Deep Phenotyping of Yield-Related Traits in Wheat
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experiment and Plant Sampling
2.2. Spectral Measurements and Data Preparation
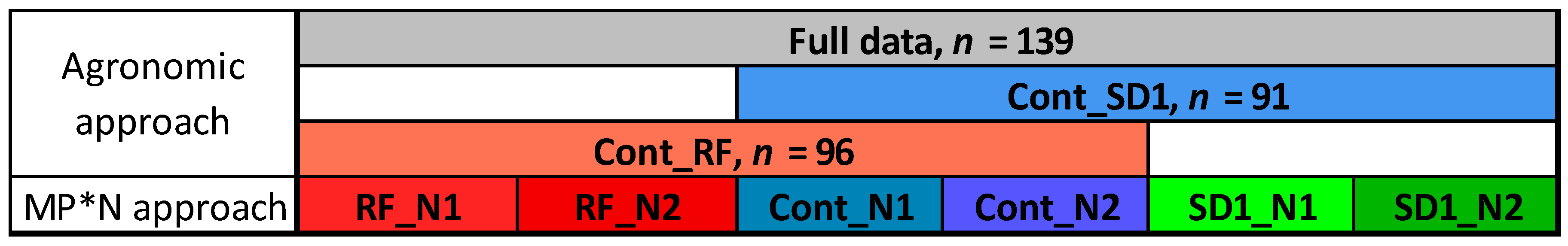
2.3. Statistical Analysis
3. Results
3.1. Optimized Index and Date Selection Considering the Contributing Treatments
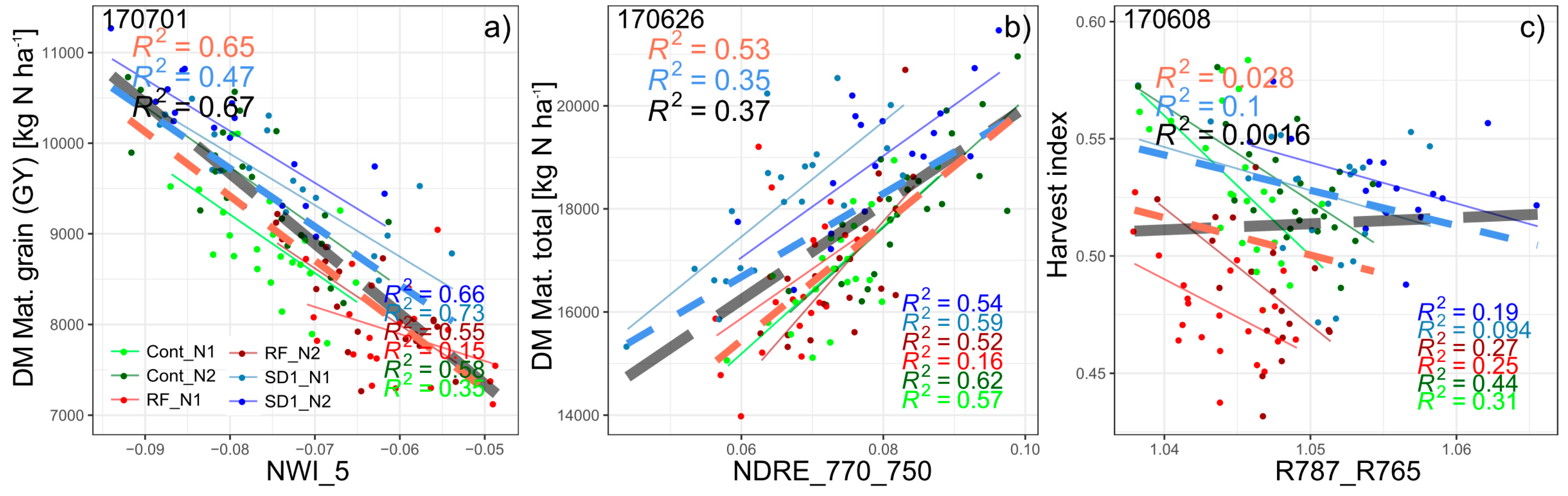
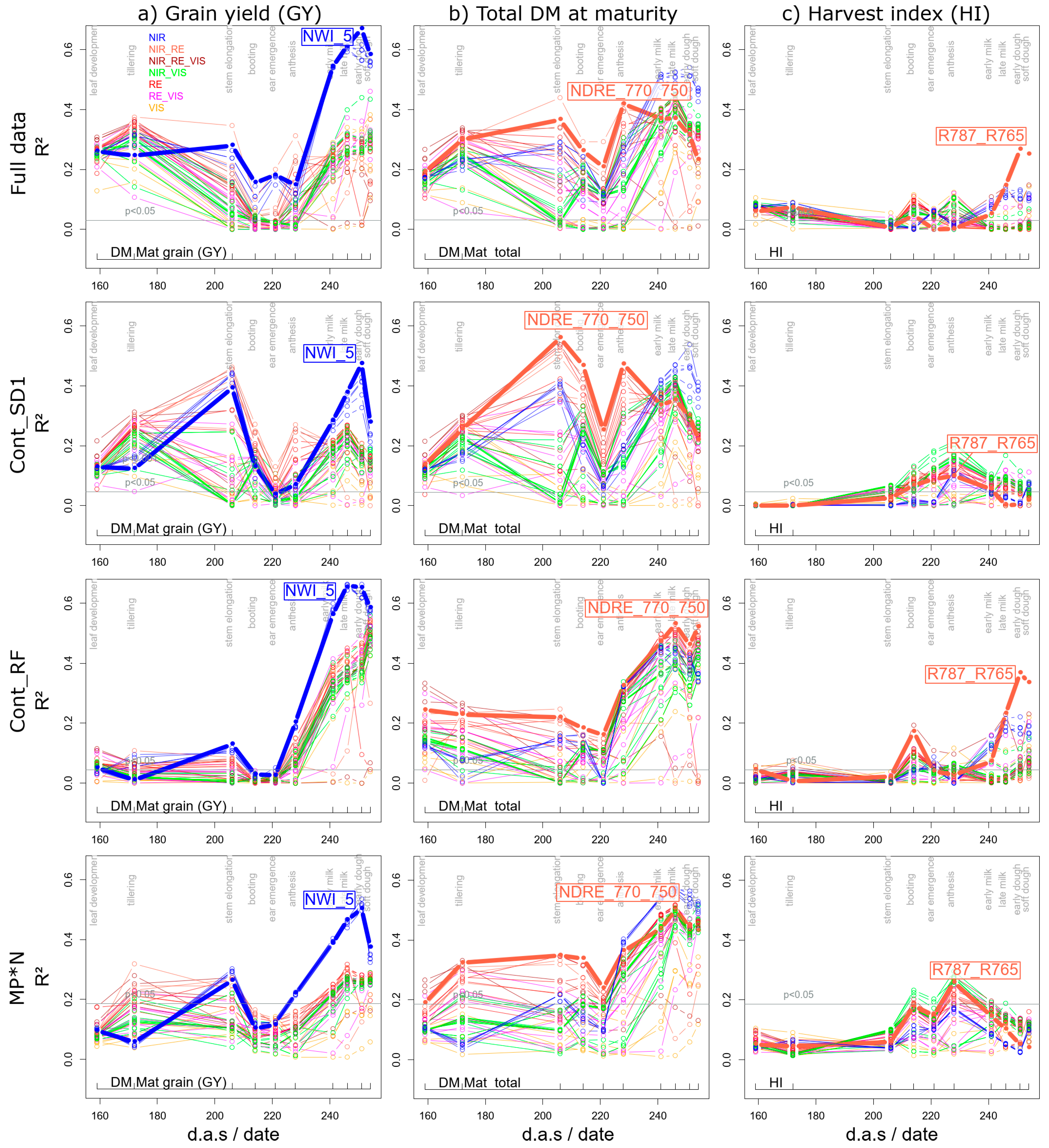
3.1.1. Grain Yield
3.1.2. Further Direct DM Traits
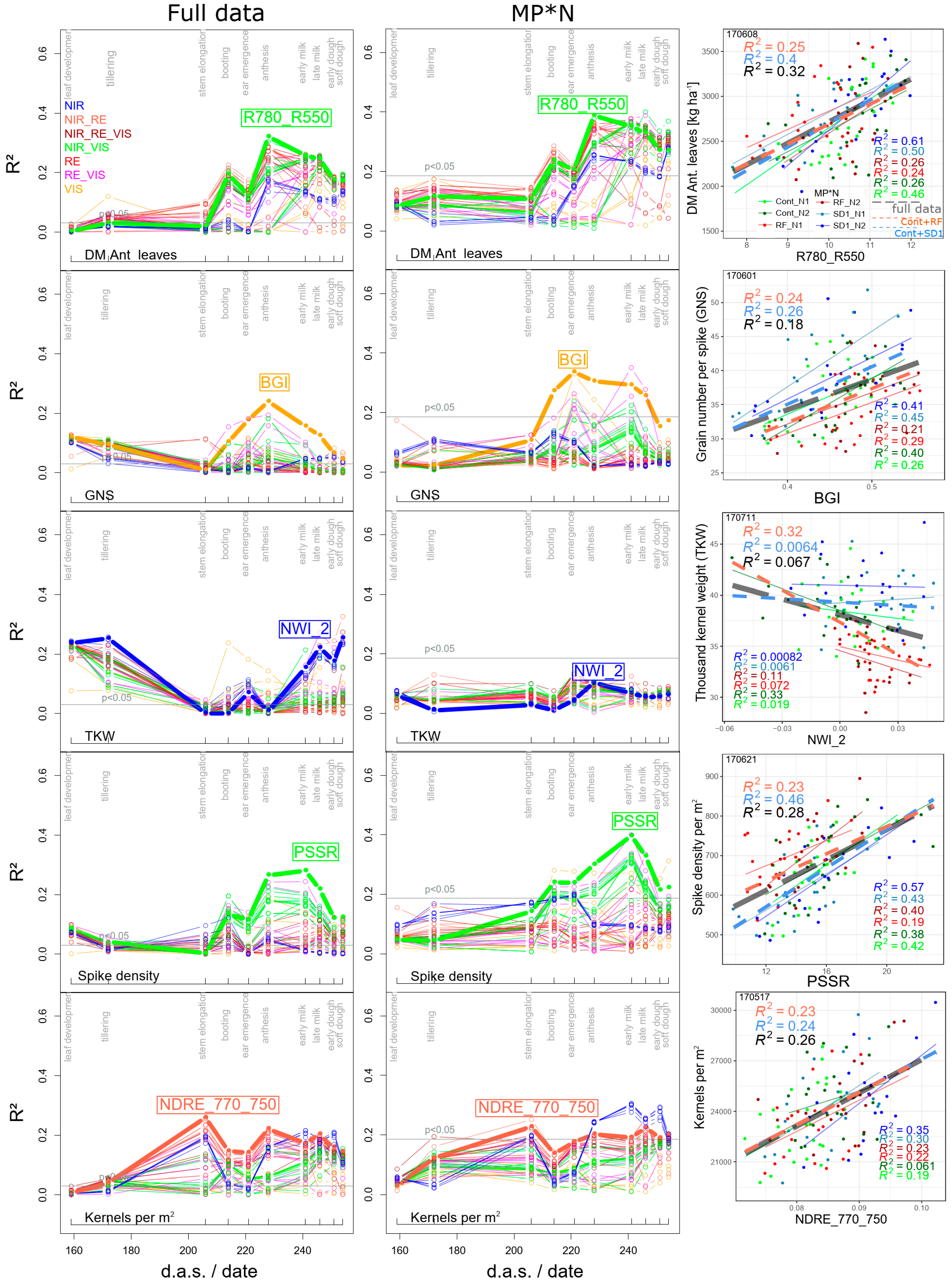
3.1.3. Derived DM Traits
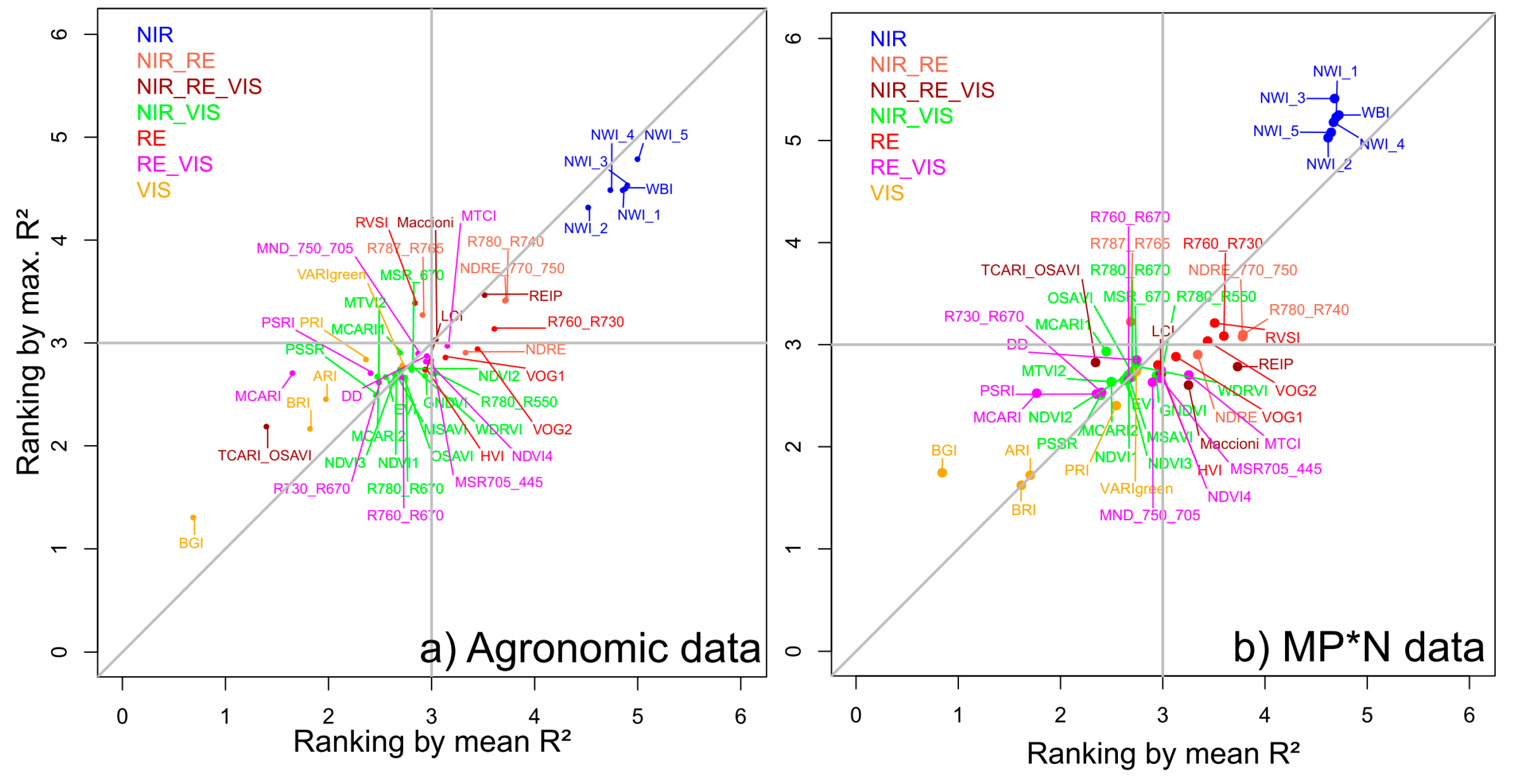
3.2. Index Ranking According to Traits and Datasets
4. Discussion
4.1. In-Season Estimation of Grain Yield and Contributing DM Traits
4.2. In-Season Estimation of Yield Components
4.3. Suitability of the R787_R765 and TCARI_OSAVI for the Agronomic Approach
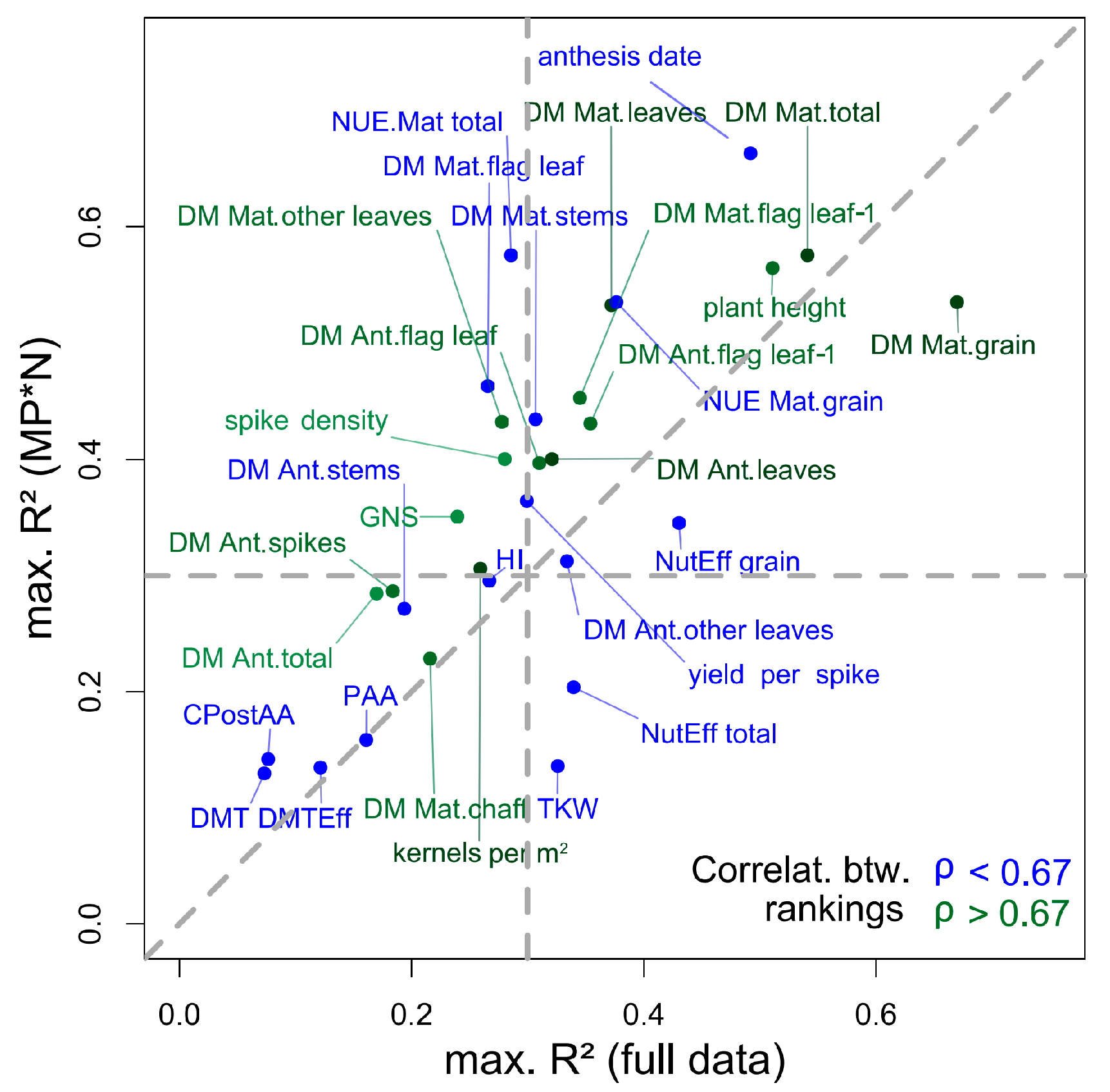
4.4. Stability of Index Rankings According to Dataset
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ehdaie, B.; Waines, J. Sowing date and nitrogen rate effects on dry matter and nitrogen partitioning in bread and durum wheat. Field Crop. Res. 2001, 73, 47–61. [Google Scholar] [CrossRef]
- Ferrise, R.; Triossi, A.; Stratonovitch, P.; Bindi, M.; Martre, P. Sowing date and nitrogen fertilisation effects on dry matter and nitrogen dynamics for durum wheat: An experimental and simulation study. Field Crop. Res. 2010, 117, 245–257. [Google Scholar] [CrossRef]
- Gooding, M.J.; Gregory, P.J.; Ford, K.E.; Pepler, S. Fungicide and cultivar affect post-anthesis patterns of nitrogen uptake, remobilization and utilization efficiency in wheat. J. Agric. Sci. 2005, 143, 503–518. [Google Scholar] [CrossRef]
- Ruske, R.E.; Gooding, M.J.; Jones, S.A. The effects of triazole and strobilurin fungicide programmes on nitrogen uptake, partitioning, remobilization and grain N accumulation in winter wheat cultivars. J. Agric. Sci. 2003, 140, 395–407. [Google Scholar] [CrossRef]
- Parry, M.A.J.; Reynolds, M.; Salvucci, M.E.; Raines, C.; Andralojc, P.J.; Zhu, X.G.; Price, G.D.; Condon, A.G.; Furbank, R.T. Raising yield potential of wheat. II. Increasing photosynthetic capacity and efficiency. J. Exp. Bot. 2011, 62, 453–467. [Google Scholar] [CrossRef]
- Cormier, F.; Faure, S.; Dubreuil, P.; Heumez, E.; Beauchêne, K.; Lafarge, S.; Praud, S.; Le Gouis, J. A multi-environmental study of recent breeding progress on nitrogen use efficiency in wheat (Triticum aestivum L.). Theor. Appl. Genet. 2013, 126, 3035–3048. [Google Scholar] [CrossRef]
- Prey, L.; Kipp, S.; Hu, Y.; Schmidhalter, U. Nitrogen Use Efficiency and Carbon Traits of High-Yielding European Hybrid vs. Line Winter Wheat Cultivars: Potentials and Limitations. Front. Plant Sci. 2019, 9, 1988. [Google Scholar] [CrossRef] [Green Version]
- Prey, L.; Hu, Y.; Schmidhalter, U. Temporal dynamics and the contribution of plant organs in a phenotypically diverse population of high-yielding winter wheat: Evaluating concepts for disentangling yield formation and nitrogen use efficiency. Front. Plant Sci. 2019, 10, 1295. [Google Scholar] [CrossRef]
- Ding, D.; Feng, H.; He, J.Q.; Zou, F.; Jin, J.M. Modifying Winter Wheat Sowing Date as an Adaptation to Climate Change on the Loess Plateau. Agron. J. 2016, 53–63. [Google Scholar] [CrossRef] [Green Version]
- Yin, L.; Dai, X.; He, M. Delayed sowing improves nitrogen utilization efficiency in winter wheat without impacting yield. Field Crop. Res. 2018, 221, 90–97. [Google Scholar] [CrossRef]
- Rasmussen, I.S.; Thorup-Kristensen, K. Does earlier sowing of winter wheat improve root growth and N uptake? Field Crop. Res. 2016, 196, 10–21. [Google Scholar] [CrossRef]
- Milford, G.F.J.; Penny, A.; Prew, R.D.; Darby, R.J.; Todd, A.D. Effects of previous crop, sowing date, and winter and spring applications of nitrogen on the growth, nitrogen uptake and yield of winter wheat. J. Agric. Sci. 1993, 121, 1–12. [Google Scholar] [CrossRef]
- Van Eeuwijk, F.A.; Bustos-korts, D.; Millet, E.J.; Boer, M.P.; Kruijer, W. Modelling strategies for assessing and increasing the effectiveness of new phenotyping techniques in plant breeding. Plant Sci. 2018, 282, 23–39. [Google Scholar] [CrossRef] [PubMed]
- Rebetzke, G.J.; Jimenez-Berni, J.; Fischer, R.A.; Deery, D.M.; Smith, D.J. Review: High-throughput phenotyping to enhance the use of crop genetic resources. Plant Sci. 2019, 282, 40–48. [Google Scholar] [CrossRef]
- Sankaran, S.; Mishra, A.; Ehsani, R.; Davis, C. A review of advanced techniques for detecting plant diseases. Comput. Electron. Agric. 2010, 72, 1–13. [Google Scholar] [CrossRef]
- Schmid, A. Erfassung des Aktuellen Stickstoffstatus von Kulturpflanzen mit Berührungsloser Sensorik zur Optimierung der Teilflächenspezifischen Bestandesführung; Herbert Utz Verlag: Munich, Germany, 2008; ISBN 3831607745. [Google Scholar]
- Fiorani, F.; Schurr, U. Future scenarios for plant phenotyping. Annu. Rev. Plant Biol. 2013, 64, 267–291. [Google Scholar] [CrossRef] [Green Version]
- Araus, J.L.; Cairns, J.E. Field high-throughput phenotyping: The new crop breeding frontier. Trends Plant Sci. 2014, 19, 52–61. [Google Scholar] [CrossRef]
- Pommel, B.; Gallais, A.; Coque, M.; Quilleré, I.; Hirel, B.; Prioul, J.L.; Andrieu, B.; Floriot, M. Carbon and nitrogen allocation and grain filling in three maize hybrids differing in leaf senescence. Eur. J. Agron. 2006, 24, 203–211. [Google Scholar] [CrossRef]
- Gooding, M.J.; Dimmock, J.P.R.E.; France, J.; Jones, S.A. Green leaf area decline of wheat flag leaves: The influence of fungicides and relationships with mean grain weight and grain yield. Ann. Appl. Biol. 2000, 136, 77–84. [Google Scholar] [CrossRef]
- Vilmus, I.; Ecarnot, M.; Verzelen, N.; Roumet, P. Monitoring nitrogen leaf resorption kinetics by near-infrared spectroscopy during grain filling in durum wheat in different nitrogen availability conditions. Crop Sci. 2014, 54, 284–296. [Google Scholar] [CrossRef]
- Acquaah, G. Principles of Plant Genetics and Breeding; Blackwell Publishing Ltd: Malden, MA, USA, 2007; ISBN 1444309013. [Google Scholar]
- Foulkes, M.J.; Slafer, G.A.; Davies, W.J.; Berry, P.M.; Sylvester-Bradley, R.; Martre, P.; Calderini, D.F.; Griffiths, S.; Reynolds, M.P. Raising yield potential of wheat. III. Optimizing partitioning to grain while maintaining lodging resistance. J. Exp. Bot. 2011, 62, 469–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Unkovich, M.; Baldock, J.; Forbes, M. Variability in Harvest Index of Grain Crops and Potential Significance for Carbon Accounting. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Burlington, NC, USA, 2010; Volume 105, pp. 173–219. ISBN 9780123810236. [Google Scholar]
- Prey, L.; Germer, M.; Schmidhalter, U. Temporal and Organ-specific Responses in NUE Traits to N Fertilization, Fungicide Intensity and Early Sowing in Winter Wheat Cultivars. Agronomy 2019, 9, 313. [Google Scholar] [CrossRef] [Green Version]
- Prey, L.; Hu, Y.; Schmidhalter, U. High-Throughput Field Phenotyping Traits of Grain Yield Formation and Nitrogen Use Efficiency: Optimizing the Selection of Vegetation Indices and Growth Stages. Front. Plant Sci. 2020, 10, 1672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raun, W.R.; Solie, J.B.; Johnson, G.V.; Stone, M.L.; Lukina, E.V.; Thomason, W.E.; Schepers, J.S. In-Season Prediction of Potential Grain Yield in Winter Wheat Using Canopy Reflectance. Agron. J. 2001, 93, 131–138. [Google Scholar] [CrossRef] [Green Version]
- Freeman, K.W.; Raun, W.R.; Johnson, G.V.; Mullen, R.W.; Stone, M.L.; Solie, J.B. Late-season prediction of wheat grain yield and grain protein. Commun. Soil Sci. Plant Anal. 2003, 34, 1837–1852. [Google Scholar] [CrossRef]
- Babar, M.A.; van Ginkel, M.; Reynolds, M.P.; Prasad, B.; Klatt, A.R. Heritability, correlated response, and indirect selection involving spectral reflectance indices and grain yield in wheat. Aust. J. Agric. Res. 2007, 58, 432–442. [Google Scholar] [CrossRef]
- Babar, M.A.; van Ginkel, M.; Klatt, A.R.; Prasad, B.; Reynolds, M.P. The Potential of Using Spectral Reflectance Indices to Estimate Yield in Wheat Grown Under Reduced Irrigation. Euphytica 2006, 150, 155–172. [Google Scholar] [CrossRef]
- Babar, M.A.; Reynolds, M.P.; van Ginkel, M.; Klatt, A.R.; Raun, W.R.; Stone, M.L. Spectral Reflectance Indices as a Potential Indirect Selection Criteria for Wheat Yield under Irrigation. Crop Sci. 2006, 46, 578–588. [Google Scholar] [CrossRef]
- Prasad, B.; Carver, B.F.; Stone, M.L.; Babar, M.A.; Raun, W.R.; Klatt, A.R. Genetic Analysis of Indirect Selection for Winter Wheat Grain Yield Using Spectral Reflectance Indices. Crop Sci. 2007, 47, 1416–1425. [Google Scholar] [CrossRef] [Green Version]
- Prasad, B.; Carver, B.F.; Stone, M.L.; Babar, M.A.; Raun, W.R.; Klatt, A.R. Potential use of spectral reflectance indices as a selection tool for grain yield in winter wheat under great plains conditions. Crop Sci. 2007, 47, 1426–1440. [Google Scholar] [CrossRef] [Green Version]
- Gizaw, S.A.; Garland-Campbell, K.; Carter, A.H. Evaluation of agronomic traits and spectral reflectance in Pacific Northwest winter wheat under rain-fed and irrigated conditions. Field Crop. Res. 2016, 196, 168–179. [Google Scholar] [CrossRef] [Green Version]
- Gizaw, S.A.; Garland-Campbell, K.; Carter, A.H. Use of spectral reflectance for indirect selection of yield potential and stability in Pacific Northwest winter wheat. Field Crop. Res. 2016, 196, 199–206. [Google Scholar] [CrossRef] [Green Version]
- Montesinos-López, O.A.; Montesinos-López, A.; Crossa, J.; los Campos, G.; Alvarado, G.; Suchismita, M.; Rutkoski, J.; González-Pérez, L.; Burgueño, J. Predicting grain yield using canopy hyperspectral reflectance in wheat breeding data. Plant Methods 2017, 13, 4. [Google Scholar] [CrossRef] [Green Version]
- Gutierrez, M.; Reynolds, M.P.; Raun, W.R.; Stone, M.L.; Klatt, A.R. Spectral water indices for assessing yield in elite bread wheat genotypes under well-irrigated, water-stressed, and high-temperature conditions. Crop Sci. 2010, 50, 197–214. [Google Scholar] [CrossRef]
- Christopher, J.T.; Veyradier, M.; Borrell, A.K.; Harvey, G.; Fletcher, S.; Chenu, K. Phenotyping novel stay-green traits to capture genetic variation in senescence dynamics. Funct. Plant Biol. 2014, 41, 1035–1048. [Google Scholar] [CrossRef]
- Becker, E.; Schmidhalter, U. Evaluation of Yield and Drought Using Active and Passive Spectral Sensing Systems at the Reproductive Stage in Wheat. Front. Plant Sci. 2017, 8, 379. [Google Scholar] [CrossRef] [Green Version]
- Aparicio, N.; Villegas, D.; Casadesus, J.; Araus, J.L.; Royo, C. Spectral Vegetation Indices as Nondestructive Tools for Determining Durum Wheat Yield. Agron. J. 2000, 92, 83–91. [Google Scholar] [CrossRef]
- Pavuluri, K.; Chim, B.K.; Griffey, C.A.; Reiter, M.S.; Balota, M.; Thomason, W.E. Canopy spectral reflectance can predict grain nitrogen use efficiency in soft red winter wheat. Precis. Agric. 2015, 16, 405–424. [Google Scholar] [CrossRef]
- Barmeier, G.; Schmidhalter, U. High-Throughput Field Phenotyping of Leaves, Leaf Sheaths, Culms and Ears of Spring Barley Cultivars at Anthesis and Dough Ripeness. Front. Plant Sci. 2017, 8, 1920. [Google Scholar] [CrossRef] [Green Version]
- Frels, K.; Guttieri, M.; Joyce, B.; Leavitt, B.; Baenziger, P.S. Evaluating canopy spectral reflectance vegetation indices to estimate nitrogen use traits in hard winter wheat. Field Crop. Res. 2018, 217, 82–92. [Google Scholar] [CrossRef]
- Mistele, B.; Schmidhalter, U. Tractor-based quadrilateral spectral reflectance measurements to detect biomass and total aerial nitrogen in winter wheat. Agron. J. 2010, 102, 499–506. [Google Scholar] [CrossRef]
- Erdle, K.; Mistele, B.; Schmidhalter, U. Comparison of active and passive spectral sensors in discriminating biomass parameters and nitrogen status in wheat cultivars. Field Crop. Res. 2011, 124, 74–84. [Google Scholar] [CrossRef]
- Simko, I.; Jimenez-Berni, J.A.; Sirault, X.R.R. Phenomic approaches and tools for phytopathologists. Phytopathology 2016, 107, 6–17. [Google Scholar] [CrossRef] [Green Version]
- Prey, L.; Schmidhalter, U. Temporal and Spectral Optimization of Vegetation Indices for Estimating Grain Nitrogen Uptake and Late-Seasonal Nitrogen Traits in Wheat. Sensors 2019, 19, 4640. [Google Scholar] [CrossRef] [Green Version]
- Hatfield, J.L.; Gitelson, A.A.; Schepers, J.S.; Walthall, C.L. Application of Spectral Remote Sensing for Agronomic Decisions. Agron. J. 2008, 100, 117–131. [Google Scholar] [CrossRef] [Green Version]
- Prey, L.; von Bloh, M.; Schmidhalter, U. Evaluating RGB Imaging and Multispectral Active and Hyperspectral Passive Sensing for Assessing Early Plant Vigor in Winter Wheat. Sensors 2018, 18, 2931. [Google Scholar] [CrossRef] [Green Version]
- Kipp, S.; Mistele, B.; Baresel, P.; Schmidhalter, U. High-throughput phenotyping early plant vigour of winter wheat. Eur. J. Agron. 2014, 52, 271–278. [Google Scholar] [CrossRef]
- Prey, L.; Schmidhalter, U. Simulation of satellite reflectance data using high-frequency ground based hyperspectral canopy measurements for in-season estimation of grain yield and grain nitrogen status in winter wheat. ISPRS J. Photogramm. Remote Sens. 2019, 149, 176–187. [Google Scholar] [CrossRef]
- Rischbeck, P.; Elsayed, S.; Mistele, B.; Barmeier, G.; Heil, K.; Schmidhalter, U. Data fusion of spectral, thermal and canopy height parameters for improved yield prediction of drought stressed spring barley. Eur. J. Agron. 2016, 78, 44–59. [Google Scholar] [CrossRef]
- Erdle, K.; Mistele, B.; Schmidhalter, U. Spectral assessments of phenotypic differences in spike development during grain filling affected by varying N supply in wheat. J. Plant Nutr. Soil Sci. 2013, 176, 952–963. [Google Scholar] [CrossRef]
- Erdle, K.; Mistele, B.; Schmidhalter, U. Spectral high-throughput assessments of phenotypic differences in biomass and nitrogen partitioning during grain filling of wheat under high yielding Western European conditions. Field Crop. Res. 2013, 141, 16–26. [Google Scholar] [CrossRef]
- Elsayed, S.; Rischbeck, P.; Schmidhalter, U. Comparing the performance of active and passive reflectance sensors to assess the normalized relative canopy temperature and grain yield of drought-stressed barley cultivars. Field Crop. Res. 2015, 177, 148–160. [Google Scholar] [CrossRef]
- Shearman, V.J.; Sylvester-Bradley, R.; Scott, R.K.; Foulkes, M.J. Physiological processes associated with wheat yield progress in the UK. Crop Sci. 2005, 45, 175–185. [Google Scholar] [CrossRef]
- Ustin, S.L.; Gitelson, A.A.; Jacquemoud, S.; Schaepman, M.; Asner, G.P.; Gamon, J.A.; Zarco-tejada, P. Remote Sensing of Environment Retrieval of foliar information about plant pigment systems from high resolution spectroscopy. Remote Sens. Environ. 2009, 113, S67–S77. [Google Scholar] [CrossRef] [Green Version]
- Garriga, M.; Romero-Bravo, S.; Estrada, F.; Escobar, A.; Matus, I.A.; del Pozo, A.; Astudillo, C.A.; Lobos, G.A. Assessing Wheat Traits by Spectral Reflectance: Do We Really Need to Focus on Predicted Trait-Values or Directly Identify the Elite Genotypes Group? Front. Plant Sci. 2017, 8, 280. [Google Scholar] [CrossRef] [Green Version]
- Fava, F.; Colombo, R.; Bocchi, S.; Meroni, M.; Sitzia, M.; Fois, N.; Zucca, C. Identification of hyperspectral vegetation indices for Mediterranean pasture characterization. Int. J. Appl. Earth Obs. Geoinf. 2009, 11, 233–243. [Google Scholar] [CrossRef]
- Haboudane, D.; Miller, J.R.; Tremblay, N.; Zarco-Tejada, P.J.; Dextraze, L. Integrated narrow-band vegetation indices for prediction of crop chlorophyll content for application to precision agriculture. Remote Sens. Environ. 2002, 81, 416–426. [Google Scholar] [CrossRef]
- Yang, G.; Liu, J.; Zhao, C.; Li, Z.; Huang, Y.; Yu, H.; Xu, B.; Yang, X.; Zhu, D.; Zhang, X.; et al. Unmanned Aerial Vehicle Remote Sensing for Field-Based Crop Phenotyping: Current Status and Perspectives. Front. Plant Sci. 2017, 8, 1111. [Google Scholar] [CrossRef]



| Date (Month/Day) | d.a.s. (Cont, RF) | Growth Stage |
|---|---|---|
| 03/31 | 159 | leaf development |
| 04/13 | 172 | tillering |
| 05/17 | 206 | stem elongation |
| 05/25 | 214 | booting |
| 06/01 | 221 | ear emergence |
| 06/08 | 228 | anthesis |
| 06/21 | 241 | early milk |
| 06/26 | 246 | late milk |
| 07/01 | 251 | early dough |
| 07/04 | 255 | soft dough |
| Seasonal Best R2-Value | Optimum Date | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trait Group | Trait | Best SVI | WMMRS | Full Data | Cont_SD1 | Cont_RF | MP*N | Full Data | Cont_SD1 | Cont_RF | MP*N | ||||
| DM [kg ha−1] | Ant. spikes | BRI | 17 | 0.18 | *** | 0.26 | *** | 0.19 | *** | 0.28 | ** | 06/26 | 07/01 | 06/26 | 07/04 |
| Ant. stems | GNDVI | 13 | 0.16 | *** | 0.23 | *** | 0.17 | *** | 0.27 | ** | 05/25 | 05/25 | 06/08 | 06/08 | |
| Ant. flag leaf | R780_R550 | 13 | 0.31 | *** | 0.45 | *** | 0.26 | *** | 0.39 | *** | 06/26 | 06/21 | 06/26 | 07/04 | |
| Ant. flag leaf -1 | LCI | 12 | 0.34 | *** | 0.41 | *** | 0.33 | *** | 0.40 | *** | 06/08 | 06/08 | 06/08 | 06/21 | |
| Ant. other leaves | R787_R765 | 12 | 0.33 | *** | 0.34 | *** | 0.16 | *** | 0.29 | ** | 06/08 | 06/08 | 06/08 | 06/08 | |
| Ant. leaves | R780_R550 | 13 | 0.32 | *** | 0.40 | *** | 0.25 | *** | 0.39 | *** | 06/08 | 06/08 | 06/08 | 06/08 | |
| Mat. grain | NWI_5 | 15 | 0.67 | *** | 0.47 | *** | 0.65 | *** | 0.50 | *** | 07/01 | 07/01 | 06/26 | 07/01 | |
| Mat. chaff | BRI | 14 | 0.21 | *** | 0.22 | *** | 0.25 | *** | 0.22 | ** | 06/21 | 06/26 | 06/21 | 06/21 | |
| Mat. stems | NDRE_770_750 | 14 | 0.31 | *** | 0.37 | *** | 0.26 | *** | 0.36 | *** | 05/25 | 05/17 | 05/25 | 06/21 | |
| Mat. flag leaf | NDRE_770_750 | 12 | 0.27 | *** | 0.41 | *** | 0.20 | *** | 0.40 | *** | 07/04 | 07/01 | 07/04 | 07/04 | |
| Mat. flag leaf -1 | R780_R550 | 11 | 0.32 | *** | 0.37 | *** | 0.34 | *** | 0.39 | *** | 06/26 | 07/01 | 07/04 | 07/01 | |
| Mat. other leaves | GNDVI | 13 | 0.28 | *** | 0.30 | *** | 0.31 | *** | 0.43 | *** | 06/08 | 06/08 | 06/08 | 06/08 | |
| Mat. leaves | R780_R550 | 12 | 0.37 | *** | 0.46 | *** | 0.32 | *** | 0.50 | *** | 06/26 | 06/26 | 06/26 | 06/26 | |
| Ant. total | LCI | 13 | 0.16 | *** | 0.21 | *** | 0.18 | *** | 0.28 | ** | 06/08 | 06/01 | 06/08 | 06/08 | |
| Mat. total | NDRE_770_750 | 12 | 0.42 | *** | 0.56 | *** | 0.53 | *** | 0.50 | *** | 06/08 | 05/17 | 06/26 | 06/26 | |
| derived DM | HI | R787_R765 | 18 | 0.27 | *** | 0.10 | ** | 0.37 | *** | 0.26 | ** | 07/01 | 06/08 | 07/01 | 06/08 |
| PAA | NWI_3 | 16 | 0.16 | *** | 0.15 | *** | 0.12 | ** | 0.13 | n.s. | 07/01 | 05/17 | 05/17 | 06/26 | |
| CPostAA | NWI_2 | 18 | 0.08 | ** | 0.10 | ** | 0.09 | ** | 0.09 | n.s. | 05/17 | 05/17 | 05/17 | 06/26 | |
| DMTEff | TCARI_OSAVI | 25 | 0.12 | *** | 0.13 | *** | 0.11 | ** | 0.09 | n.s. | 06/26 | 06/26 | 07/01 | 06/26 | |
| DMT | TCARI_OSAVI | 21 | 0.07 | ** | 0.07 | * | 0.05 | * | 0.08 | n.s. | 07/04 | 07/04 | 06/08 | 06/08 | |
| GNS | BGI | 30 | 0.24 | *** | 0.27 | *** | 0.31 | *** | 0.34 | *** | 06/08 | 06/08 | 06/21 | 06/01 | |
| TKW | NWI_2 | 13 | 0.25 | *** | 0.11 | ** | 0.43 | *** | 0.10 | n.s. | 07/04 | 06/08 | 07/04 | 06/08 | |
| NutEff total | REIP | 17 | 0.32 | *** | 0.40 | *** | 0.31 | *** | 0.16 | * | 06/26 | 06/26 | 05/17 | 05/25 | |
| NutEff grain | REIP | 15 | 0.34 | *** | 0.46 | *** | 0.36 | *** | 0.26 | ** | 05/25 | 06/26 | 05/25 | 07/04 | |
| NUE Mat. total | RVSI | 24 | 0.26 | *** | 0.29 | *** | 0.19 | *** | 0.42 | *** | 07/01 | 07/01 | 07/01 | 06/21 | |
| NUE Mat. grain | R787_R765 | 25 | 0.38 | *** | 0.53 | *** | 0.30 | *** | 0.32 | *** | 06/26 | 06/26 | 06/26 | 04/13 | |
| spike density | PSSR | 20 | 0.28 | *** | 0.46 | *** | 0.23 | *** | 0.40 | *** | 06/21 | 06/21 | 06/21 | 06/21 | |
| yield per spike | BGI | 14 | 0.27 | *** | 0.30 | *** | 0.21 | *** | 0.36 | *** | 03/31 | 06/08 | 06/21 | 05/25 | |
| kernels per m2 | NDRE_770_750 | 15 | 0.26 | *** | 0.24 | *** | 0.23 | *** | 0.23 | ** | 05/17 | 05/17 | 05/17 | 05/17 | |
| others | anthesis date | Maccioni | 12 | 0.40 | *** | 0.54 | *** | 0.49 | *** | 0.59 | *** | 06/21 | 07/04 | 06/21 | 06/21 |
| plant height | REIP | 15 | 0.51 | *** | 0.55 | *** | 0.45 | *** | 0.56 | *** | 05/25 | 05/25 | 05/25 | 06/21 | |
| DM | Derived DM | ||||
|---|---|---|---|---|---|
| Ant. spikes | 0.90 | *** | HI | 0.23 | |
| Ant. stems | 0.77 | *** | PAA | 0.82 | *** |
| Ant. flag leaf | 0.90 | *** | CPostAA | −0.13 | |
| Ant. flag leaf-1 | 0.90 | *** | DMTEff | 0.16 | |
| Ant. other leaves | 0.82 | *** | DMT | 0.47 | *** |
| Ant. leaves | 0.98 | *** | GNS | 0.86 | *** |
| Mat. grain | 0.98 | *** | TKW | −0.24 | |
| Mat. chaff | 0.93 | *** | NutEff total | 0.70 | *** |
| Mat. stems | 0.76 | *** | NutEff grain | 0.81 | *** |
| Mat. flag leaf | 0.73 | *** | NUE Mat. total | −0.19 | |
| Mat. flag leaf-1 | 0.90 | *** | NUE Mat. grain | −0.03 | |
| Mat. other leaves | 0.92 | *** | spike density | 0.86 | *** |
| Mat. leaves | 0.94 | *** | yield per spike | 0.63 | *** |
| Ant. total | 0.86 | *** | kernels per m2 | 0.95 | *** |
| Mat. total | 0.97 | *** | |||
| other traits | |||||
| anthesis date | 0.81 | *** | |||
| plant height | 0.91 | *** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prey, L.; Schmidhalter, U. Deep Phenotyping of Yield-Related Traits in Wheat. Agronomy 2020, 10, 603. https://doi.org/10.3390/agronomy10040603
Prey L, Schmidhalter U. Deep Phenotyping of Yield-Related Traits in Wheat. Agronomy. 2020; 10(4):603. https://doi.org/10.3390/agronomy10040603
Chicago/Turabian StylePrey, Lukas, and Urs Schmidhalter. 2020. "Deep Phenotyping of Yield-Related Traits in Wheat" Agronomy 10, no. 4: 603. https://doi.org/10.3390/agronomy10040603
APA StylePrey, L., & Schmidhalter, U. (2020). Deep Phenotyping of Yield-Related Traits in Wheat. Agronomy, 10(4), 603. https://doi.org/10.3390/agronomy10040603





