Influence of Exogenous Hydrogen Peroxide on Plant Physiology, Leaf Anatomy and Rubisco Gene Expression of the Ficus deltoidea Jack var. Deltoidea
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site and Plant Propagation
2.2. Treatment Application
2.3. Measurements of Growth and Physiological Parameters
2.4. Measurement of Chlorophyll Content, Chlorophyll Fluorescence and Photosynthetic Parameters
2.5. Determination of Total Sugar, Total Phenolic Content, Flavonoid Content, Antioxidant Activity and Pigment Content
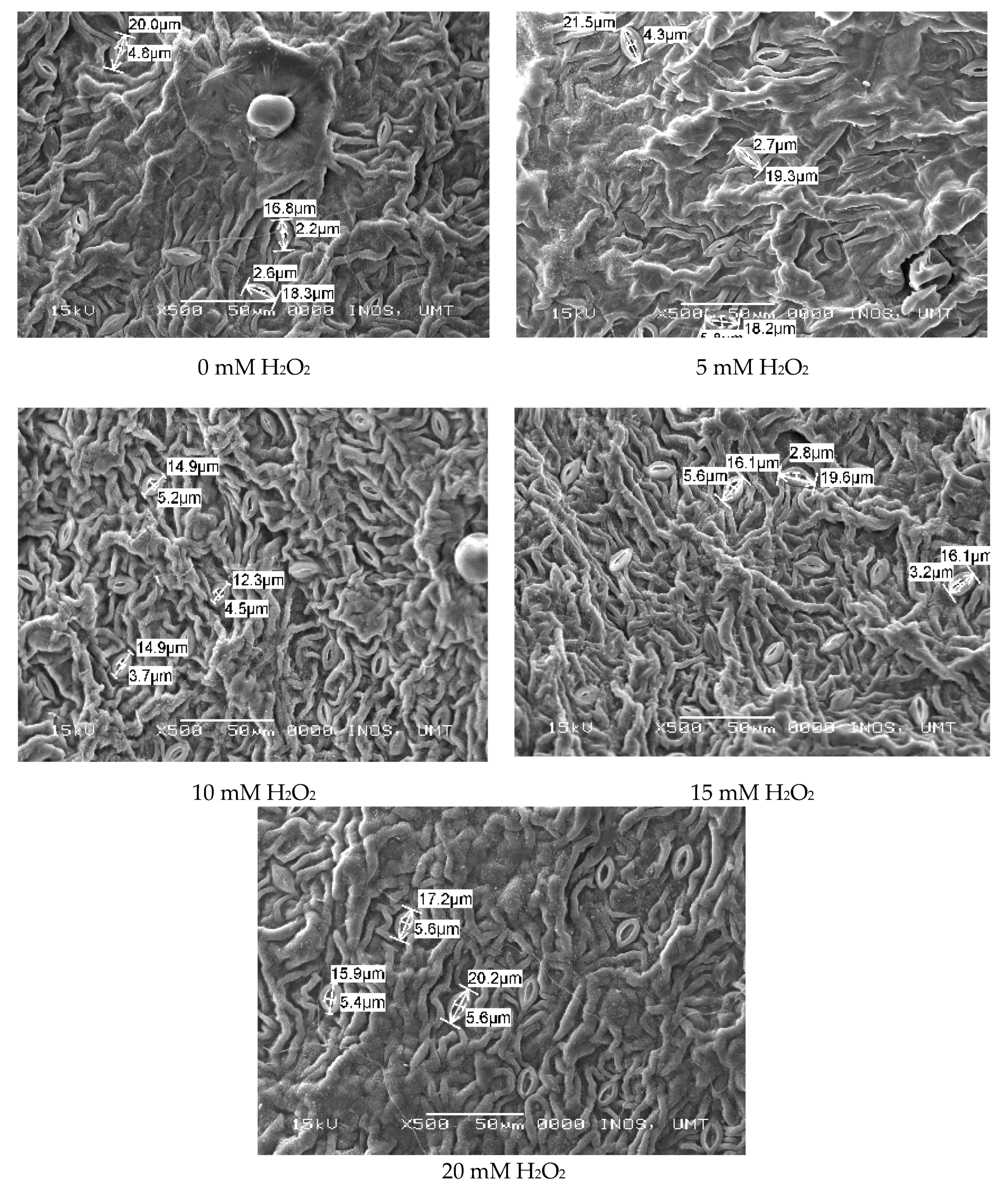
2.6. Leaf Histological Study (SEM)
2.7. RNA Extraction and Purification, Synthesis of cDNA and RT-PCR
2.8. Statistical Analysis
3. Results
3.1. The Effects of Hydrogen Peroxide (H2O2) on Growth and Photosynthesis
3.2. Regulatory Effects of Hydrogen Peroxide (H2O2) on Biochemical Properties
3.3. Regulatory Effects of Hydrogen Peroxide on Leaf Anatomy
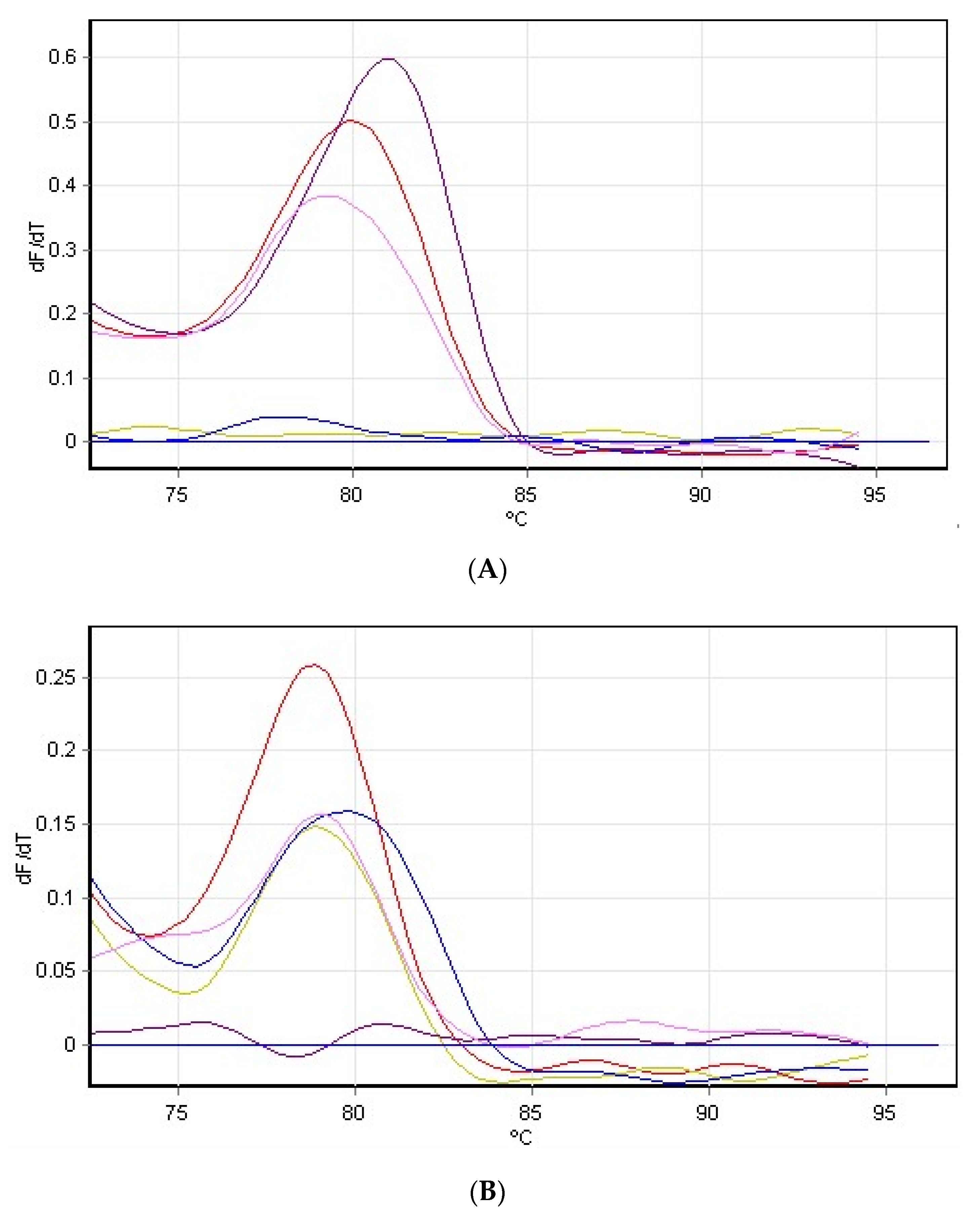
3.4. Hydrogen Peroxide on Rubisco Gene Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hasham, R.; Choi, H.K.; Sarmidi, M.R.; Park, C.S. Protective effects of a Ficus deltoidea extract against UVB-induced photoageing in skin cells. Biotechnol. Bioprocess Eng. 2013, 18, 185–193. [Google Scholar] [CrossRef]
- Fasihuddin, B.A.; Din, L.B. Medicinal Plants Used by Various Ethnic Groups in Sabah; Paper presented at the french Malaysian-symposium on natural products; Dept. of Chemistry, University of Malaya: Kuala Lumpur, Malaysia, 2002; p. 85. [Google Scholar]
- Misbah, H.; Abdul Aziz, A.; Aminudin, N. Antidiabetic and antioxidant properties of F. deltoidea fruit extracts and fractions. BMC complement. Alt. Med. 2013, 13, 118. [Google Scholar] [CrossRef] [PubMed]
- Cheeseman, J.M. Hydrogen Peroxide concentrations in leaves under natural conditions. J. Exp. Bot. 2006, 57, 2435–2444. [Google Scholar] [CrossRef]
- Kim, K.; Portis, A.R. Oxygen-dependent H2O2 production by Rubisco. FEBS Lett. 2004, 571, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Lariguet, P.; Ranocha, P.; De Meyer, M.; Barbier, O.; Penel, C.; Dunand, C. Identification of a H2O2 signalling pathway in the control of light-dependent germination in Arabidopsis. Planta 2013, 238, 381–395. [Google Scholar] [CrossRef] [PubMed]
- Khandaker, M.M.; Hossain, A.S.; Osman, N.; Boyce, A.N. Application of girdling for improved fruit retention, yield and fruit quality in Syzygium samarangense under field conditions. Int. J. Agric. Biol. 2011, 13, 18–24. [Google Scholar]
- Khandaker, M.M.; Boyce, A.N.; Osman, N. The influence of hydrogen peroxide on the growth, development and quality of wax apple (Syzygium samarangense, [Blume] Merrill & L.M. Perry var. jambu madu) fruits. Plant Physiol. Biochem. 2012, 53, 101–110. [Google Scholar] [CrossRef]
- Haq, M.; Sani, W.; Hossain, A.B.M.S.; Taha, R.M.; Monneruzzaman, K.M. Total phenolic contents, antioxidant and antimicrobial activities of Bruguiera gymnorrhiza. J. Med Plants Res. 2011, 5, 4112–4118. [Google Scholar]
- Dubois, M.; Gilles, K.; Hamilton, J.K.; Robers, P.A.; Smith, F. A colorimetric method for the determination of sugar and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Singleton, V.; Rossi, J.A.J. Colorimetry of total phenolic with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Viticult. 1965, 16, 144–158. [Google Scholar]
- Chang, C.; Yang, M.; Wen, H.; Chern, J. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar]
- Arnon, D.I. Copper enzymes in isolated chloroplasts, polyphenoxidase in beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Gowtham, H.G.; Hariprasad, P.; Brijesh, S.S.; Niranjana, S.R. Biological control of Phomopsis leaf blight of brinjal (Solanum melongena L.) with combining phylloplane and rhizosphere colonizing beneficial bacteria. Biol. Cont. 2016, 101, 123–129. [Google Scholar]
- Wang, B.; Chen, J.; Chen, L.; Wang, X.; Wang, R.; Ma, L.; Peng, S.; Luo, J.; Chen, Y. Combined drought and heat stress in Camellia oleifera cultivars: Leaf characteristics, soluble sugar and protein contents, and Rubisco gene expression. Trees 2015, 29, 1483–1492. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Anjum, S.A.; Xiao-yu, X.; Long-chang, W.; Muhammad, F.S.; Chen, M.; Wang, L. Morphological, physiological and biochemical responses of plants to drought stress. Afr. J. Agril. Res. 2011, 6, 2026–2032. [Google Scholar] [CrossRef]
- Barba-Espín, G.; Diaz-Vivancos, P.; Job, D.; Belghazi, M.; Job, C.; Hernández, J.A. Understanding the role of H2O2 during pea seed germination: A combined proteomic and hormone profiling approach. Plant Cell Environ. 2011, 34, 1907–1919. [Google Scholar] [CrossRef]
- Neill, S.; Desikan, R.; Hancock, J. Hydrogen peroxide signalling. Current Opinion in Plant Biol. 2002, 5, 388–395. [Google Scholar] [CrossRef]
- Watanabe, K.; Yachi, C.; Song, X.J.; Kakuyama, S.; Nishibe, M.; Michigami, S. Measurements of atmospheric hydroperoxides at a rural site in central Japan. J. Atmos. Chem. 2018, 75, 71–84. [Google Scholar] [CrossRef]
- Mittler, R.; Vanderauwera, S.; Gollery, M.; Breusegem, F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004, 9, 490–498. [Google Scholar] [CrossRef]
- Ozaki, K.A.U.; Tomoko, T.; Shinagawaa, F.; Yoshito, T.; Takabeb, T.; Takahisa, H.; Tasuku, H.; Ashwani, K.R. Enrichment of sugar content in melon fruits by hydrogen peroxide treatment. J. Plant Physiol. 2009, 166, 569–578. [Google Scholar] [CrossRef]
- Gil, P.M.; Ferreyra, R.E.; Barrera, C.M.; Zuniga, C.E.; Gurovich, L.R. Effects of injecting hydrogen peroxide into heavy clay loam soil on plant water status, net CO2 assimilation, biomass, and vascular anatomy of avocado trees. Chilean J. Agril. Res. 2009, 69, 97–106. [Google Scholar] [CrossRef]
- Konstantinos, P.A.; Imene, T.; Panagiotis, M.N.; Roubelakis-Angelakis, K.A. ABA-dependent amine oxidases-derived H2O2 affects stomata conductance. Plant Signal. Behavior. 2010, 5, 1153–1156. [Google Scholar] [CrossRef] [PubMed]
- Gondim, F.A.; Miranda, R.S.; Filho, E.G.; Prisco, J.T. Enhanced salt tolerance in maize plants induced by H2O2 leaf spraying is associated with improved gas exchange rather than with non-enzymatic antioxidant system. Theoret. Exp. Plant Physiol. 2013, 25, 251–260. [Google Scholar] [CrossRef]
- Butcher, J.D.; Charles, P.L.; Johannes, C.C. A study of oxygenation techniques and the chlorophyll responses of pelargonium tomentosum grown in deep water culture hydroponics. Hort. Sci. 2017, 52, 952–957. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Khan, N.A.; Masood, A.; Per, T.S.; Asgher, M. Hydrogen Peroxide Alleviates Nickel-Inhibited Photosynthetic Responses through Increase in Use-Efficiency of Nitrogen and Sulfur, and Glutathione Production in Mustard. Front. Plant Sci. 2016, 7, 44. [Google Scholar] [CrossRef] [PubMed]
- Kautsky, H.; Appel, W.; Amann, H. Chlorophyll fluorescence and carbon assimilation. Biochem. Zeit. 1960, 322, 277–292. [Google Scholar]
- Jeong, J.; Lee, I.; Kim, S.W.; Park, Y.H. Stimulation of β-carotene synthesis by hydrogen peroxide in Blakeslea trispora. Biotechnol. Lett. 1999, 21, 683–686. [Google Scholar] [CrossRef]
- Kim, H.J.; Fonseca, J.M.; Kubota, C.; Choi, J.H. Effect of hydrogen peroxide on quality of fresh-cut tomato. J. Food Sci. 2007, 72, S463–S467. [Google Scholar] [CrossRef]
- Nyathi, Y.; Baker, A. Plant peroxisomes as a source of signalling molecules. Biochim. Biophys. Acta. 2006, 1763, 1478–1495. [Google Scholar] [CrossRef]
- Uchida, A.; Jagendorf, A.T.; Hibino, T.; Takabe, T.; Takabe, T. Effects of hydrogen peroxide and nitric oxide on both salt and heat stress tolerance in rice. Plant Sci. 2002, 163, 515–523. [Google Scholar] [CrossRef]
- Zhang, X.L.; Jia, X.F.; Yu, B.; Gao, Y.; Bai, J.G. Exogenous hydrogen peroxide influences antioxidant enzyme activity and lipid peroxidation in cucumber leaves at low light. Sci. Hort. 2011, 129, 656–662. [Google Scholar] [CrossRef]
- Pei, Z.M.; Murata, Y.; Benning, G.; Thomine, S.; Klusener, B.; Allen, G.J.; Grill, E.; Schroeder, J.I. Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature 2000, 406, 731–734. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, Y.; Yamaguchi, H.; Yuasa, T.; Iwaya-Inoue, M.; Arima, S.; Zheng, S.H. Hydrogen peroxide spraying alleviates drought stress in soybean plants. J. Plant Physiol. 2011, 168, 1562–1567. [Google Scholar] [CrossRef] [PubMed]
- Doke, N.; Miura, Y.; Sanchez, L.M.; Park, H.J.; Noritake, T.; Yoshioka, H.; Kawakita, K. The oxidative burst protects plants against pathogen attack: Mechanism and role as an emergency signal for plant bio-defence. Rev. Gene 1996, 179, 45–51. [Google Scholar] [CrossRef]
- Dempsey, D.A.; Klessig, D.F. Signals in plant disease resistance. Bull. de l’Inst. Pasteur 1995, 93, 167–186. [Google Scholar] [CrossRef]
- Bradley, D.J.; Kjellbom, P.; Lamb, C.J. Elicitor-and wound-induced oxidative cross-linking of a proline-rich plant cell wall protein: A novel, rapid defense response. Cell 1992, 70, 21–30. [Google Scholar] [CrossRef]
- Orozco-Cárdenas, M.L.; Ryan, C. Hydrogen peroxide is generated systemically in plant leaves by wounding and systemin via the octadecanoid pathway. Proc. Nat. Acad. Sci. USA 1999, 96, 6553–6557. [Google Scholar] [CrossRef]
- Jafariyan, T.; Mohammad, J.Z. Hydrogen peroxide affects plant growth promoting effects of Azospirillum. J. Crop Sci. Biotechnol. 2016, 19, 167–175. [Google Scholar] [CrossRef]
- Thordal-Christensen, H.; Shang, Z.; Wei, Y.; Collinge, D.B. Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley powdery mildew interaction. Plant J. 1997, 11, 1187–1194. [Google Scholar] [CrossRef]
- Kasai, M.; Koide, K.; Ichikawa, Y. Effect of Pot Size on Various Characteristics Related to Photosynthetic Matter Production in Soybean. Plants. Int. J. Agron 2012, 2012, 1–7. [Google Scholar] [CrossRef]
- Cui, K.; Xing, G.; Liu, X.; Wang, Y.; Kairong, C.; Gengsheng, X.; Yafu, W. Effect of hydrogen peroxide on somatic embryogenesis of Lycium barbarum L. Plant Sci. 1999, 146, 9–16. [Google Scholar] [CrossRef]
- Chen, M.P.; Yang, S.H.; Chou, C.H.; Yang, K.C.; Wu, C.C.; Cheng, Y.H.; Lin, F.H. The chondroprotective effects of ferulic acid on hydrogen peroxide-stimulated chondrocytes: Inhibition of hydrogen peroxide-induced pro-inflammatory cytokines and metalloproteinase gene expression at the mRNA level. Inflam. Res. Off. J. Eur. His. Res. Soc. 2010, 59, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wakao, S.; Fischer, B.B.; Niyogi, K.K. Sensing and responding to excess light. Ann. Rev. Plant Biol. 2009, 60, 239–260. [Google Scholar] [CrossRef]







| H2O2 (mM) | Number of Leaves | Plant Height (cm) | Leaf Area (cm2) | Photo. Rate (µmol CO2 m−2 s−1) | Transp. Rate (mmolm−2 s−1) | Stomat. cond. (mol H2O m−2 s−1) |
|---|---|---|---|---|---|---|
| 0 | 500 c | 60 b | 6.70 a | 2.38 c | 0.30 a | 16.38 c |
| 5 | 528 b | 63 b | 6.80 a | 2.52 b | 0.28 a | 21.03 b |
| 10 | 540 b | 63 b | 7.25 a | 2.64 b | 0.27 a | 29.08 b |
| 15 | 570 b | 65 a | 7.30 a | 2.67 b | 0.25 a | 34.09 a |
| 20 | 600 a | 67 a | 7.40 a | 2.86 a | 0.25 a | 37.20 a |
| H2O2 (mM) | Int CO2 Conc (µmol mol−1) | Chloro Cont. (SPAD) | Lower Fluo. (F0) | Higher Fluo. (Fm) | Relative Fluo. (Fv) | Quantum Yield (Fv/FM) |
|---|---|---|---|---|---|---|
| 0 | 612 c | 52.90 a | 710 a | 3604 a | 2894 b | 0.80 a |
| 5 | 620 b | 54.70 a | 715 a | 3623 a | 2908 a | 0.80 a |
| 10 | 650 a | 56.50 a | 730 a | 3754 a | 3024 a | 0.81 a |
| 15 | 658 a | 57.85 a | 728 a | 3793 a | 3065 a | 0.81 a |
| 20 | 678 a | 58.82 a | 735 a | 3809 a | 3074 a | 0.81 a |
| H2O2 (mM) | Chlorophyll a (mg/L FE) | Chlorophyll b (mg/L FE) | Carotenoid (µg/g FE) | TPC (mg GAE/g DE) | TFC (mg QE/g DE) | TSC (GE µmol/g DE) | ABTS (mmol Trolox/g DE) |
|---|---|---|---|---|---|---|---|
| 0 | 11.54 a | 4.61a | 1.66 a | 132 a | 14.1 a | 1.62 a | 3.08 a |
| 5 | 13.81 b | 6.15 b | 1.72 b | 136 a | 15.21 a | 2.72 b | 3.74 a |
| 10 | 14.75 c | 6.93 bc | 1.71 b | 157 b | 17.36 b | 2.84 c | 3.74 a |
| 15 | 18.10 d | 7.33 c | 2.03 c | 166 bc | 20.08 c | 2.83 c | 3.90 a |
| 20 | 18.61 e | 7.72 c | 2.13 c | 168 c | 25.21 d | 3.07 d | 3.94 a |
| H2O2 | Pfaffl | 2−∆∆CT |
|---|---|---|
| Control | 0.0 | 0.0 |
| 15 mM | 4.796 ± 0.23 | 0.11582 ± 0.3 |
| 20 mM | 0.576 ± 0.4 | 0.534 ± 0.27 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jamaludin, R.; Mat, N.; Suryati Mohd, K.; Afiza Badaluddin, N.; Mahmud, K.; Hailmi Sajili, M.; Khandaker, M.M. Influence of Exogenous Hydrogen Peroxide on Plant Physiology, Leaf Anatomy and Rubisco Gene Expression of the Ficus deltoidea Jack var. Deltoidea. Agronomy 2020, 10, 497. https://doi.org/10.3390/agronomy10040497
Jamaludin R, Mat N, Suryati Mohd K, Afiza Badaluddin N, Mahmud K, Hailmi Sajili M, Khandaker MM. Influence of Exogenous Hydrogen Peroxide on Plant Physiology, Leaf Anatomy and Rubisco Gene Expression of the Ficus deltoidea Jack var. Deltoidea. Agronomy. 2020; 10(4):497. https://doi.org/10.3390/agronomy10040497
Chicago/Turabian StyleJamaludin, Rosnah, Nashriyah Mat, Khamsah Suryati Mohd, Noor Afiza Badaluddin, Khairil Mahmud, Mohammad Hailmi Sajili, and Mohammad Moneruzzaman Khandaker. 2020. "Influence of Exogenous Hydrogen Peroxide on Plant Physiology, Leaf Anatomy and Rubisco Gene Expression of the Ficus deltoidea Jack var. Deltoidea" Agronomy 10, no. 4: 497. https://doi.org/10.3390/agronomy10040497
APA StyleJamaludin, R., Mat, N., Suryati Mohd, K., Afiza Badaluddin, N., Mahmud, K., Hailmi Sajili, M., & Khandaker, M. M. (2020). Influence of Exogenous Hydrogen Peroxide on Plant Physiology, Leaf Anatomy and Rubisco Gene Expression of the Ficus deltoidea Jack var. Deltoidea. Agronomy, 10(4), 497. https://doi.org/10.3390/agronomy10040497





