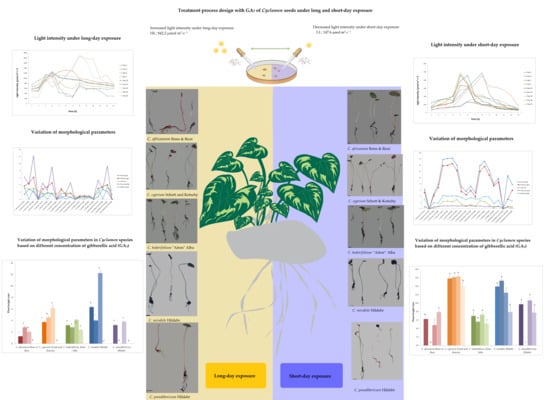
Gibberellic Acid Can Improve Seed Germination and Ornamental Quality of Selected Cyclamen Species Grown Under Short and Long Days
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Experiment Design and GA3 Application on Selected Cyclamen Species
2.3. Effect of Exogenous GA3 on Morphological and Physiological Traits Under Different Light Exposure Treatments
2.4. Statistical Analysis
3. Results
3.1. Variation in Germination Parameters with Gibberellic Acid (GA3) Application Under Long-Day Exposure
3.2. Variation in Plant Growth and Morpho-Agronomic Parameters of Species Under Long-Day exposure
3.3. Variation in Germination Parameters with Gibberellic Acid (GA3) Application and Short-Day Exposure
3.4. Variation in Plant Growth and Morpho-Agronomic Parameters of Short-Day Exposed Cyclamen Species
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Grey-Wilson, C. Cyclamen (A Guide for Gardeners, Horticulturists and Botanists); B.T. Bastsford Publishers: London, UK, 2015; pp. 182–197. ISBN 9781849942218. [Google Scholar]
- Schweizer, F.; Hasinger, O. Cyclamen purpurascens. The IUCN Red List of Threatened Species 2014: e.T196750A2475951. Available online: https://www.iucnredlist.org/species/196750/2475951 (accessed on 31 January 2020). [CrossRef]
- Moser, D.M.; Gygax, A.; Bäumler, B.; Wyler, N.; Palese, R. Liste Rouges des Espèces Menacées de Suisse. Fougères et Plantes à Fleurs. Berne et Genève, Office fédéral de L’environnement, des Forêts et du Paysage (OFEFP); Centre du Réseau Suisse de Floristique (CRSF) et Conservatoire et Jardin Botaniques de Genève: Chambésy, Switzerland, 2002; p. 120. [Google Scholar]
- Kathe, W.; Honnef, S.; Heym, A. Medicinal and Aromatic Plants in Albania, Bosnia-Herzegovina, Bulgaria, Croatia and Romania; Bundesamt für Naturschutz BfN Skripten: Bonn, Germany, 2003; p. 91. [Google Scholar]
- Lee, J.W.; Kim, Y.C.; Kim, J.U.; Jo, I.H.; Kim, K.H.; Kim, D.H. Effects of gibberellic acid and alternating temperature on breaking seed dormancy of Panax ginseng CA Meyer. Korean J. Med. Crop Sci. 2016, 4, 284–293. [Google Scholar] [CrossRef]
- Urbanova, T.; Leubner-Metzger, G. Gibberellins and seed germination. Annu. Plant Rev. 2018, 49, 253–284. [Google Scholar]
- Oh, W.; Kim, K.S. Light intensity and temperature regulate petiole elongation by controlling the content of and sensitivity to gibberellin in Cyclamen persicum. Hortic. Environ. Biotechnol. 2014, 55, 175–182. [Google Scholar] [CrossRef]
- Oh, W.; Kim, J.; Kim, Y.H.; Lee, I.J.; Kim, K.S. Shoot elongation and gibberellin contents in Cyclamen persicum are influenced by temperature and light intensity. Hortic. Environ. Biotechnol. 2015, 56, 762–768. [Google Scholar] [CrossRef]
- Alshakhaly, Z.M.; Qrunfleh, M.M. Effect of plant growth regulators on flower development and quality of five Cyclamen persicum hybrids. In Proceedings of the XXX International Horticultural Congress IHC2018: International Symposium on Ornamental Horticulture and XI International, Istanbul, Turkey, 12–16 August 2018; Volume 1263, pp. 215–222. [Google Scholar]
- Dong, B.; Deng, Y.; Wang, H.; Gao, R.; Stephen, G.U.; Chen, S.; Chen, F. Gibberellic acid signaling is required to induce flowering of chrysanthemums grown under both short and long days. Int. J. Mol. Sci. 2017, 18, 1259. [Google Scholar] [CrossRef] [Green Version]
- Hisamatsu, T.; Koshioka, M.; Kubota, S.; Fujime, Y.; King, R.W.; Mander, L.N. The role of gibberellin biosynthesis in the control of growth and flowering in Matthiola incana. Physiol. Plant. 2000, 109, 97–105. [Google Scholar] [CrossRef]
- Mutasa-Göttgens, E.; Hedden, P. Gibberellin as a factor in floral regulatory networks. J. Exp. Bot. 2009, 60, 1979–1989. [Google Scholar] [CrossRef] [Green Version]
- Wilkie, J.D.; Sedgley, M.; Olesen, T. Regulation of floral initiation in horticultural trees. J. Exp. Bot. 2008, 59, 3215–3228. [Google Scholar] [CrossRef] [Green Version]
- Wilson, R.N.; Heckman, J.W.; Somerville, C.R. Gibberellin is required for flowering in Arabidopsis thaliana under short days. Plant Physiol. 1992, 100, 403–408. [Google Scholar] [CrossRef] [Green Version]
- Wahyuni, S.; Krisantini, S.; Johnston, M.E. Plant growth regulators and flowering of Brunonia and Calandrinia sp. Sci. Hortic. 2011, 128, 141–145. [Google Scholar] [CrossRef]
- Sumanasiri, H.; Krishnarajah, S.A.; Eeswara, J.P. Effect of gibberellic acid on growth and flowering of Henckelia humboldtianus Gardner (Ceylon Rock Primrose). Sci. Hortic. 2013, 159, 29–32. [Google Scholar] [CrossRef]
- Al-Khassawneh, N.M.; Karam, N.S.; Shibli, R.A. Growth and flowering of black iris (Iris nigricans Dinsm.) following treatment with plant growth regulators. Sci. Hortic. 2006, 107, 187–193. [Google Scholar] [CrossRef]
- Bergmann, B.A.; Dole, J.M.; McCall, I. Gibberellic acid shows promise for promoting flower stem length in four field-grown cut flowers. HortTechnology 2016, 26, 287–292. [Google Scholar] [CrossRef] [Green Version]
- Toscano, S.; Trivellini, A.; Ferrante, A.; Romano, D. Physiological mechanisms for delaying the leaf yellowing of potted geranium plants. Sci. Hortic.-Amst. 2018, 242, 146–154. [Google Scholar] [CrossRef]
- Skene, K.G.M.; Lang, A. Native Gibberellins+ Flower Formation in Bryophyllum daigremontianum. Plant Physiol. 1964, 39, 37. [Google Scholar]
- Zeevaart, J.A.D. Changes in the gibberellin content of Bryophyllum daigremontianum in connection with floral induction. Neth. J. Agric. Sci. 1969, 17, 215–220. [Google Scholar]
- Huang, C.H. Studies on Flowering Physiology, Interspecies Hybridization and ISSR Analysis of Kalanchoe Species Native in Taiwan. Ph.D. Thesis, National Chung Hsing University, Taichung, Taiwan, 2007. [Google Scholar]
- The Cyclamen Society. Available online: https://www.cyclamen.org (accessed on 16 August 2019).
- Al-Ansari, F.; Ksiksi, T. A Quantitative Assessment of Germination Parameters: The Case of Crotalaria Persica and Tephrosia apollinea. Open Ecol. J. 2016, 9, 13–21. [Google Scholar] [CrossRef] [Green Version]
- Kader, M.A. A comparison of seed germination calculation formulae and the associated interpretation of resulting data. J. Proc. R. Soc. N. S. W. 2005, 138, 65–75. [Google Scholar]
- Ellis, R.H.; Roberts, E.H. Towards a rational basis for testing seed quality. In Seed Production; Hebblethwaite, P.D., Ed.; Butterworths: London, UK, 1980; pp. 605–635. [Google Scholar]
- Abdul-Baki, A.A.; Anderson, J.D. Relationship between Decarboxylation of Glutamic Acid and Vigor in Soybean Seed 1. Crop Sci. 1973, 13, 227–232. [Google Scholar] [CrossRef]
- Al-Mudaris, M.A. Notes on various parameters recording the speed of seed germination. Der Tropenlandwirt-J. Agric. Trop. Subtrop. 1998, 99, 147–154. [Google Scholar]
- Ranal, M.A.; Santana, D.G.D. How and why to measure the germination process? Braz. J. Bot. 2006, 29, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Janmohammadi, M.; Sabaghnia, N. Effect of pre-sowing seed treatments with silicon nanoparticles on germinability of sunflower (Helianthus annuus). Bot. Lith. 2015, 21, 13–21. [Google Scholar] [CrossRef] [Green Version]
- Yasmeen, A.; Basra, S.M.A.; Wahid, A.; Nouman, W.; Rehman, H.U. Exploring the potential of Moringa oleifera leaf extract (MLE) as a seed priming agent in improving wheat performance. Turk. J. Bot. 2013, 37, 512–520. [Google Scholar]
- Kumar, S.; Malik, T.P.; Mor, V.S.; Kumar, P. Effect of gibberellic acid on seed quality of coriander (Coriandrum sativum L.). J. Pharmacogn. Phytochem. 2018, 7, 830–832. [Google Scholar]
- Zulfiqar, F.; Younis, A.; Abideen, Z.; Francini, A.; Ferrante, A. Bioregulators Can Improve Biomass Production, Photosynthetic Efficiency, and Ornamental Quality of Gazania rigens L. Agronomy 2019, 9, 773. [Google Scholar] [CrossRef] [Green Version]
- Hanyan, X.; Du Hongmei, H.D. Effects of GA_3 on the Seed Germination of Cyclamen Persicum. Seed 2006, 4, 62. [Google Scholar]
- Sgamma, T. Juvenility, 2nd ed.; Thomas, B., Murphy, D.J., Murray, B.G., Eds.; Encyclopedia of Applied Plant Sciences; Academic Press: Cambridge, MA, USA, 2017; Volume 1, pp. 437–441. [Google Scholar]
- Barratt, N.M.; Davies, P.J. Developmental changes in the gibberellin-induced growth response in stem segments of light-grown pea genotypes. Plant Growth Regul. 1997, 21, 127–134. [Google Scholar] [CrossRef]
- Sansberro, P.A.; Mroginski, L.A.; Masciarelli, O.A.; Bottini, R. Shoot growth in Ilex paraguariensis plants grown under varying photosynthetically active radiation is affected through gibberellin levels. Plant Growth Regul. 2002, 38, 231–236. [Google Scholar] [CrossRef]
- Metzger, J.D. Gibberellin and light regulated petiole growth in Thlaspi arvense L. Plant Physiol. 1988, 86, 237–240. [Google Scholar] [CrossRef] [Green Version]
- Pinthus, M.J.; Abraham, M. Effects of light, temperature, gibberellin (GA3) and their interaction on coleoptile and leaf elongation of tall, semi-dwarf and dwarf wheat. Plant Growth Regul. 1996, 18, 239–247. [Google Scholar] [CrossRef]
- Song, Y.H.; Ito, S.; Imaizumi, T. Flowering time regulation: Photoperiod-and temperature-sensing in leaves. Trends Plant Sci. 2013, 18, 575–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shim, J.S.; Imaizumi, T. Circadian clock and photoperiodic response in Arabidopsis: From seasonal flowering to redox homeostasis. Biochemistry 2014, 54, 157–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asil, M.H.; Roein, Z.; Abbasi, J. Response of tuberose (Polianthes tuberose L.) to gibberellic acid and benzyladenine. Hortic. Environ. Biotechnol. 2011, 52, 46. [Google Scholar] [CrossRef]
- Malik, S.A.; Neelofar, Z.A.; Nazki, I.T.; Mir, S.A.; Khan, F.A.; Pukhta, M.S. Effect of Gibberellic acid, spacing and nutrient sprays on growth and flowering in snapdragon (Antirrhinum majus L.) cv. Rocket Pink. Int. J. Plant Soil Sci. 2011, 28, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Alcalá-Rico, J.S.G.J.; López-Benítez, A.; Vázquez-Badillo, M.E.; Sánchez-Aspeytia, D.; Rodríguez-Herrera, S.A.; Pérez-Rodríguez, M.Á.; Ramírez-Godina, F. Seed Physiological Potential of Capsicum annuum var. glabriusculum Genotypes and Their Answers to Pre-Germination Treatments. Agronomy 2019, 9, 325. [Google Scholar] [CrossRef] [Green Version]
- Fagge, A.A.; Manga, A.A. Effect of Sowing Media and Gibberellic Acid on the Growth and Seedling Establishment of Bougainvillea glabra, Ixora coccinea and Rosa chinensis. 2. Root Characters. Bayero J. Pure Appl. Sci. 2011, 4, 155–159. [Google Scholar] [CrossRef]
- Punetha, P.; Rawat, T.; Bohra, M.; Trivedi, H. Effects of various concentrations of GA3 and NAA on cuttings of hydrangea under shade net conditions. J. Hill Agric. 2018, 9, 260–264. [Google Scholar] [CrossRef]
- Arteca, R.N.; Schlagnhaufer, C.D.; Arteca, J.M. Root applications of gibberellic acid enhance growth of seven Pelargonium cultivars. HortScience 1991, 26, 555–556. [Google Scholar] [CrossRef] [Green Version]
- Ravnjak, B.; Bavcon, J.; Osterc, G. Physiological response of local populations of species Cyclamen purpurascens Mill. to forest gaps. Appl. Ecol. Environ. Res. 2019, 17, 11489–11508. [Google Scholar] [CrossRef]
- Osterc, G.; Petkovsek, M.M.; Stampar, F.; Ravnjak, B.; Bavcon, J. Impact of specific environmental characteristics of the site of origin (shady, sunny) on anthocyanin and flavonol contents of replanted plants at common cyclamen (Cyclamen purpurascens Mill.). Acta Physiol. Plant 2017, 39, 64. [Google Scholar] [CrossRef]
- Huang, J.; Zhao, X.; Chory, J. The Arabidopsis Transcriptome Responds Specifically and Dynamically to High Light Stress. Cell Rep. 2019, 29, 4186–4199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, W.; Kim, K.S. Temperature and light intensity induce morphological and anatomical changes of leaf petiole and lamina in Cyclamen persicum. Hortic. Environ. Biotechnol. 2010, 51, 494–500. [Google Scholar]
- Oh, W.; Kim, K.S. Developmental stage and temperature influence elongation response of petiole to low irradiance in Cyclamen persicum. Korean J. Hortic. Sci. 2010, 28, 719–727. [Google Scholar]
- Cheon, I.H.; Oh, W.; Park, J.H.; Kim, K.S. Long day and high photosynthetic photon flux promote the growth and flowering of Cyclamen persicum. Hortic. Environ. Biotechnol. 2006, 47, 353–358. [Google Scholar]
- Aparna, V.; Prakash, K.; Neema, M.; Ajay, A.; Kumar, N.; Singh, M.C. Effect of Gibberellic Acid on plant growth and flowering of Chrysanthemum cv. Thai Chen Queen under short day planting conditions. Int. J. Agric. Sci. 2018, 10, 6274–6278. [Google Scholar]
- Simsek, O.; Curuk, P.; Aslan, F.; Bayramoglu, M.; Izgu, T.; da Silva, J.A.T.; Mendi, Y.Y. Molecular characterization of Cyclamen species collected from different parts of Turkey by RAPD and SRAP markers. Biochem. Genet. 2017, 55, 87–102. [Google Scholar] [CrossRef]
- Thompson, J.D.; Gauthier, P.; Papuga, G.; Pons, V.; Debussche, M.; Farris, E. The conservation significance of natural hybridisation in Mediterranean plants: From a case study on Cyclamen (Primulaceae) to a general perspective. Plant Biol. 2018, 20, 128–138. [Google Scholar] [CrossRef] [Green Version]
- Yesson, C.; Culham, A.A. Phyloclimatic study of Cyclamen. BMC Evol. Biol. 2006, 6, 72. [Google Scholar] [CrossRef] [Green Version]
- Curuk, P.; Sogut, Z.; Bozdogan, E.; Izgu, T.; Sevindik, B.; Tagipur, E.M.; Mendi, Y.Y. Morphological characterization of Cyclamen sp. grown naturally in Turkey: Part I. S. Afr. J. Bot. 2015, 100, 7–15. [Google Scholar] [CrossRef]
- Curuk, P.; Sogut, Z.; Izgu, T.; Sevindik, B.; Tagipur, E.M.; da Silva, J.A.T.; Mendi, N.Y. Morphological characterization of Cyclamen sp. grown naturally in Turkey: Part II. Acta Sci. Pol. Hortorum Cultus 2016, 15, 205–224. [Google Scholar] [CrossRef]









| Parameter | Genotype | GA3 Concentrations (mg/L) | |||
|---|---|---|---|---|---|
| 0 | 50 | 100 | 150 | ||
| Germination percentage (GP%) | C. africanum (African cyclamen) | 33.33 ± 0.76 c | 73.33 ± 0.8 a | 40.00 ± 1.0 b | 0.0 ± 0.0 d |
| C. cyprium (Cyprus cyclamen) | 53.33 ± 0.8 a | 40.00 ± 0.9 b | 53.33 ± 0.7 ac | 0.0 ± 0.0 c | |
| C. hederifolium ‘Alba’ (ivy-leaved cyclamen) | 80.00 ± 0.7 b | 93.33 ± 0.8 a | 93.33 ± 0.76a | 53.33 ± 0.8 c | |
| C. mirabile (sowbread) | 73.33 ± 0.8 a | 53.33 ± 0.7 b | 73.33 ± 0.8 a | 0.00 ± 0.0 c | |
| C. pseudibericum Hildebr (false Iberian cyclamen) | 0.00 ± 0.0 b | 33.33 ± 0.76 a | 0.00 ± 0.0 b | 33.33 ± 0.8 a | |
| Mean germination time–MGT (days) | C. africanum (African cyclamen) | 33.62 ± 1.2 c | 23.90 ± 0.7 b | 42.72 ± 1.5 d | 0.00 ± 0.0 a |
| C. cyprium (Cyprus cyclamen) | 31.22 ± 0.8 c | 47.50 ± 1.0 d | 32.70 ± 0.9 b | 0.00 ± 0.0 a | |
| C. hederifolium ‘Alba’ (ivy-leaved cyclamen) | 49.22 ± 1.3 d | 29.36 ± 0.9 b | 19.57 ± 0.4 a | 32.07 ± 0.6 c | |
| C. mirabile (sowbread) | 73.59 ± 2.8 b | 55.64 ± 1.4 d | 43.32 ± 0.4 c | 0.00 ± 0.0 a | |
| C. pseudibericum (false Iberian cyclamen) | 37.51 ± 1.9 b | 0.00 ± 0.0a | 53.9 ± 1.8 c | 0.00 ± 0.0 a | |
| Seedling vigor index I (SVI) | C. africanum (African cyclamen) | 20.0 ± 2.6 b | 185.5 ± 2.7 d | 82.6 ± 4.2 c | 0.0 ± 0.0 a |
| C. cyprium (Cyprus cyclamen) | 190.8 ± 3.2 b | 223.8 ± 3.2 c | 346.4 ± 4.0 d | 0.0 ± 0.0 a | |
| C. hederifolium ‘Alba’ (ivy-leaved cyclamen) | 114.8 ± 2.3 b | 328.2 ± 3.2 d | 312.4 ± 2.9 c | 110.4 ± 2.6 a | |
| C. mirabile (sowbread) | 461.9 ± 3.0 c | 187.3 ± 3.7 b | 812.4 ± 2.5 d | 0.0 ± 0.0 a | |
| C. pseudibericum (false Iberian cyclamen) | 63.6 ± 2.2 b | 0.0 ± 0.0 a | 100.4 ± 1.9 c | 0.0 ± 0.0 a | |
| Parameter | Genotype | GA3 Concentrations (mg/L) | |||
|---|---|---|---|---|---|
| 0 GA3 | 50 GA3 | 100 GA3 | 150 GA3 | ||
| Germination percentage (GP%) | C. africanum | 33.33 ± 0.75 b | 0.00 ± 0.0 c | 73.33 ± 0.7 a | 33.33 ± 0.8 b |
| C. cyprium | 33.33 ± 0.8 b | 73.33 ± 1.0 a | 73.33 ± 0.76 a | 73.33 ± 1.0 a | |
| C. hederifolium “‘Alba’ | 33.33 ± 1.2 d | 93.33 ± 0.7 a | 73.33 ± 0.8 b | 60.00 ± 1.1 c | |
| C. mirabile | 93.33 ± 0.7 a | 93.33 ± 0.8 a | 53.33 ± 0.76 a | 53.33 ± 0.8 a | |
| C. pseudibericum | 53.33 ± 0.75 a | 0.00 ± 0.0 b | 53.33 ± 0.8 a | 0.00 ± 0.0 b | |
| Mean germination time–MGT (days) | C. africanum | 64.3 ± 0.4 b | 0.0 ± 0.0 d | 13.9 ± 0.6 a | 37.6 ± 1.9 c |
| C. cyprium | 34.4 ± 0.7 d | 74.4 ± 1.4 a | 33.2 ± 0.9 c | 47.7 ± 1.2 b | |
| C. hederifolium ‘Alba’ | 41.3 ± 0.8 a | 26.1 ± 0.4 c | 30.5 ± 0.7 b | 30.7 ± 0.4 b | |
| C. mirabile | 28.3 ± 0.7 c | 50.8 ± 0.9 a | 38.6 ± 0.4 b | 51.7 ± 0.6 a | |
| C. pseudibericum | 35.4 ± 1.9 a | 0.0 ± 0.0 c | 23.2 ± 1.4 b | 23.9 ± 1.4 b | |
| Seedling vigor index I (SVI) | C. africanum | 206.4 ± 1.5 a | 0.0 ± 0.0 d | 178.0 ± 3.4 c | 134.4 ± 2.2 b |
| C. cyprium | 1123.8 ± 0.6 a | 417.8 ± 0.5 d | 611.6 ± 0.4 b | 517.9 ± 0.8 c | |
| C. hederifolium ‘Alba’ | 143.5 ± 0.8 d | 304.8 ± 0.1 a | 234.9 ± 0.4 b | 198.2 ± 0.3 c | |
| C. mirabile | 640.9 ± 0.3 a | 577.0 ± 0.5 b | 456.8 ± 0.7 c | 245.3 ± 1.2 d | |
| C. pseudibericum | 176.3 ± 0.5 b | 0.0 ± 0.0 d | 248.4 ± 0.0 a | 75.2 ± 1.2 c | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cornea-Cipcigan, M.; Pamfil, D.; Sisea, C.R.; Mărgăoan, R. Gibberellic Acid Can Improve Seed Germination and Ornamental Quality of Selected Cyclamen Species Grown Under Short and Long Days. Agronomy 2020, 10, 516. https://doi.org/10.3390/agronomy10040516
Cornea-Cipcigan M, Pamfil D, Sisea CR, Mărgăoan R. Gibberellic Acid Can Improve Seed Germination and Ornamental Quality of Selected Cyclamen Species Grown Under Short and Long Days. Agronomy. 2020; 10(4):516. https://doi.org/10.3390/agronomy10040516
Chicago/Turabian StyleCornea-Cipcigan, Mihaiela, Doru Pamfil, Cristian Radu Sisea, and Rodica Mărgăoan. 2020. "Gibberellic Acid Can Improve Seed Germination and Ornamental Quality of Selected Cyclamen Species Grown Under Short and Long Days" Agronomy 10, no. 4: 516. https://doi.org/10.3390/agronomy10040516
APA StyleCornea-Cipcigan, M., Pamfil, D., Sisea, C. R., & Mărgăoan, R. (2020). Gibberellic Acid Can Improve Seed Germination and Ornamental Quality of Selected Cyclamen Species Grown Under Short and Long Days. Agronomy, 10(4), 516. https://doi.org/10.3390/agronomy10040516