A Comparison of Selected Biochemical and Physical Characteristics and Yielding of Fruits in Apple Cultivars (Malus domestica Borkh.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Description of Climatic Conditions
2.3. Determination of Total Saccharide Content
2.4. Determination of Soluble Sugars and Organic Acids by HPLC
2.5. Determination of Sorbitol by Gas Chromatography Coupled with Mass Spectrometry
2.5.1. Extraction
2.5.2. Derivatization
2.5.3. GC-MS Analyses
2.6. Determination of Semiquinone Radicals
2.7. Detection of Abscisic Acid (ABA)
2.8. Determination of Membrane Injury Index
2.9. Yield and Determination of Fruit Quality
- -
- fruit weight: 100 fruits were collected from each experimental variant and weighed accurate to 1 g;
- -
- fruit firmness: measurements were taken on 100 fruits per four replication (a total of 400 fruits) for each experimental variant on the shaded side and intensive blush using a Fruit Hardness Tester (FHT-803) by Silverado Company, China;
- -
- Soluble solid content was measured on the same fruits, on which firmness was measured. Soluble solid contents were evaluated using a Digital Handheld Refractometer DR 101-60 (A. KRUSS Optronic GmbH, Hamburg Germany). Slices of fruit flesh were cut from apples on opposite sides of fruits, from which juice was pressed on a refractometer plate. Values of measurements were expressed in % oBrix;
- -
- Potential acidity was measured using a pH-meter. The juice was pressed from apples, from which 5 mL samples were collected, 45 mL distilled water was added and 0.1 N NaOH were titrated until pH 7.4 was reached. Based on the amount of used NaOH, the acid percentage content was given in malic acid;
- -
- Fruit size was determined based on their calibration. A total of 100 fruits from each experimental variant were evaluated. Fruit diameter was measured using a template ruler graduated to 0.5 cm in a 5-point scale: 1–below 6.5 cm; 2–6.6–7.0 cm; 3–7.1–7.5 cm; 4–7.6–8.0 cm; 5–over 8.1 cm;
- -
- Fruit color was assessed using a 5-point scale, taking into account the blush area. Evaluation was performed on 100 fruits from each experimental variant immediately after fruit harvest as a relative number (percentage of all assessed fruits). For color assessment, the percentage area of the skin blush was analyzed, i.e., 0–fruits with no blush; 1–<25% skin area covered by blush; 2–26–50% skin area covered by blush; 3–51–75% skin area covered by blush; 4–>75% skin area covered by blush; 5—100% skin area covered by blush.
2.10. Statistical Analysis
3. Results
3.1. Sugar Levels in Organs of Apple Trees
3.1.1. Total Saccharide Content in Organs of Apple Trees
3.1.2. Soluble Sugars in Fruits of Apple Trees
3.2. Concentration of Organic Acids in Fruits of Apple Trees
3.3. Amounts of Sorbitol in Fruits of Apple Trees
3.4. Total Solid Substance (TSS) Contents in Fruits of Apple Trees
3.5. Concentration of Semiquinone Radicals in Organs of Apple Trees
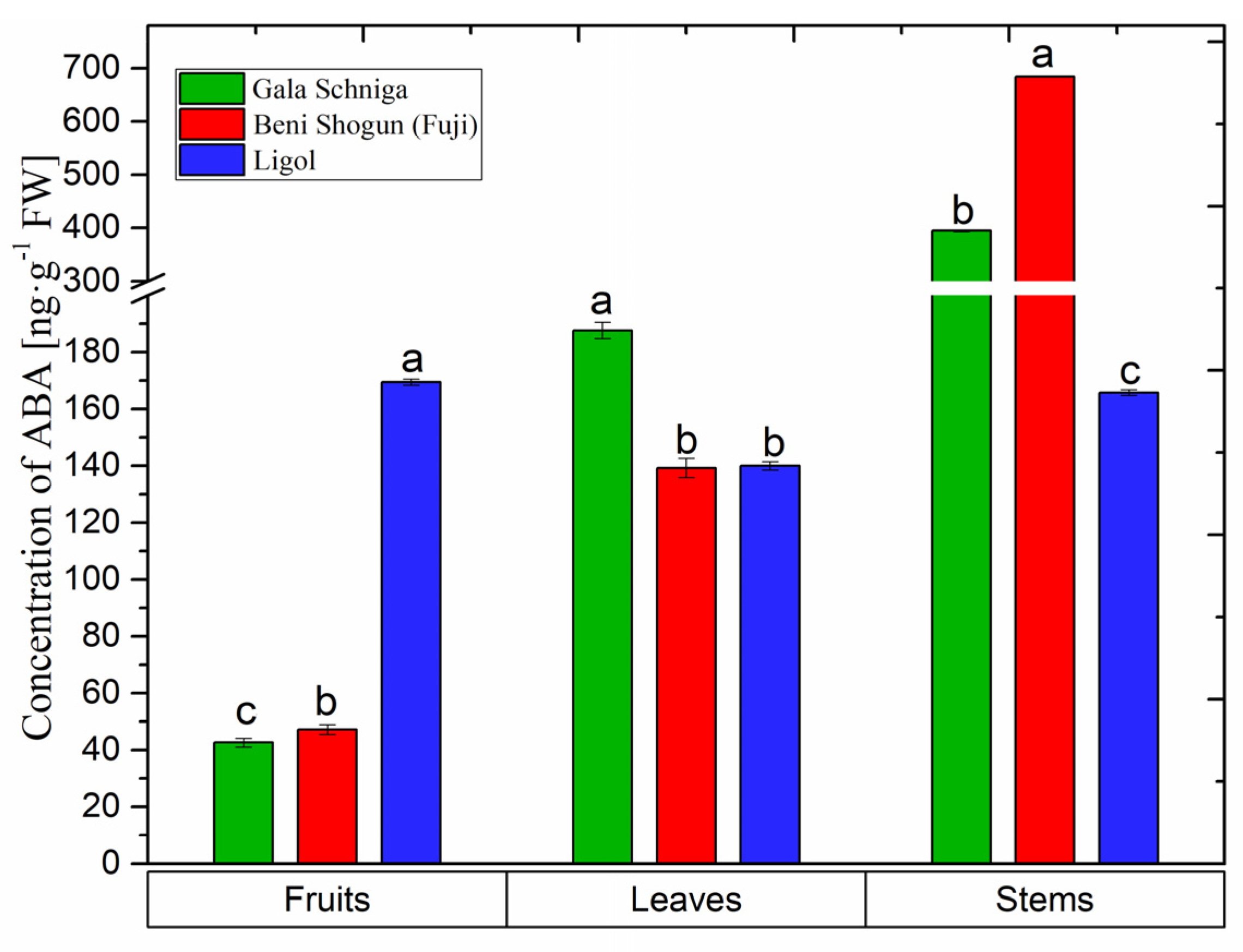
3.6. Abscisic Acid Levels in Organs of Apple Trees
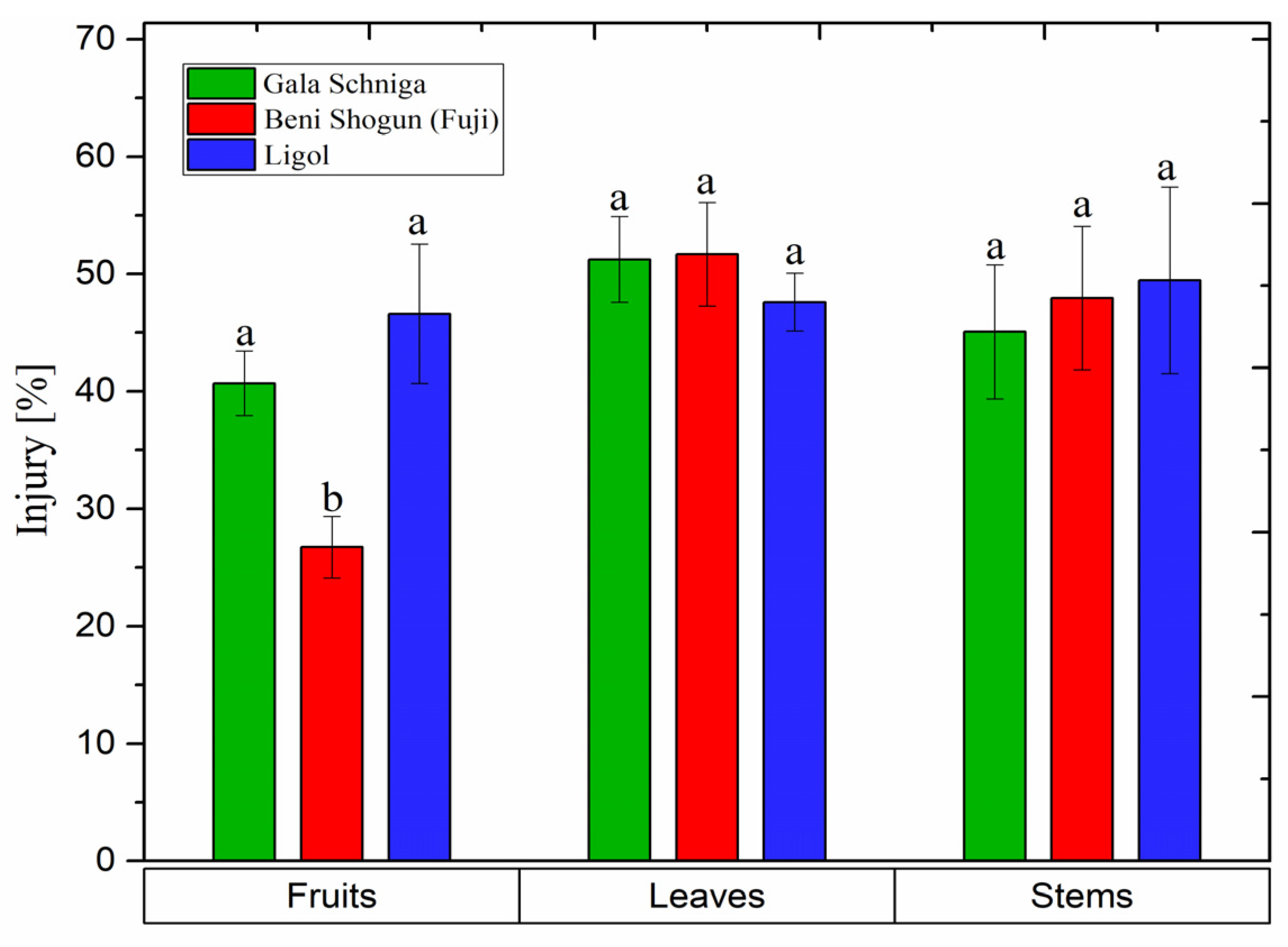
3.7. Release of Electrolytes from Organs of Apple Trees
3.8. Yields and Quality of Fruits
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Goddrie, P.W.; Kemp, H. Cultivar testing with apple. Wilherminadorp Res. St Ann. Rep. 1991, 48–54. [Google Scholar]
- Ugolik, M.; Kantorowicz-Bąk, M.; Ugolik, D. Evaluation of the Growth and Yield of Several New Apple Varieties on the M.9 Rootstock. In Proceedings of the 33 Polish National Scientific Conference of Institute of Pomology and Floriculture, Skierniewice, Poland, 30 August–1 September 1994; pp. 86–87. [Google Scholar]
- Wrona, D.; Sadowski, A.; Dziuban, R. Comparison of Seven Varieties of VF Apple on M.9 Rootstock. In Proceedings of the 33 Polish National Scientific Conference of Institute of Pomology and Floriculture, Skierniewice, Poland, 30 August–1 September 1994; pp. 81–89. [Google Scholar]
- Włodarczyk, P. Selected Factors Affecting the Early Start Fruiting Apple Trees; Akademia Rolnicza Lublin: Lublin, Poland, 1996. [Google Scholar]
- Błaszczyk, J.; Poniedziałek, W. Growth and Yielding of 20 Apple Varieties in the Kraków Region. In Proceedings of the 37 Polish National Scientific Conference of Judical Institute of Pomology and Floriculture, Skierniewice, Poland, 25–27 August 1998; pp. 363–365. [Google Scholar]
- Makosz, E. The Vision of the Fruit Market in Poland. In Proceedings of the 44 Congress of the Fruit-Growers’ Market Fruit, Skierniewice, Poland, 27 October 2005; pp. 5–13. [Google Scholar]
- Kapłan, M.; Wociór, S. The study of the yield of four apple varieties on the Sandomierska Upland. Zesz Nauk. Inst. Sadow. Kwiac. Ski. 2002, 69–73. [Google Scholar]
- Lewandowski, M.; Żurawicz, E. Effect of genotype on germination of Malus domestica seeds. In Natural and Induced Variability in the Genetic Improvement of Horticultural Plants; UTP in Bydgoszcz: Bydgoszcz, Poland, 2007; pp. 73–80. [Google Scholar]
- Laurens, F. Review of the current apple breeding programmes in the world: Objectives for scion cultivar improvement. Acta Hortic. 1998, 484, 163–170. [Google Scholar] [CrossRef]
- Sansavini, S.; Donati, F.; Costa, F.; Tartarini, S. Advances in apple breeding for enhanced fruit quality and resistance to biotic stresses:new varieties for the european market. J. Fruit Ornam. Plant Res. 2004, 12, 40. [Google Scholar]
- Kellerhals, M. Introduction to Apple (Malus × domestica). In Genetics and Genomics of Rosaceae; Folta, K.M., Gardiner, S.E., Eds.; Springer: New York, NY, USA, 2009; pp. 73–84. ISBN 978-0-387-77490-9. [Google Scholar]
- Kumar, S.; Volz, R.K.; Chagné, D.; Gardiner, S. Breeding for apple (Malus × domestica Borkh.) fruit quality traits in the genomics era. In Genomics of Plant Genetic Resources; Tuberosa, R., Graner, A., Frison, E., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 387–416. ISBN 978-94-007-7574-9. [Google Scholar]
- Bekbergen, A. Marker Assisted Breeding and Screening of Apple Scab Resistance (VF Gene) from Columnar Apple Seedlings by PCR. Master’s Thesis, University of Eastern Finland, Joensuu/Kuopio, Finland, 2016. [Google Scholar]
- Sofla, H.S.; Zamani, Z.; Talaei, A.R.; Fatahi, M.R.; Nazari, S.A.; Farokhzad, A.R.; Gharghani, A.; Asgarzadeh, M. Introduction of New Promising Apple Genotypes: A Study of Quality Attributes of Apple in Crosses between Iranian Early Ripening and Exotic Late Ripening Apple Cultivars. Int. J. Fruit Sci. 2016, 16, 210–224. [Google Scholar] [CrossRef]
- Foster, T.M.; Celton, J.-M.; Chagné, D.; Tustin, D.S.; Gardiner, S.E. Two quantitative trait loci, Dw1 and Dw2, are primarily responsible for rootstock-induced dwarfing in apple. Hortic. Res. 2015, 2, 15001. [Google Scholar] [CrossRef]
- Tang, C.; He, H.; Li, E.; Li, H. Multispectral imaging for predicting sugar content of ‘Fuji’ apples. Opt. Laser Technol. 2018, 106, 280–285. [Google Scholar] [CrossRef]
- Choi, D.; Cho, H.-T.; Lee, Y. Expansins: Expanding importance in plant growth and development. Physiol. Plant. 2006, 126, 511–518. [Google Scholar] [CrossRef]
- Aprea, E.; Charles, M.; Endrizzi, I.; Laura Corollaro, M.; Betta, E.; Biasioli, F.; Gasperi, F. Sweet taste in apple: The role of sorbitol, individual sugars, organic acids and volatile compounds. Sci. Rep. 2017, 7, 44950. [Google Scholar] [CrossRef]
- Hu, W.; Sun, D.-W.; Pu, H.; Pan, T. Recent developments in methods and techniques for rapid monitoring of sugar metabolism in fruits: Rapid monitoring of sugar metabolism. Compr. Rev. Food Sci. Food Saf. 2016, 15, 1067–1079. [Google Scholar] [CrossRef]
- Leopold, L.; Diehl, H.; Socaciu, C. Quantification of glucose, fructose and sucrose in apple juices using ATR-MIR spectroscopy coupled with chemometry. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca Agric. 2009, 66, 9. [Google Scholar]
- Krost, C.; Petersen, R.; Schmidt, E.R. The transcriptomes of columnar and standard type apple trees (Malus x domestica)—A comparative study. Gene 2012, 498, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Rock, C.; Quatrano, R. The role of hormones during seed development. In Plant Horomnes: Physiology, Biochemistry and Molecular Biology; Davies, P.J., Ed.; Kluwer Academic Publishers: Norwell, MA, USA, 1995; pp. 671–697. [Google Scholar]
- Davies, W.J.; Zhang, J. Root Signals and the Regulation of Growth and Development of Plants in Drying Soil. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1991, 42, 55–76. [Google Scholar] [CrossRef]
- Brenner, M.L. The role of hormones in photosynthate partitioning and seed filling. In Plant Hormones and Their Role in Plant Growth and Development; Davies, P.J., Ed.; Martinus-Nijhoff: Dordrecht, The Netherlands, 1987; pp. 474–493. [Google Scholar]
- Wayne, H.L.; John, D.E. Sugar alcohol metabolism in sinks and sources. In Photoassimilate Distribution in Plants and Crops: Source-Sink Relationships; Zamski, E., Schaffer, A.A., Eds.; Marcel Dekker Inc.: New York, NY, USA, 1996; pp. 185–207. [Google Scholar]
- Peng, Y.-B.; Lu, Y.-F.; Zhang, D.-P. Abscisic acid activates ATPase in developing apple fruit especially in fruit phloem cells. Plant Cell Environ. 2003, 26, 1329–1342. [Google Scholar] [CrossRef]
- Lu, Y.M.; Zhang, D.P.; Yan, H.Y. Sugar unloading mechanism in the developing apple fruit. Acta Hortic. Sin. 1999, 26, 141–146. [Google Scholar]
- Barbehenn, R.V.; Poopat, U.; Spencer, B. Semiquinone and ascorbyl radicals in the gut fluids of caterpillars measured with EPR spectrometry. Insect Biochem. Mol. Biol. 2003, 33, 125–130. [Google Scholar] [CrossRef]
- Formela, M.; Samardakiewicz, S.; Marczak, Ł.; Nowak, W.; Narożna, D.; Bednarski, W.; Kasprowicz-Maluśki, A.; Morkunas, I. Effects of endogenous signals and Fusarium oxysporum on the mechanism regulating genistein synthesis and accumulation in yellow lupine and their impact on plant cell cytoskeleton. Molecules 2014, 19, 13392–13421. [Google Scholar] [CrossRef]
- Mai, V.C.; Bednarski, W.; Borowiak-Sobkowiak, B.; Wilkaniec, B.; Samardakiewicz, S.; Morkunas, I. Oxidative stress in pea seedling leaves in response to Acyrthosiphon pisum infestation. Phytochemistry 2013, 93, 49–62. [Google Scholar] [CrossRef]
- Morkunas, I.; Bednarski, W.; Kopyra, M. Defense strategies of pea embryo axes with different levels of sucrose to Fusarium oxysporum and Ascochyta pisi. Physiol. Mol. Plant Pathol. 2008, 72, 167–178. [Google Scholar] [CrossRef]
- Morkunas, I.; Bednarski, W. Fusarium oxysporum-induced oxidative stress and antioxidative defenses of yellow lupine embryo axes with different sugar levels. J. Plant Physiol. 2008, 165, 262–277. [Google Scholar] [CrossRef]
- Esteban-Carrasco, A.; Lopez-Serrano, M.; Zapata, J.M.; Sabater, B.; Martin, M. Oxidation of pehnolic compounds from Aloe barbadensis by peroxidase activity: Possible involvement in defence rection. Plant Physiol. Biochem. 2001, 39, 521–527. [Google Scholar] [CrossRef]
- Hammerschmidt, R. Phenols and plant-pathogen interactions: The saga continues. Physiol. Mol. Plant Pathol. 2005, 66, 77–78. [Google Scholar] [CrossRef]
- Barbehenn, R.; Cheek, S.; Gasperut, A.; Lister, E.; Maben, R. Phenolic Compounds in Red Oak and Sugar Maple Leaves Have Prooxidant Activities in the Midgut Fluids of Malacosoma disstria and Orgyia leucostigma Caterpillars. J. Chem. Ecol. 2005, 31, 969–988. [Google Scholar] [CrossRef] [PubMed]
- Pearce, N.J.G.; Perkins, W.T.; Westgate, J.A.; Gorton, M.P.; Jackson, S.E.; Neal, C.R.; Chenery, S.P. A compilation of new and published major and trace element data for NIST SRM 610 and NIST SRM 612 glass reference materials. Geostand. Newsl. J. Geostand. Geoanalysis 1997, 21, 115–144. [Google Scholar] [CrossRef]
- Borowiak-Sobkowiak, B.; Woźniak, A.; Bednarski, W.; Formela, M.; Samardakiewicz, S.; Morkunas, I. Brachycorynella asparagi (Mordv.) induced—Oxidative stress and antioxidative defenses of Asparagus officinalis L. Int. J. Mol. Sci. 2016, 17, 1740. [Google Scholar] [CrossRef]
- Zydlik, Z.; Rutkowski, K.; Świerczyński, S.; Morkunas, I.; Yoon, H.-K.; Seo, J.-H.; Kang, K.; Kleiber, T. The effect of climatic conditions in successive plant growing seasons on the response of selected varieties of apple trees (Malus domestica Borkh.). J. Elem. 2020, 25, 205–224. [Google Scholar]
- Vercammen, J. Search for a more dwarfing rootstock for apple. Acta Hortic. 2004, 658, 313–318. [Google Scholar] [CrossRef]
- Robinson, T. Advances in apple culture worldwide. Rev. Bras. Frutic. 2011, 33, 37–47. [Google Scholar] [CrossRef]
- Yordanov, A.; Tabakov, S.; Kaymakanov, P. Comparative study of Wavit® rootstock with two plum and two apricot cultivars in nursery. J. Agric. Sci. Belgrade 2015, 60, 159–168. [Google Scholar] [CrossRef]
- Webster, A.D.; Wertheim, S.J. Comparisons of species and hybrid rootstocks for European plum cultivars. J. Hortic. Sci. 1993, 68, 861–869. [Google Scholar] [CrossRef]
- Marini, R.P.; Fazio, G. Apple rootstocks: History, physiology, management, and breeding. Hortic. Rev. 2018, 45, 197–312. [Google Scholar]
- Björnesjö, K.B. Analysis of protein-bound serum polysaccharides with anthrone reagent. Scand. J. Clin. Lab. Invest. 1955, 6, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Miron, D.; Schaffer, A.A. Sucrose Phosphate Synthase, Sucrose Synthase, and Invertase Activities in Developing Fruit of Lycopersicon esculentum Mill. and the Sucrose Accumulating Lycopersicon hirsutum Humb. and Bonpl. Plant Physiol. 1991, 95, 623–627. [Google Scholar] [CrossRef] [PubMed]
- Bozan, B.; Tunalier, Z.; Kosar, M.; Altintas, A.; Baser, K.H.C. Quantitative Analysis of Vitamin C in Rose Hip Products Collected from Local Markets in Turkey. In Proceedings of the XI Symposium Plant Originated Crude Drugs, Ankara, Turkey, 22–24 May 1996; pp. 258–266. [Google Scholar]
- Morkunas, I.; Woźniak, A.; Formela, M.; Mai, V.C.; Marczak, Ł.; Narożna, D.; Borowiak-Sobkowiak, B.; Kühn, C.; Grimm, B. Pea aphid infestation induces changes in flavonoids, antioxidative defence, soluble sugars and sugar transporter expression in leaves of pea seedlings. Protoplasma 2016, 253, 1063–1079. [Google Scholar] [CrossRef] [PubMed]
- Bednarski, W.; Ostrowski, A.; Waplak, S. Low temperature short-range ordering caused by Mn2 + doping of Rb3H(SO4)2. J. Phys. Condens. Matter 2010, 22, 225901. [Google Scholar] [CrossRef] [PubMed]
- Morkunas, I.; Garnczarska, M.; Bednarski, W.; Ratajczak, W.; Waplak, S. Metabolic and ultrastructural responses of lupine embryo axes to sugar starvation. J. Plant Physiol. 2003, 160, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Morkunas, I.; Bednarski, W.; Kozłowska, M. Response of embryo axes of germinating seeds of yellow lupine to Fusarium oxysporum. Plant Physiol. Biochem. 2004, 42, 493–499. [Google Scholar] [CrossRef]
- Morkunas, I.; Formela, M.; Floryszak-Wieczorek, J.; Marczak, Ł.; Narożna, D.; Nowak, W.; Bednarski, W. Cross-talk interactions of exogenous nitric oxide and sucrose modulates phenylpropanoid metabolism in yellow lupine embryo axes infected with Fusarium oxysporum. Plant Sci. 2013, 211, 102–121. [Google Scholar] [CrossRef]
- Vine, J.H.; Noiton, D.; Plummer, J.A.; Baleriola-Lucas, C.; Mullins, M.G. Simultaneous quantitation of indole 3-acetic acid and abscisic acid in small samples of plant tissue by gas chromatography/mass spectrometry/selected ion monitoring. Plant Physiol. 1985, 85, 419–422. [Google Scholar] [CrossRef]
- Dexter, S.T.; Tottingham, W.E.; Graber, L.F. Investigation of the hardiness of the plants by measurement of electricial conductivity. Plant Physiol. 1932, 7, 63–78. [Google Scholar] [CrossRef]
- Bandurska, H. Does proline accumulated in leaves of water deficit stressed barley plants confine cell membrane injuries? II. Proline accumulation during hardening and its involvement in reducing membrane injuries in leaves subjected to severe osmotic stress. Acta Physiol. Plant. 2001, 23, 483–490. [Google Scholar] [CrossRef]
- Sullivan, C.Y. Techniqes for measuring plant drought stress. In Drought Injury an Resistance in Crops; Larson, K.L., Eastin, J.D., Eds.; Crop Science Society of America: Madison, WI, USA, 1971; pp. 1–18. [Google Scholar]
- Wosiacki, G.; Nogueira, A.; Denardi, F.; Vieira, R.G. Sugar composition of depectinized apple juices. Semin. Ciênc. Agrár. 2007, 28, 645–652. [Google Scholar] [CrossRef]
- Endrizzi, I.; Torri, L.; Corollaro, M.L.; Demattè, M.L.; Aprea, E.; Charles, M.; Biasioli, F.; Gasperi, F. A conjoint study on apple acceptability: Sensory characteristics and nutritional information. Food Qual. Prefer. 2015, 40, 39–48. [Google Scholar] [CrossRef]
- Bonany, J.; Buehler, A.; Carbó, J.; Codarin, S.; Donati, F.; Echeverria, G.; Egger, S.; Guerra, W.; Hilaire, C.; Höller, I.; et al. Consumer eating quality acceptance of new apple varieties in different European countries. Food Qual. Prefer. 2013, 30, 250–259. [Google Scholar] [CrossRef]
- Füzfai, Z.; Katona, Z.F.; Kovács, E.; Molnár-Perl, I. Simultaneous identification and quantification of the wugar, Sugar Alcohol, and Carboxylic Acid Contents of Sour Cherry, Apple, and Ber Fruits, as Their Trimethylsilyl Derivatives, by Gas Chromatography−Mass Spectrometry. J. Agric. Food Chem. 2004, 52, 7444–7452. [Google Scholar] [CrossRef] [PubMed]
- Kopcewicz, J.; Lewak, S. Plant Physiology; Wydawnictwo Naukowe PWN: Warsaw, Poland, 2002. [Google Scholar]
- Woźniak, A.; Drzewiecka, K.; Kęsy, J.; Marczak, Ł.; Narożna, D.; Grobela, M.; Motała, R.; Bocianowski, J.; Morkunas, I. The influence of lead on generation of signalling molecules and accumulation of flavonoids in pea seedlings in response to pea aphid infestation. Molecules 2017, 22, 1404. [Google Scholar] [CrossRef]
- Leng, P.; Yuan, B.; Guo, Y. The role of abscisic acid in fruit ripening and responses to abiotic stress. J. Exp. Bot. 2013, 65, 4577–4588. [Google Scholar] [CrossRef]
- Frankowski, K.; Wilmowicz, E.; Kućko, A.; Sidłowska, M.; Kesy, J.; Kopcewicz, J. Abscisic acid metabolism. Postepy Biochem. 2013, 59, 83–88. [Google Scholar]
- Kitahata, N.; Asami, T. Chemical biology of abscisic acid. J. Plant Res. 2011, 124, 549–557. [Google Scholar] [CrossRef]
- Onik, J.C.; Hu, X.; Lin, Q.; Wang, Z. Comparative transcriptomic profiling to understand pre- and post-ripening hormonal regulations and anthocyanin biosynthesis in early ripening apple fruit. Molecules 2018, 23, 1908. [Google Scholar] [CrossRef]
- Seymour, G.B.; Østergaard, L.; Chapman, N.H.; Knapp, S.; Martin, C. Fruit ripening and development. Annu. Rev. Plant Biol. 2013, 64, 219–241. [Google Scholar] [CrossRef]
- McAtee, P.; Karim, S.; Schaffer, R.; David, K. A dynamic interplay between phytohormones is required for fruit development, maturation, and ripening. Front. Plant Sci. 2013, 4, 79. [Google Scholar] [CrossRef]
- Giovannoni, J.J. Fruit ripening mutants yield insights into ripening control. Curr. Opin. Plant Biol. 2007, 10, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Lech, W. Flowering and fruiting of fruit plants. Zeszyty Naukowe Akademii Rolniczej im. Hugona Kołłątaja w Krakowie 2000, 70, 5–17. [Google Scholar]
- Żurawicz, E. Pomology, Varieties of Fruit Plants; PWRiL: Warsaw, Poland, 2003. [Google Scholar]
- Failla, O.; Treccani, C.P.; Mignani, I. Water status, growth and calcium nutrition of apple trees in relation to bitter pit. Sci. Hortic. 1990, 42, 55–64. [Google Scholar] [CrossRef]
- Ahmadi-Afzadi, M. Genetic and Biochemical Properties of Apples that Affect Storability and Nutritional Value. Ph.D. Thesis, Balsgård, Swedish University of Agricultural Sciences, Uppsala, Sweden, 2012. [Google Scholar]
- Mizani, A.; Hajnajari, H. Genetic stability assessment of apple mutants “Fuji Kiku 8” and “Gala Schniga” during adaptation trials in Iran. Acta Hortic. 2015, 1074, 111–118. [Google Scholar] [CrossRef]
- Mika, A.; Buler, Z. Modifying apple spindle trees to improve fruit quality. Acta Sci. Pol. Hortorum Cultus 2015, 14, 12. [Google Scholar]








| Month | The Sum of Rainfall in mm | Average Temperature in °C | ||||
|---|---|---|---|---|---|---|
| Average from 1982–2012 | In 2017 | Change in Relation to the Long-Term Average | Average from 1982–2012 | In 2017 | Change in Relation to the Long-Term Average | |
| January | 31.1 | 17.6 | −13.5 | −0.8 | −2.7 | −1.9 |
| February | 26.3 | 28.0 | +1.7 | −0.1 | 0.0 | +0.1 |
| March | 34.3 | 35.0 | +0.7 | 3.6 | 5.9 | +2.3 |
| April | 28.0 | 36.4 | +8.4 | 9.3 | 7.0 | −2.3 |
| May | 48.0 | 31.2 | −16.8 | 14.6 | 13.3 | −1,3 |
| June | 63.5 | 85.6 | +22.1 | 17.2 | 17.5 | +0.3 |
| July | 78.8 | 182.4 | +103.6 | 19.5 | 17.8 | −1.7 |
| August | 61.9 | 80.0 | +18.1 | 18.9 | 18.2 | −0.7 |
| September | 41.0 | 47.2 | +6.2 | 14.1 | 12.9 | −1.2 |
| October | 32.0 | 56.8 | +24.8 | 9.0 | 10.2 | +1.2 |
| November | 37.2 | 35.0 | −2.2 | 3.7 | 2.7 | −1.0 |
| December | 39.0 | 40.6 | +1.6 | 0.2 | 2.2 | +2.0 |
| Total/ Average | 520.1 | 644.6 | +124.5 | 9.1 | 8.8 | -0.3 |
| Variety | Mean | Minimum | Maximum | Standard Deviation |
|---|---|---|---|---|
| Yield (kg per tree) | ||||
| Gala Schniga | 12.86 b | 3.50 | 28.40 | 4.71 |
| Fuji Benishogun | 6.49 a | 1.00 | 19.50 | 4.21 |
| Ligol | 6.21 a | 0.40 | 18.30 | 4.30 |
| Average fruit weight (g) | ||||
| Gala Schniga | 156.4 a | 121.2 | 242.6 | 24.9 |
| Fuji Benishogun | 194.9 b | 151.4 | 263.1 | 30.6 |
| Ligol | 295.9 c | 208.6 | 420.9 | 45.2 |
| Firmness kg/cm2 | ||||
| Gala Schniga | 7.18 c | 5.50 | 8.90 | 0.60 |
| Fuji Benishogun | 6.02 a | 4.90 | 7.60 | 0.54 |
| Ligol | 6.22 b | 4.50 | 7.90 | 0.65 |
| Total soluble solid content °Brix | ||||
| Gala Schniga | 12.13 a | 10.30 | 13.80 | 0.66 |
| Fuji Benishogun | 12.86 b | 10.90 | 15.80 | 1.01 |
| Ligol | 12.63 b | 10.60 | 15.50 | 0.89 |
| pH of juice | ||||
| Gala Schniga | 4.10 c | 4.13 | 4.07 | 5.50 |
| Fuji Benishogun | 4.01 b | 4.03 | 3.99 | 5.56 |
| Ligol | 3.70 a | 3.72 | 3.69 | 5.40 |
| Acidity as malic acid content % | ||||
| Gala Schniga | 0.33 a | 0.31 | 0.37 | 0.02 |
| Fuji Benishogun | 0.37 b | 0.33 | 0.43 | 0.03 |
| Ligol | 0.52 c | 0.49 | 0.54 | 0.02 |
| Variety | No Blush | <25% | 26–50% | 51–75% | >75% | 100% |
|---|---|---|---|---|---|---|
| % | ||||||
| Gala Schniga | 0 | 0 | 0 | 0 | 25 | 75 |
| Fuji Benishogun | 0 | 2 | 17 | 61 | 20 | 0 |
| Ligol | 0 | 24 | 39 | 31 | 6 | 0 |
| Variety | 6.5 cm | 7.0 cm | 7.5 cm | 8.0 cm | 8.5 cm | 9.0 cm | >9.0 cm |
|---|---|---|---|---|---|---|---|
| % | |||||||
| Gala Schniga | 41 | 46 | 8 | 5 | 0 | 0 | 0 |
| Fuji Benishogun | 0 | 12 | 53 | 24 | 11 | 0 | 0 |
| Ligol | 0 | 0 | 5 | 15 | 11 | 11 | 58 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, H.-K.; Kleiber, T.; Zydlik, Z.; Rutkowski, K.; Woźniak, A.; Świerczyński, S.; Bednarski, W.; Kęsy, J.; Marczak, Ł.; Seo, J.-H.; et al. A Comparison of Selected Biochemical and Physical Characteristics and Yielding of Fruits in Apple Cultivars (Malus domestica Borkh.). Agronomy 2020, 10, 458. https://doi.org/10.3390/agronomy10040458
Yoon H-K, Kleiber T, Zydlik Z, Rutkowski K, Woźniak A, Świerczyński S, Bednarski W, Kęsy J, Marczak Ł, Seo J-H, et al. A Comparison of Selected Biochemical and Physical Characteristics and Yielding of Fruits in Apple Cultivars (Malus domestica Borkh.). Agronomy. 2020; 10(4):458. https://doi.org/10.3390/agronomy10040458
Chicago/Turabian StyleYoon, Hong-Ki, Tomasz Kleiber, Zofia Zydlik, Krzysztof Rutkowski, Agnieszka Woźniak, Sławomir Świerczyński, Waldemar Bednarski, Jacek Kęsy, Łukasz Marczak, Jeong-Hak Seo, and et al. 2020. "A Comparison of Selected Biochemical and Physical Characteristics and Yielding of Fruits in Apple Cultivars (Malus domestica Borkh.)" Agronomy 10, no. 4: 458. https://doi.org/10.3390/agronomy10040458
APA StyleYoon, H.-K., Kleiber, T., Zydlik, Z., Rutkowski, K., Woźniak, A., Świerczyński, S., Bednarski, W., Kęsy, J., Marczak, Ł., Seo, J.-H., Choi, T.-Y., Kang, K.-J., Kafkas, N. E., Bocianowski, J., Jeandet, P., & Morkunas, I. (2020). A Comparison of Selected Biochemical and Physical Characteristics and Yielding of Fruits in Apple Cultivars (Malus domestica Borkh.). Agronomy, 10(4), 458. https://doi.org/10.3390/agronomy10040458