Abstract
Biochars have long been associated with elevating plant productivity. An increasing number of studies, however, report that char application might also impair plant nutrient availability and reduce yields. In particular, char accompanying compounds as well as a hypothesized immobilization of nitrogen have been identified as playing a significant role in possibly diminishing plant productivity following char application. Herein, we tested the fertilizing effects of modified biochars in order to derive knowledge required to develop tailor-made chars, which predictably affect plant nutrition. Slow-pyrolysis maize cob biochar was modified by washing with either ethanol or hydrochloric acid to remove ash and organic compounds or by loading it with nutrient-rich residues in the form of digestate from the bioenergy sector. Maize plants were grown for 35 days on biochar-amended sand. We analyzed both substrate properties (pH, total carbon, and nitrogen, available magnesium and potassium) and plant functional traits (biomass, leaf area, root to shoot ratio, specific leaf area). Our results suggest that total plant biomass production remained unaffected by the application of biochar and its washed forms. Contrastingly, nutrient-loaded biochar induced a significant increase in productivity at similar nutrient levels due to improved plant nutrient uptake. Further research is required to understand the role of biochar modifications that facilitated improvements in plant productivity.
1. Introduction
Biochar is the porous, poly-aromatic product of an incomplete thermochemical conversion of organic biomass, which is increasingly used as a soil amendment [1] to increase crop yields [2,3]. However, depending on the chars’ physical and chemical properties their impact on plant productivity remains largely unpredictable due to complex interactions between soil and environment [4,5,6]. As described elsewhere, the application of biochar had a positive effect on crop productivity, increasing it by 10% on average [7]. Nevertheless, recently published studies indicated that above-ground productivity of plants in biochar amended soils varies due to accompanying organic and inorganic compounds that might affect germination and plant nutrition negatively [6,8,9,10]. As a result, tailor-made modified chars have been introduced in order to predictably improve plant performance [11,12].
Modification techniques include (i) chemical (e.g., amination, coatings, loadings), (ii) physical (e.g., steam activation), and (iii) biological (e.g., composting) modifications to increase the biochars’ sorption capacity, modify porosity, load them with nutrients or remove superfluous compounds [13,14,15,16,17]. Previously, chemical pre-treatments, including washing with HCl, NaOH, deionized water and ethanol significantly modified the surface and chemical characteristics of biochar [18]. While simpler approaches such as water leaching resulted in only small improvements in removing organic matter from biochar [19], more elaborate approaches such as washing with acidic solutions or ethanol might show promise in the future. Already, organic contaminants in biochar such as phenols, benzene, toluene, ethylbenzene, and xylenes have been successfully removed by leaching with organic solvents such as dichloromethane and methanol [20,21]. Ethanol, as an organic solvent, could also be used to dissolve organic compounds in biochar. Similarly, HCl has been used previously to remove acid-soluble inorganic components, including ashes [22]. Acid modification of biochars also increased the water solubility of plant nutrients and plant availability of the nutrients in the soil [23].
Notwithstanding pre-treatment, plant nutrient availability in the soil or lack thereof might be the reason why the overall positive effect of biochar application is generally low, as pure biochar application does not directly enrich the soil with nutrients but elevates its C/N ratio [7]. Hence, biological pre-treatments, such as mixing biochar with nutrient-rich organic material (e.g., digestate) prior to application to the soil might be an important strategy in (i) providing missing nutrients, and (ii) overcoming a biochar-induced immobilization of plant-available nitrogen—especially in N-limited systems [24].
Digestate is the residual product of feedstock degradation by anaerobic bacteria during biogas production [25,26]. (Re-)using it in concert with biochars offers the unique chance to recycle and dispose of this residue while closing local nutrient loops, by reusing waste material (i.e. maize-based digestate) in fertilizing future feedstock (i.e. maize plants). Its chemical characteristics vary with feedstock characteristics and digester conditions. Digestates can improve soil quality through increases in hydraulic conductivity and water retention and decreases in bulk density. The sludge can be separated into a solid phase, which is typically high in organic carbon, phosphorus, total nitrogen, and organic nitrogen and a liquid phase, which serves as a source for plant-available nitrogen, mostly ammonium-N [27]. The positive effects of pure maize-based digestate application on the nutrient content in a marginal sandy substrate and plant growth are well studied [28,29], whereas we lack important knowledge of its interactions with biochar. In fact, in an earlier study under the same experimental conditions (identical substrate and digestate), maize-based biogas digestate was applied in varying amounts to the sandy substrate to evaluate its effect on maize growth [30]. Results of this study suggested that digestate application has positive fertilization effects in low-fertility substrates, similar to mineral fertilizer, even though digestate application may have a negative impact on the permeability in sandy substrates that could interfere with germination. This drawback could be overcome by incorporating nutrient-loaded biochars, increasing the porosity and permeability of the substrate. In general, digestates of various feedstocks can be applied as valuable fertilizers for crop production, particularly when directly incorporated into the soil [25,30].
In this study we tested the effects of biochar and its modified forms on the performance of maize (Zea mays L.) on a nutrient-deficient, sandy substrate. Biochars were washed with either (i) hydrochloric acid (HCl) to remove ash or (ii) ethanol to remove organic compounds or (iii) loaded with soluble nutrients from maize digestate.
The main aim of this study was to assess the effects of biochar modifications on marginal sandy substrate properties and in turn, on plant productivity. We hypothesized that (i) biochars affect substrate pH and nutrient levels and thus (ii) plant productivity and traits.
2. Materials and Methods
2.1. Experimental Set-Up
A pot experiment was established in the greenhouse facilities at the Forschungszentrum Jülich GmbH, Institute of Bio- and Geosciences, IBG-2: Plant Sciences, Germany. In the greenhouse, light intensities were set to a minimum of 400 µmols m−2 s−1, provided 16 h a day by natural light complemented by sodium-vapor lamps (SON-T AGRO 400, Phillips). The temperature was set to an average of 19 °C during the day and 17 °C at night, both at 60% relative humidity. Following a 6-day germination phase, plants were transplanted into plastic pots 12 cm in diameter and filled to a depth of 9 cm with sand and biochar at a mixing ratio of 19:1 to a total dry weight of 560 g pot−1, accounting for approximately 5% of biochar in each pot. Throughout the experiment, plants were watered three times a week in order to keep the substrate water content around 50% of the pre-determined water holding capacity (WHC) of sand and sand-biochar mixture [31]. Biochar amendment increased WHC from 24% to 35%.
Four treatments were tested: Non-modified biochar (NM-BC), HCl-washed biochar (HCl-BC), ethanol-washed biochar (EtOH-BC), and digestate-loaded biochar (Dig-BC). Pure sand served as negative control, NM-BC served as the positive control (see below). Per treatment, eight plants (n = 8) were destructively harvested 21, 28, and 35 days after transplantation. Pot placement was completely randomized following sampling 21 and 28 days after transplantation in order to avoid systematic edge or cumulative bias among the treatments.
2.2. Substrate and Biochar Properties
Nutrient-deficient fine sand with a particle size ranging between 0 and 1 mm, non-detectable amounts of N, P and K and a pH of 7.1 was sourced at a local gravel extraction plant in Inden, Germany, and used as the base substrate (Rheinische Baustoffwerke GmbH, Inden, Germany). Biochar was produced from whole maize cobs at University of Hohenheim using a self-purging pyrolysis reactor at a pyrolysis temperature of 450 °C, with a heating rate of 10 °C min−1 and a residence time of 1 h at final temperature [32]. The resulting biochar was crushed to a 2 mm particle size using a universal cutting mill (Pulverisette 19, Fritsch GmbH, Idar-Oberstein, Germany).
In order to produce ethanol- and HCl-modified biochars, biochar was washed with either 0.1 M ethanol or 0.1 M HCl at a ratio of 1:9. The comparatively mild acid wash was chosen for its supposed ability to induce mineral leaching [33]. Ethanol concentrations were adjusted accordingly. The HCl-char mixture was shaken for 2 h at 30 r.p.m. (HS 500, Janke & Kunkel IKA Labortechnik, Staufen, Germany). Both types of biochar were then vacuum-extracted from solution and dried at 80 °C until constant weight. In order to remove residual HCl solution, the HCl-washed biochar was treated with pH-adjusted (NaHCO3, pH of 7.5) deionized water before drying.
Nutrient-loaded biochar was produced by wrapping biochar in polypropylene fleece (18 g m−2), and submerging it in maize silage digestate obtained from the digestate storage tank of a commercially operating, thermophilic biogas facility (NaturPower GmbH and Co. KG, Titz-Ameln, Germany) allowing only the liquid fraction to penetrate for 7 d. As described earlier, the used digestate was obtained after the anaerobic digestion of pure maize silage for biogas production and originated from a commercially operating, thermophilic biogas facility with a fermenter volume of 2500 m3 and a hydraulic retention time of the biomass of 72 days [30]. The digestate was used as received and contained 0.53% total nitrogen (including 0.32% ammonium-N), 0.19% phosphate (P2O5), 0.71% potassium (K2O), 0.14% CaO and 0.06% MgO. In addition, the digestate contained 6.9% dry matter (consisting of 41.1% total carbon), and had a pH value of 7.9 (CaCl2) and a C/N ratio of 5 [30]. The resulting nutrient-loaded biochar was subsequently dried before further use.
2.3. Substrate, Biochar, and Plant Analyses
At each harvest, plants were separated into stem, leaf, and root biomass prior to drying. Plant and substrate samples were dried until constant weight at 70 and 40 °C, respectively. Samples were then homogenized and analyzed for their C and N content, using an elemental analyzer (vario Max CNS, Elementar GmbH, Langenselbold, Germany). Major inorganic elements (i.e., K and Mg) were estimated from the aqua regia extract using the inductively coupled plasma-optical emission spectrometer (ICP-OES, iCAP7600, Thermo Scientific, Dreieich, Germany). Substrate pH values were determined following a 2 h equilibration phase in a 0.1 M CaCl2 solution, prepared at a ratio of 1:2.5, using a pH meter (HQ40D, Hach Company, Loveland, CO, USA).
Above- (stem and leaves) and below-ground (roots) plant biomass was quantified following the destructive harvest and subsequent drying. Biomass data was used to calculate the root to shoot ratio. Leaf area measurements were performed using a leaf area meter (LI-3100 Area meter, LI-COR Inc., Lincoln, NE, USA) and used to derive specific leaf area values. Specific leaf area (SLA) was calculated as SLA = leaf area (m2)/leaf DW (kg−1).
2.4. Statistical Analysis
Relative treatment effects were quantified as RE (%) = [((treatment/control) − 1) ∗ 100] [34]. This conversion was applied to two sets of data: (i) where sand served as the negative control and all four biochar amendments represented the ‘treatment’ group to examine whether effect directionality following soil amendment was similar among the four biochar treatments, and (ii) where NM-BC served as the positive control for all three modified biochars (EtOH-BC, HCl-BC and Dig-BC) to separately assess the relative effects of biochar modification only.
We applied parametric ANCOVAs to both sets of data, with time as a co-variate. Tukey’s multiple comparison tests (‘glht’) were applied. Following an initial assessment via the Shapiro Wilks test, data was log-transformed, if applicable. Non-transformed data is shown in the table and the figures. All analyses were performed using R! 3.6.3 [35], installing additional ‘car’ [36] and ‘multcomp’ packages [37].
3. Results
3.1. pH, C/N Ratio, and Nutrient Content in the Substrate
Out of the six examined soil parameters, soil pH, the soil C/N ratio, soil C concentration and soil N concentration exhibited a uniform pattern in effect directionality, i.e., relative effects (RE) of biochar amendment in contrast to the negative control (sand) were either all positive or all negative for the four treatments (Table 1). Treatment effect sizes decreased in the following order: soil C concentration ≥ C/N ratio ≥ soil N concentration ≥ soil pH.

Table 1.
Relative effects (RE, %) of biochar amendment via NM-BC, EtOH-BC, HCl-BC, and Dig-BC on all examined soil and plant parameters in contrast to the negative control (unamended sand). Uniformity of response (UNI) indicates whether all four treatments exhibit the same effect directionality. Y: Yes, N: No. DW: dry weight, SR: shoot to root ratio, LA: leaf area, SLA: specific leaf area. Superscript letters indicate the results of the Tukey test (α = 0.05).
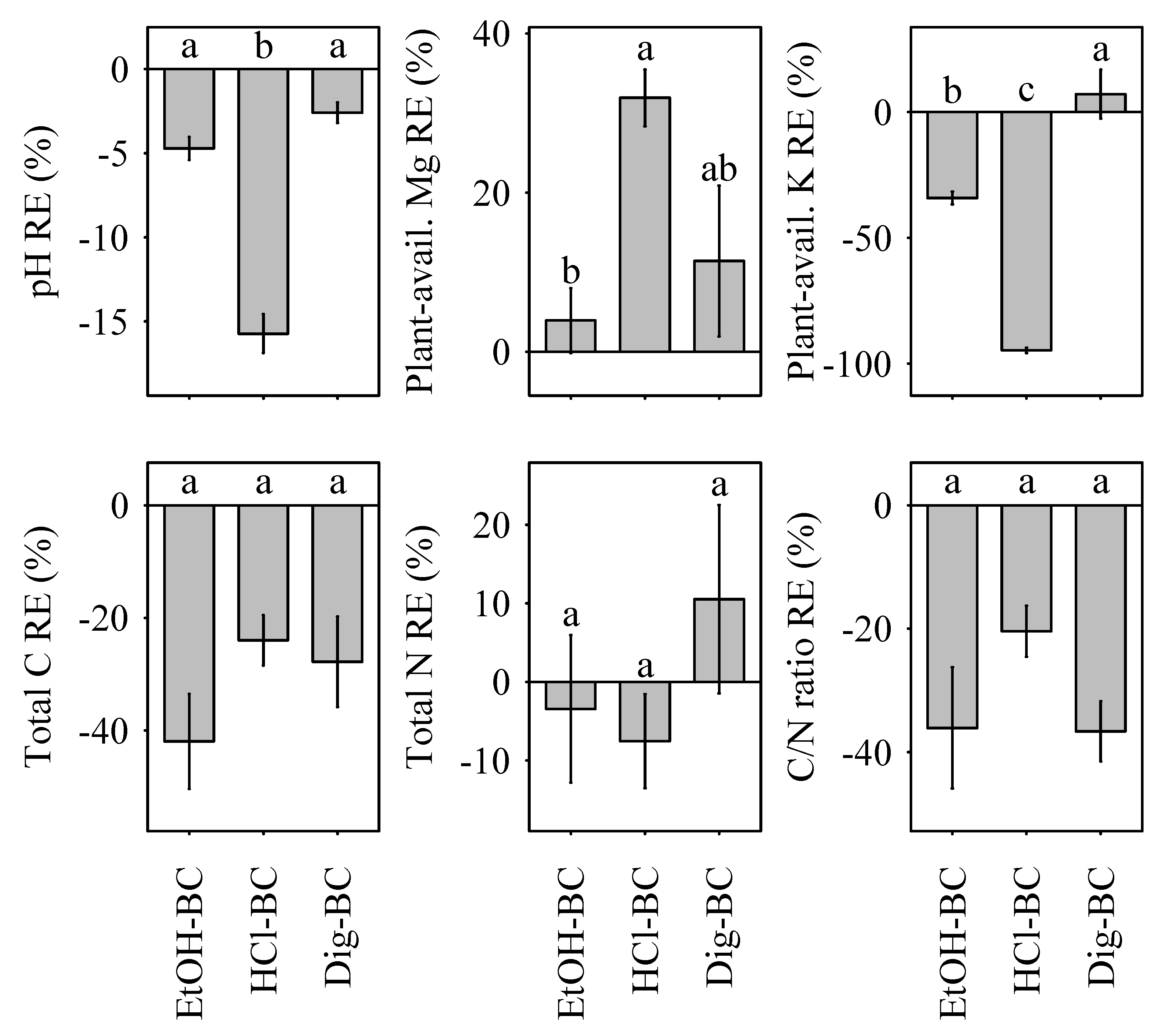
Increases in pH ranged from 0.4 (HCl-BC) to 1.5 (NM-BC) units. Following the initial pH assessment, soil pH remained constant for the duration of the experiment (0–35 days after transplantation). Relative modification effects were pronounced, as pH RE decreased most strongly in HCl-BC (Figure 1).
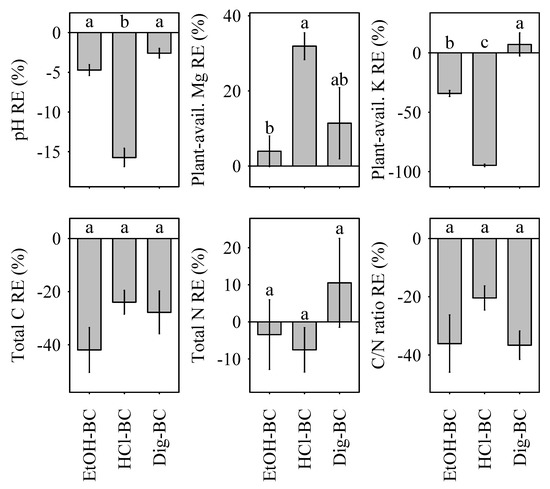
Figure 1.
Relative effects (RE, %) of biochar modification on soil pH, total carbon, total nitrogen, plant-available magnesium, and plant-available potassium relative to the non-modified NM-BC. Different lower case letters indicate significant differences (p < 0.05) between biochar modification effects, namely EtOH-BC, HCl-BC and Dig-BC. Data is shown as means with standard error (n = 24).
Similarly, the C/N ratio increased by 45% (NM-BC), 52% (EtOH-BC), 58% (HCl-BC), and 39% (Dig-BC) following biochar amendment. Despite increasing soil N concentrations to a comparative level to that observed in NM-BC, EtOH-BC and HCl-BC, a lower increase in soil C content in Dig-BC amended substrate resulted in a less pronounced increase of the C/N ratio. Relative effects of biochar modification were negative for soil total carbon and total nitrogen content and the C/N ratio, with the exception of Dig-BC, where positive modification effects were the result of an increase in soil N concentrations in comparison to NM-BC (Figure 1).
Three biochar amendments (NM-BC, EtOH and Dig-BC) exhibited uniformity in their response in soil K and soil Mg concentrations. In contrast to sand, biochar amendment had a positive effect on soil K concentrations and a negative effect on soil Mg concentrations (Table 1). The only treatment not adhering to this pattern, i.e., exhibiting lower values than the negative control, when all other biochar treatments exhibit higher values than the negative control or vice versa, was HCl-BC. HCl-BC had a positive effect on soil Mg and a negative effect on soil K concentrations in contrast to sand (Table 1).
While the observed effects in all other soil parameters remained stable over the experimental period the available soil K concentrations and soil Mg concentrations increased, most likely due to watering. In the marginal sandy substrate, soil K concentrations increased by a factor of 12, while in the biochar-treatments (except HCl-BC, factor of 3) K availability increased by a factor of 21 over the experimental period (Table 2). At the last harvest, biochar amendments had increased soil K availability by a factor ranging between 9 (EtOH-BC) to 14.9 (Dig-BC) compared to pure sand.

Table 2.
pH and nutrient content of the used biochar, the pure sand (control), and its mixtures with biochars (BC). Biochar was treated prior to substrate application by washing with ethanol (EtOH-BC) and hydrochloric acid (HCl-BC) to remove ashes and labile organic compounds. Another portion of the used biochar was loaded with nutrients by using digestate (Dig-BC). Substrate properties (i.e., pH; total C and N; plant-available K and Mg) are shown for the beginning of the experiment (day 0) and the last day of this experiment (day 35). The table shows means and standard deviations (n = 24).
Mg availability increased equally for all treatments by a factor of 20 over the experimental period. Relative effects of biochar modification were uniformly positive for soil Mg and uniformly negative for soil K, with the exception of Dig-BC, which exhibited slightly positive effects of biochar modification on soil K (Figure 1).
3.2. Plant Growth
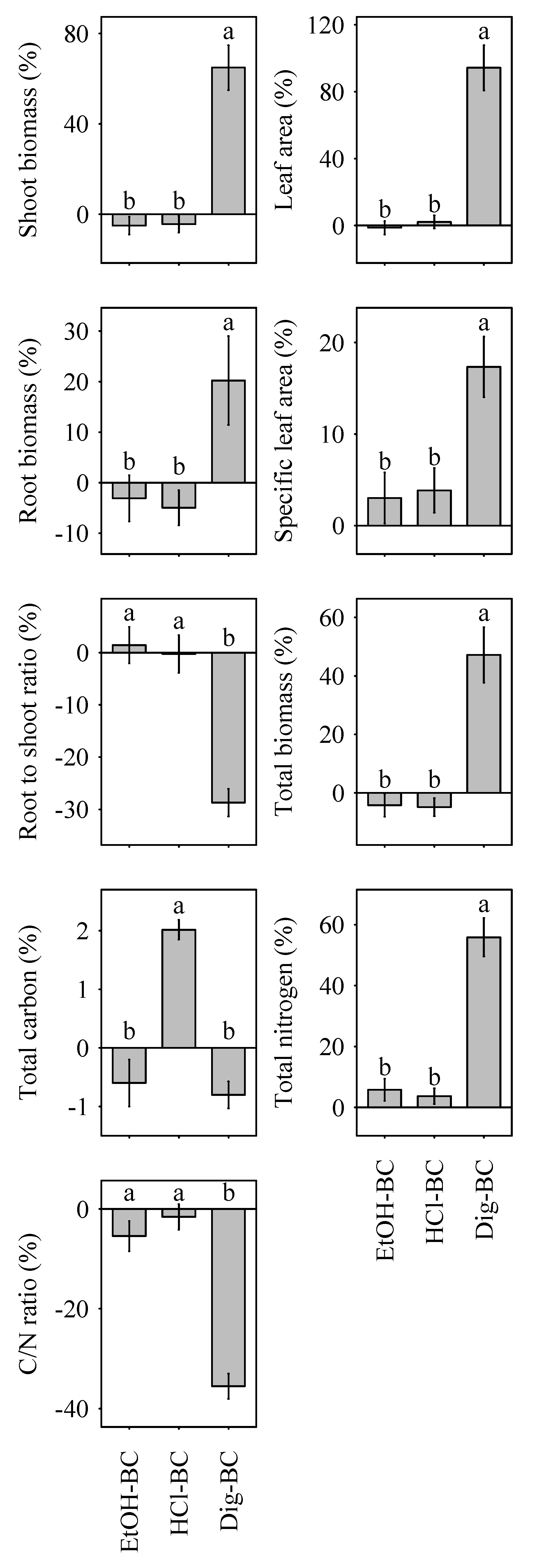
Biochar amendment uniformly increased plant leaf area, shoot biomass, and total biomass. Accordingly, the root-to shoot ratio uniformly decreased (Table 1). Effects of biochar amendment on plant productivity were most pronounced in Dig-BC. Following the addition of Dig-BC plant biomass more than doubled, compared to the sand control (Table 1). Similarly, leaf area values of plants treated with Dig-BC were significantly increased, while those grown in pots amended with other biochar forms remained unaffected. The root to shoot ratio was significantly lower for plants treated with Dig-BC, indicating a shift from below-ground to above-ground biomass production, although overall below-ground productivity remained higher in Dig-BC than NM-BC, EtOH-BC and HCl-BC (Table 1). Specific leaf area increased in plants treated with Dig-BC. All other biochar amendments resulted in a decrease of specific leaf area values. This immense response in plant parameters following Dig-BC amendment was reflected in the relative effects of biochar modification, with Dig-BC being the most productive out of the three modified biochars (Figure 2). In contrast, modification via ethanol and HCl washing had null effect on overall plant productivity in contrast to the non-modified NM-BC (Figure 2).
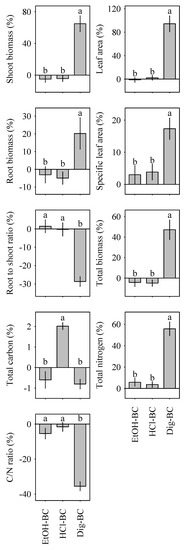
Figure 2.
Relative effects (RE, %) of biochar modification on plant functional traits (i.e., leaf area, specific leaf area, root to shoot ratio, and biomass) and plant elemental concentrations (i.e., total carbon, total nitrogen) relative to the non-modified NM-BC. Different lower-case letters indicate significant differences (p < 0.05) between biochar modification effects, namely EtOH-BC, HCl-BC, and Dig-BC. Data is shown as means with standard error (n = 24).
3.3. Nutrient Uptake
Except for HCl-washed biochar, all biochars induced a reduced net carbon accumulation in plants. Nitrogen uptake was significantly enhanced by Dig-BC, while other biochars reduced or maintained nitrogen uptake relative to that of the negative sand control (Table 1). In consequence, C/N ratios in plant tissue tended to increase for all biochar treatments except Dig-BC, which exhibited a significant decline of C/N ratios, mirroring effects observed in the soil. In turn, relative effects of biochar modification illustrated the increase of N nutrition and decrease in plant C/N ratio in contrast to the other two modified biochars (Figure 2).
4. Discussion
In line with recent reports, the addition of biochars herein increased the total substrate carbon (C) and nitrogen (N) concentrations [38,39]. Except for HCl-washed biochars, biochar addition also increased the availability of potassium (K) and reduced that of magnesium (Mg) in comparison to pure sand. This is most likely due to a surplus of easily soluble K supplied by biochar accompanying ash in unwashed biochars [8,40]. A surplus of K can induce a nutrient imbalance and reduce the availability of Mg due to excessive competition for sorption sites [8]. Although we observed treatment-specific variations in K and Mg, it is likely that watering with non-deionized water introduced additional Mg and K into the system. However, given the same amount of water was applied to each biochar treatment, varying effects between treatments are likely negligible. Still, in order to mimic the effects of watering via natural precipitation in the field, water lacking in these specific nutrients should be used in future experiments.
While results of other studies and meta-analyses reported that plant productivity may remain unaffected after biochar application [2,4,8], untreated biochar addition as used in our study resulted in a biomass increase of 10% compared with the pure sand substrate, which is in line with previous results of a meta-analysis by Jeffrey et al. [41]. However, the relative increase of below-ground biomass production in comparison to traditional biochar, by plants growing on substrate amended with washed biochars, indicates foraging behavior. Although initial N concentrations following amendment are slightly higher in washed biochar amended substrate, this boost does not translate into higher tissue N concentrations at harvest. This indicates, that while washing might increase soil N background concentrations, these nutrients are likely not available for plant uptake. Instead, plants actively search for nutrients in order to balance out potential deficiencies during the experimental period. However, as nutrients potentially become available over longer time periods, future experiments, testing for the interactions between plant ontogeny, and an initial dose of biochar on nutrient-deficient substrate should cover an entire growing season. Based on the presented data, we would expect Dig-BC amended plants to fare best, assuming the soil nutrient pools are not depleted as a result of quicker soil exploration and enhanced nutrient uptake. The only hurdle currently preventing its industrial long-term usage are hygienic considerations, as digestates could potentially introduce additional phytotoxic compounds and/or trace metal elements into the soil long-term [27].
Initially, pre-treatment was applied in order to avoid the pervasive issue of potentially phytotoxic mobile organic compounds [9,42,43,44] and N immobilization following biochar amendment [34,45,46] commonly resulting in null or even negative effects of biochar addition [41]. However, contrasting the elaborate production process of the washed chars to its null effect, it seems unlikely that a large-scale production of these biochars is feasible. Instead modifying biochars via natural weathering and aging processes by exposing them to the elements might prove an alternative, especially at larger scales [47].
In contrast to the two washed chars and despite similar background concentrations, we observed a doubling of above-ground biomass for the Dig-BC treatment. This fertilizing effect indicates, in contrast to the observed effects in soils amended with washed biochars, that the existing N is plant-available. This is further supported by observably lower root to shoot ratios in Dig-BC amended substrates, which strongly suggests an improved plant nutrition [48]. Similarly, specific leaf area markedly increased, which in turn is strongly related to the net enhancement of plant-available N uptake [49]. In line with this argument, plants grown on digestate loaded biochars are enriched in nitrogen (i.e., increased N concentration and decreased C/N ratio). The overall fertilizing and plant promoting effect of the used maize silage digestate in a sandy substrate both as used in this study was presented earlier [30]. This complementary experiment was conducted as a positive control to evaluate the effects of the used digestate on maize germination, plant growth, and performance in the sandy substrate—also in comparison with a mineral NPK fertilizer [30]. As previously observed, digestate from plant material such as maize silage, supplied organic matter to the marginal sandy substrate facilitating the improvement of its structure and water-holding capacity [50]. In these previous studies, the employed digestate met the nutritional requirements of maize, increasing the fertility of the marginal sandy substrate used, and reduced nutrient leaching as compared with a mineral NPK fertilizer [28,30]. The threat of N immobilization caused by high C/N ratio could be avoided by the high concentration of mineralized N in ammonium form in the digestate absorbed by the Dig-BC during the incubation process. Its success at similar total N concentrations is then simply a function of N availability to the plant.
5. Conclusions
We conclude that loading biochars with nutrients from nutrient-rich biogenic residues (e.g., digestates) can improve plant productivity by improving plant nutrition, which promotes the formation of photosynthetic plant parts and uptake of nutrients. Using biogenic residues such as biochars made of maize cobs and digestates also strengthens the concept of local nutrient recycling, which might be a promising strategy for plant production on nutrient-poor sandy soils. However, this experiment was a short-term experiment, and thus further interdisciplinary research is required to assess the effects of modified biochars on plant productivity in the long-term, particularly at field scale.
Author Contributions
The experiment was conceptualized by N.D.J., S.L., M.A.R. and J.M., and the methodology was applied by C.C.D., M.A.R., A.A.R.-A. and N.D.J.; software was employed by C.C.D. and A.A.R.-A.; the manuscript was validated by N.D.J., C.C.D., S.L., K.I., M.A.R., and J.M.; formal analysis was mainly conducted by C.C.D., M.A.R., and A.A.R.-A.; resources were acquired and provided by N.D.J. and J.M.; data curation was conducted by M.A.R., C.C.D., and A.A.R.-A.; writing—original draft preparation, C.C.D., N.D.J., and A.A.R.-A.; writing—review and editing, N.D.J., C.C.D., A.A.R.-A., M.A.R., S.L., K.I., and J.M.; data visualization was realized by C.C.D.; supervision of the project was conducted by N.D.J., S.L., and J.M.; project administration, N.D.J., S.L., and J.M.; funding acquisition, N.D.J., S.L., and J.M. All authors have read and agreed to the published version of the manuscript.
Funding
C.C.D. received funding by the German Federal Ministry of Education and Research (BMBF) within PURESBio project (grant reference number 031A289A). A.A.R.-A. was funded by the European Community’s Framework Programme (FP7/2007–2013), grant number 603744.
Acknowledgments
We appreciate the institutional support by the Institute of Bio- and Geosciences, IBG-2: Plant Sciences, Forschungszentrum Jülich GmbH and the University of Hohenheim, Germany, making this research possible.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zimmermann, M.; Bird, M.I.; Wurster, C.; Smernik, R. Rapid degradation of pyrogenic carbon. Glob. Chang. Biol. 2012, 18, 3306–3316. [Google Scholar] [CrossRef]
- Crane-Droesch, A.; Abiven, S.; Jeffery, S. Heterogeneous global crop yield response to biochar: A meta-regression analysis. Environ. Res. Lett. 2013, 8, 044049. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, A.; Ji, C. Biochar’s effect on crop productivity and the dependence on experimental conditions—A meta-analysis of literature data Biochar’s effect on crop productivity and the dependence on experimental conditions—A meta-analysis of literature data. Plant Soil 2013, 373, 583–594. [Google Scholar] [CrossRef]
- Biedermann, L.A.; Harpole, W.S. Biochar and its effects on plant productivity and nutrient cycling: A meta-analysis. GCB Bioenergy 2013, 5, 202–214. [Google Scholar] [CrossRef]
- Kloss, S.; Zehetner, F.; Wimmer, B.; Buecker, J.; Rempt, F.; Soja, G. Biochar application to temperate soils: Effects on soil fertility and crop growth under greenhouse conditions. J. Plant Nutr. Soil Sci. 2014, 177, 3–15. [Google Scholar] [CrossRef]
- Macdonald, L.M.; Farrell, M.; Van Zwieten, L.; Krull, E.S. Plant growth responses to biochar addition: An Australian soils perspective. Biol. Fertil. Soils 2014, 50, 1035–1045. [Google Scholar] [CrossRef]
- Schulz, H.; Dunst, G.; Glaser, B. Positive effects of composted biochar on plant growth and soil fertility. Agron. Sustain. Dev. 2013, 33, 817–827. [Google Scholar] [CrossRef]
- Borchard, N.; Siemens, J.; Ladd, B.; Möller, A.; Amelung, W. Application of biochars to sandy and silty soil failed to increase maize yield under common agricultural practice. Soil Tillage Res. 2014, 144, 184–194. [Google Scholar] [CrossRef]
- Buss, W.; Mašek, O. Mobile organic compounds in biochar—A potential source ofcontamination—Phytotoxic effects on cress seed (Lepidiumsativum) germination. J. Environ. Manag. 2014, 137, 111–119. [Google Scholar] [CrossRef]
- Buss, W.; Mašek, O.; Graham, M.; Wüst, D. Inherent organic compounds in biochar-Their content, composition and potential toxic effects. J. Environ. Manag. 2015, 156, 150–157. [Google Scholar] [CrossRef]
- Joseph, S.; Chia, C.; Munroe, P.; Donne, S.; Thomas, T.; Nielsen, S.; Marjo, C.; Rutlidge, H.; Li, L.; Taylor, P.; et al. Shifting paradigms: Development of high-efficiency biochar fertilizers based on nano-structures and soluble components. Carbon Manag. 2013, 4, 323–343. [Google Scholar] [CrossRef]
- Novak, J.M.; Cantrell, K.B.; Watts, D.W.; Busscher, W.J.; Johnson, M.G. Designing relevant biochars as soil amendments using lignocellulosic-based and manure-based feedstocks. J. Soils Sediments 2014, 14, 330–343. [Google Scholar] [CrossRef]
- Hu, X.; Ding, Z.; Zimmerman, A.R.; Wang, S.; Gao, B. Batch and column sorption of arsenic onto iron-impregnated biochar synthesized through hydrolysis. Water Res. 2014, 68, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Prost, K.; Borchard, N.; Siemens, J.; Kautz, T. Biochar Affected by Composting with Farmyard Manure. J. Environ. Qual. 2013, 42, 164–172. [Google Scholar] [CrossRef]
- Qian, W.; Zhao, A.; Xu, R. Sorption of As(V) by Aluminum-Modified Crop Straw-Derived Biochars. Water Air Soil Pollut. 2013, 224, 1610. [Google Scholar] [CrossRef]
- Shafeeyan, M.S.; Mohd, W.; Wan, A.; Houshmand, A.; Shamiri, A. A review on surface modification of activated carbon for carbon dioxide adsorption. J. Anal. Appl. Pyrolysis 2010, 89, 143–151. [Google Scholar] [CrossRef]
- Shen, W.; Li, Z.; Liu, Y. Surface Chemical Functional Groups Modification of Porous Carbon. Recent Patents Chem. Eng. 2008, 1, 27–40. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, H.; Thring, R.W.; Arocena, J.M. Chemical Pretreatment of Rice Straw Biochar: Effect on Biochar Properties and Hexavalent Chromium Adsorption. Int. J. Environ. Res. 2019, 13, 91–105. [Google Scholar] [CrossRef]
- Wu, H.; Yip, K.; Kong, Z.; Li, C.Z.; Liu, D.; Yu, Y.; Gao, X. Removal and recycling of inherent inorganic nutrient species in mallee biomass and derived biochars by water leaching. Ind. Eng. Chem. Res. 2011, 50, 12143–12151. [Google Scholar] [CrossRef]
- Bernardo, M.; Lapa, N.; Gonçalves, M.; Barbosa, R.; Mendes, B.; Pinto, F.; Gulyurtlu, I. Toxicity of char residues produced in the co-pyrolysis of different wastes. Waste Manag. 2010, 30, 628–635. [Google Scholar] [CrossRef]
- Lievens, C.; Mourant, D.; Hu, X.; Wang, Y.; Wu, L.; Rossiter, A.; Gunawan, R.; He, M.; Li, C.Z. A case study: What is leached from mallee biochars as a function of pH? Environ. Monit. Assess. 2018, 190, 294. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Luo, L.; Xiao, D.; Lv, J.; Wen, B.; Ma, Y.; Zhang, S. Selected dark sides of biomass-derived biochars as environmental amendments. J. Environ. Sci. (China) 2017, 54, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Sahin, O.; Taskin, M.B.; Kaya, E.C.; Atakol, O.; Emir, E.; Inal, A.; Gunes, A. Effect of acid modification of biochar on nutrient availability and maize growth in a calcareous soil. Soil Use Manag. 2017, 33, 447–456. [Google Scholar] [CrossRef]
- Lehmann, J.; Pereira, J.; Steiner, C.; Nehls, T.; Zech, W.; Glaser, B. Nutrient availability and leaching in an archaeological Anthrosol and a Ferralsol of the Central Amazon basin: Fertilizer, manure and charcoal amendments. Plant Soil 2003, 249, 343–357. [Google Scholar] [CrossRef]
- Möller, K.; Müller, T. Effects of anaerobic digestion on digestate nutrient availability and crop growth: A review. Eng. Life Sci. 2012, 12, 242–257. [Google Scholar] [CrossRef]
- Möller, K. Effects of anaerobic digestion on soil carbon and nitrogen turnover, N emissions, and soil biological activity. A review. Agron. Sustain. Dev. 2015, 35, 1021–1041. [Google Scholar] [CrossRef]
- Nkoa, R. Agricultural benefits and environmental risks of soil fertilization with anaerobic digestates: A review. Agron. Sustain. Dev. 2014, 34, 473–492. [Google Scholar] [CrossRef]
- Nabel, M.; Temperton, V.M.; Poorter, H.; Lücke, A.; Jablonowski, N.D. Energizing marginal soils—The establishment of the energy crop Sida hermaphrodita as dependent on digestate fertilization, NPK, and legume intercropping. Biomass Bioenergy 2016, 87, 9–16. [Google Scholar] [CrossRef]
- Nabel, M.; Schrey, S.D.; Poorter, H.; Koller, R.; Nagel, K.A.; Temperton, V.M.; Dietrich, C.C.; Briese, C.; Jablonowski, N.D. Coming Late for Dinner: Localized Digestate Depot Fertilization for Extensive Cultivation of Marginal Soil With Sida hermaphrodita. Front. Plant Sci. 2018, 9, 1–14. [Google Scholar] [CrossRef]
- Robles-Aguilar, A.A.; Temperton, V.M.; Jablonowski, N.D. Maize Silage Digestate Application Affecting Germination and Early Growth of Maize Modulated by Soil Type. Agronomy 2019, 9, 473. [Google Scholar] [CrossRef]
- Glaser, B.; Wiedner, K.; Seelig, S.; Schmidt, H.P.; Gerber, H. Biochar organic fertilizers from natural resources as substitute for mineral fertilizers. Agron. Sustain. Dev. 2015, 35, 667–678. [Google Scholar] [CrossRef]
- Intani, K.; Latif, S.; Kabir, A.K.M.R.; Müller, J. Effect of self-purging pyrolysis on yield of biochar from maize cobs, husks and leaves. Bioresour. Technol. 2016, 218, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Silber, A.; Levkovitch, I.; Graber, E.R. pH-Dependent Mineral Release and Surface Properties of Cornstraw Biochar: Agronomic Implications. Environ. Sci. Technol. 2010, 44, 9318–9323. [Google Scholar] [CrossRef] [PubMed]
- Gale, N.V.; Thomas, S.C. Dose-dependence of growth and ecophysiological responses of plants to biochar. Sci. Total Environ. 2019, 658, 1344–1354. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2020. [Google Scholar]
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 3rd ed.; Sage: Thousand Oaks, CA, USA, 2019. [Google Scholar]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous inference in general parametric models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef]
- Knicker, H. Pyrogenic organic matter in soil: Its origin and occurrence, its chemistry and survival in soil environments. Quat. Int. 2011, 243, 251–263. [Google Scholar] [CrossRef]
- Lorenz, K.; Lal, R. Biochar application to soil for climate change mitigation by soil organic carbon sequestration. J. Plant Nutr. Soil Sci. 2014, 177, 651–670. [Google Scholar] [CrossRef]
- Singh, B.; Singh, B.P.; Cowie, A.L. Characterisation and evaluation of biochars for their application as a soil amendment. Aust. J. Soil Res. 2010, 48, 516–525. [Google Scholar] [CrossRef]
- Jeffery, S.; Verheijen, F.G.A.; Van Der Velde, M.; Bastos, A.C. A quantitative review of the effects of biochar application to soils on crop productivity using meta-analysis. Agric. Ecosyst. Environ. 2011, 144, 175–187. [Google Scholar] [CrossRef]
- Spokas, K.A.; Novak, J.M.; Stewart, C.E.; Cantrell, K.B.; Uchimiya, M.; DuSaire, M.G.; Ro, K.S. Qualitative analysis of volatile organic compounds on biochar. Chemosphere 2011, 85, 869–882. [Google Scholar] [CrossRef]
- Hale, S.E.; Lehmann, J.; Rutherford, D.; Zimmerman, A.R.; Bachmann, R.T.; Shitumbanuma, V.; O’Toole, A.; Sundqvist, K.L.; Arp, H.P.H.; Cornelissen, G. Quantifying the total and bioavailable polycyclic aromatic hydrocarbons and dioxins in biochars. Environ. Sci. Technol. 2012, 46, 2830–2838. [Google Scholar] [CrossRef] [PubMed]
- Gale, N.V.; Sackett, T.E.; Thomas, S.C. Thermal treatment and leaching of biochar alleviates plant growth inhibition from mobile organic compounds. PeerJ 2016, 4, e2385. [Google Scholar] [CrossRef] [PubMed]
- Glaser, B.; Lehmann, J.; Zech, W. Ameliorating physical and chemical properties of highly weathered soils in the tropics with charcoal—A review. Biol. Fertil. Soils 2002, 35, 219–230. [Google Scholar] [CrossRef]
- Gale, N.; Halim, A.; Horsburgh, M.; Thomas, S.C. Comparative responses of early-successional plants to charcoal soil amendments. Ecosphere 2017, 8, e01933. [Google Scholar] [CrossRef]
- Spokas, K.A.; Novak, J.M.; Masiello, C.A.; Johnson, M.G.; Colosky, E.C.; Ippolito, J.A.; Trigo, C. Physical Disintegration of Biochar: An Overlooked Process. Environ. Sci. Technol. Lett. 2014, 1, 326–332. [Google Scholar] [CrossRef]
- Poorter, H.; Niklas, K.J.; Reich, P.B.; Oleksyn, J.; Poot, P.; Mommer, L. Biomass allocation to leaves, stems and roots: Meta-analyses of interspecific variation and environmental control. New Phytol. 2012, 193, 30–50. [Google Scholar] [CrossRef]
- Grassein, F.; Lemauviel-lavenant, S.; Lavorel, S.; Bahn, M.; Bardgett, R.D. Relationships between functional traits and inorganic nitrogen acquisition among eight contrasting European grass species Relationships between functional traits and inorganic nitrogen acquisition among eight contrasting European grass species. Ann. Bot. 2015, 115, 107–115. [Google Scholar] [CrossRef]
- Nabel, M.; Schrey, S.D.; Poorter, H.; Koller, R.; Jablonowski, N.D. Effects of digestate fertilization on Sida hermaphrodita: Boosting biomass yields on marginal soils by increasing soil fertility. Biomass Bioenergy 2017, 107, 207–213. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).