef] [Green Version]
- Tabatabaei, S.; Ehsanzadeh, P.; Etesami, H.; Alikhani, H.A.; Glick, B.R. Indole-3-acetic acid (IAA) producing Pseudomonas isolates inhibit seed germination and α-amylase activity in durum wheat (Triticum turgidum L.). Span. J. Agric. Res. 2016, 14, e0802. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.; Kumar, V.; Tripathi, R.B. Isolation of phosphate solubilizing microorganism (PSMs) from soil. J. Microbiol. Biotechnol. Res. 2017, 1, 90–95. [Google Scholar]
- Pham, V.T.; Rediers, H.; Ghequire, M.G.; Nguyen, H.H.; De Mot, R.; Vanderleyden, J.; Spaepen, S. The plant growth-promoting effect of the nitrogen-fixing endophyte Pseudomonas stutzeri A15. Arch. Microbiol. 2017, 199, 513–517. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Kucharova, Z.; Davranov, K.; Berg, G.; Makarova, N.; Azarova, T.; Chebotar, V.; Tikhonovich, I.; Kamilova, F.; Validov, S.Z.; et al. Bacteria able to control foot and root rot and to promote growth of cucumber in salinated soils. Biol. Fertil. Soils 2011, 47, 197–205. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Jabborova, D.; Berg, G. Synergistic interactions between Bradyrhizobium japonicum and the endophyte Stenotrophomonas rhizophila and their effects on growth, and nodulation of soybean under salt stress. Plant Soil 2016, 405, 35–45. [Google Scholar] [CrossRef]
- Tahir, H.A.; Gu, Q.; Wu, H.; Raza, W.; Hanif, A.; Wu, L.; Colman, M.V.; Gao, X. Plant growth promotion by volatile organic compounds produced by Bacillus subtilis SYST2. Front. Microbiol. 2017, 8, 171. [Google Scholar] [CrossRef] [Green Version]
- Verma, V.; Ravindran, P.; Kumar, P.P. Plant hormone-mediated regulation of stress responses. BMC Plant Biol. 2016, 16, 86. [Google Scholar] [CrossRef] [Green Version]
- Gamez, R.; Cardinale, M.; Montes, M.; Ramirez, S.; Schnell, S.; Rodriguez, F. Screening, plant growth promotion and root colonization pattern of two rhizobacteria (Pseudomonas fluorescens Ps006 and Bacillus amyloliquefaciens Bs006) on banana cv. Williams (Musa acuminata Colla). Microbiol. Res. 2019, 220, 12–20. [Google Scholar] [CrossRef]
- Lee, G.; Lee, S.H.; Kim, K.M.; Ryu, C.M. Foliar application of the leaf-colonizing yeast Pseudozyma churashimaensis elicits systemic defense of pepper against bacterial and viral pathogens. Sci. Rep. 2017, 7, 39432. [Google Scholar] [CrossRef]
- Joubert, P.M.; Doty, S.L. Endophytic yeasts: Biology, ecology and applications. In Endophytes of Forest Trees; Pirttilä, A., Frank, A., Eds.; Springer: Cham, Switzerland, 2018; pp. 3–14. [Google Scholar]
- Kandel, S.L.; Joubert, P.M.; Doty, S.L. Bacterial endophyte colonization and distribution within plants. Microorganisms 2017, 5, 77. [Google Scholar] [CrossRef] [Green Version]




| Microorganism | Strain | Lytic Activity | ||
|---|---|---|---|---|
| β-1,3-glucanase (U/mL) | ||||
| 12 h | 24 h | |||
| Bacterium | S. rhizophila | KM01 | 27 d* | 41 e |
| KM02 | 29 d | 47 d | ||
| B. subtilis | RBM01 | 7 g | 17 g | |
| RBM02 | 9 g | 14 g | ||
| B. amyloliquefaciens | 2RLBF | 8 g | 15 g | |
| 3R4CF | 11 f | 21 fg | ||
| Pseudomonas spp. | 2R6BF | 19 e | 40 e | |
| 2RE9CF | 20 e | 37 e | ||
| Yeast | D. hansenii | 1R11AB | 2589 b | 5062 b |
| 1R11CB | 2105 c | 3562 c | ||
| LL01 | 3907 a | 6062 a | ||
| C. laurentii | 2R3BF | 21 e | 40 e | |
| 2R1CB | 19 e | 37 e | ||
| Treatment | Height (cm) | Fresh Root Weight (g) | Dry Foliage Weight (g) | Dry Weight of Root (g) | Leaf Area (cm2) | Radical Volume (cm3) |
|---|---|---|---|---|---|---|
| Bacterium | ||||||
| KM01 | 10.89 b | 3.62 b | 1.19 b | 1.03 b | 110.41 b | 28.37 b |
| KM02 | 9.01 d | 1.16 e | 0.58 e | 0.51 d | 82.88 f | 20.59 d |
| RBM01 | 10.71 b | 3.56 b | 1.18 b | 1.08 b | 107.75 c | 28.75 b |
| RBM02 | 12.68 a | 4.22 a | 1.96 a | 1.51 a | 120.34 a | 35.62 a |
| Yeast | ||||||
| 2R1CB | 9.98 c | 2.86 c | 0.82 c | 0.72 c | 95.33 d | 23.25 c |
| LL01 | 9.74 c | 1.78 d | 0.75 d | 0.71 c | 90.81 e | 22.07 c |
| 1R11CB | 8.97 d | 1.22 e | 0.56 e | 0.52 d | 83.55 f | 17.37 e |
| 2R3BF | 8.85 d | 1.15 e | 0.56 e | 0.49 d | 80.32 g | 17.62 e |
| Fungicide | 7.98 e | 0.81 f | 0.35 f | 0.22 e | 17.90 h | 6.75 f |
| P. ultimum | 5.07 f | 0.49 g | 0.19 g | 0.11 f | 4.77 i | 3.11 g |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lara-Capistran, L.; Zulueta-Rodriguez, R.; Castellanos-Cervantes, T.; Reyes-Perez, J.J.; Preciado-Rangel, P.; Hernandez-Montiel, L.G. Efficiency of Marine Bacteria and Yeasts on the Biocontrol Activity of Pythium ultimum in Ancho-Type Pepper Seedlings. Agronomy 2020, 10, 408. https://doi.org/10.3390/agronomy10030408
Lara-Capistran L, Zulueta-Rodriguez R, Castellanos-Cervantes T, Reyes-Perez JJ, Preciado-Rangel P, Hernandez-Montiel LG. Efficiency of Marine Bacteria and Yeasts on the Biocontrol Activity of Pythium ultimum in Ancho-Type Pepper Seedlings. Agronomy. 2020; 10(3):408. https://doi.org/10.3390/agronomy10030408
Chicago/Turabian StyleLara-Capistran, Liliana, Ramon Zulueta-Rodriguez, Thelma Castellanos-Cervantes, Juan J. Reyes-Perez, Pablo Preciado-Rangel, and Luis G. Hernandez-Montiel. 2020. "Efficiency of Marine Bacteria and Yeasts on the Biocontrol Activity of Pythium ultimum in Ancho-Type Pepper Seedlings" Agronomy 10, no. 3: 408. https://doi.org/10.3390/agronomy10030408





