Crop Response to Low Phosphorus Bioavailability with a Focus on Tomato
Abstract
:1. Introduction
2. Phosphorus in an Agroecosystem
2.1. Phosphorus Pools and Sources
2.2. Soil Phosphorus Fluxes
2.3. Phosphorus Bioavailability Across Different Soils
2.4. Phosphorus Uptake and Integration
3. Plant Physiological Responses to Low Phosphorus Stress
3.1. Internal Phosphorus Sensing
3.2. Phosphorus Reprioritization
3.3. Cellular Phosphorus Homeostasis
4. Strategies to Enhance PAE
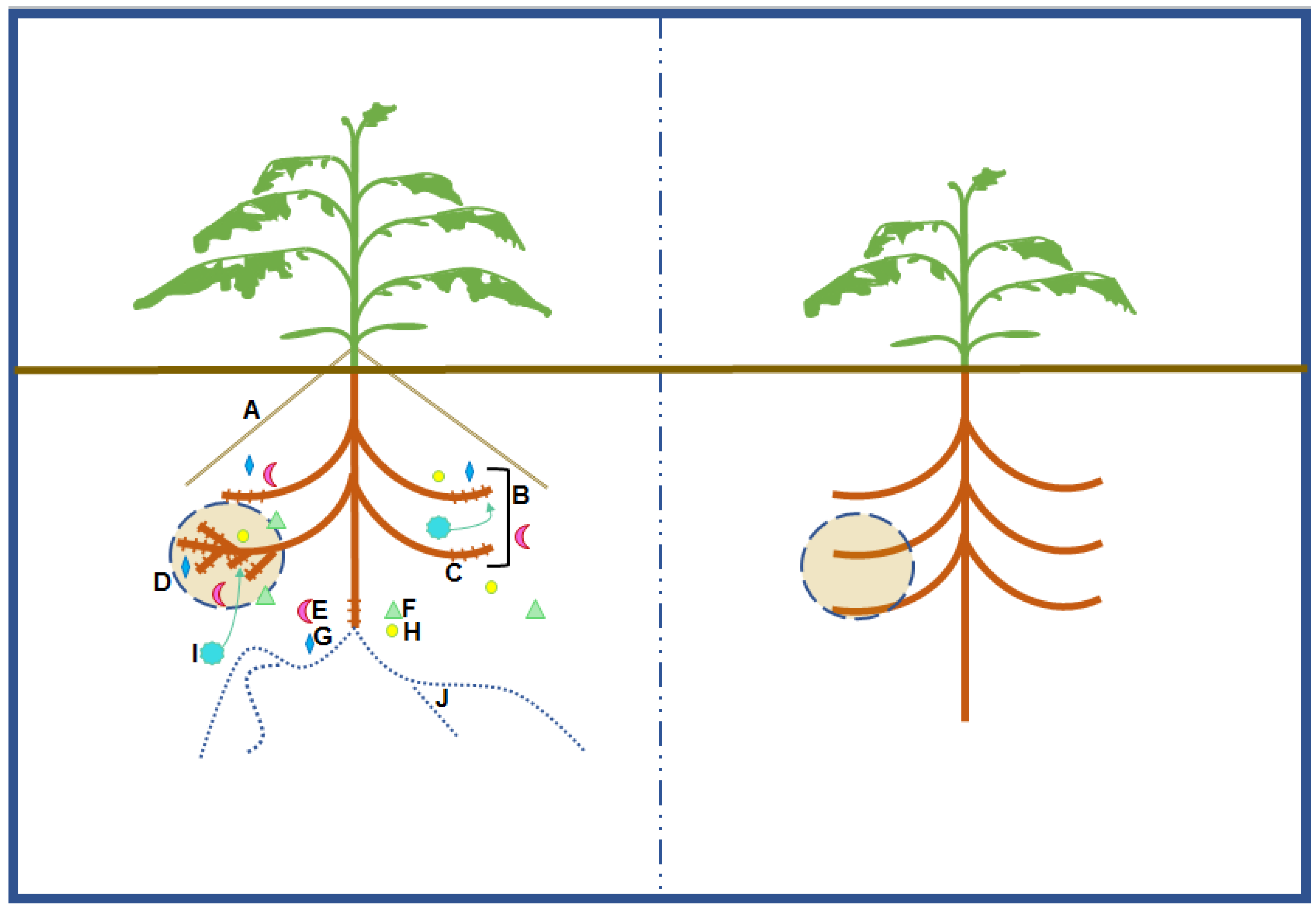
4.1. Root Morphological Responses
4.2. Exudation of Root Derived Compounds
4.3. Microbial Symbiosis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Plaxton, W.C.; Tran, H.T. Metabolic adaptations of phosphate-starved plants. Plant Physiol. 2011, 156, 1006–1015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Havlin, J.L.; Tisdale, S.L.; Nelson, W.L.; Beaton, J.D. Phosphorus. In Soil Fertility and Fertilizers, 8th ed.; Lawrensen, W., Gohn, J., Eds.; Pearson Inc.: Upper Saddle River, NJ, USA, 2014; pp. 185–221. ISBN 978-0-13-503373-9. [Google Scholar]
- Close, D.C.; Beadle, C.L. The ecophysiology of foliar anthocyanin. Bot. Rev. 2003, 69, 149–161. [Google Scholar] [CrossRef]
- Smith, G.S.; Cornforth, I.S.; Henderson, H.V. Critical leaf concentrations for deficiencies of nitrogen, potassium, phosphorus, Sulphur, and magnesium in perennial ryegrass. New Phytol. 1985, 101, 393–409. [Google Scholar] [CrossRef]
- Alsaeedi, A.H.; Elprince, A.M. Critical phosphorus levels for Salicornia growth. Agron. J. 1999, 92, 336–345. [Google Scholar] [CrossRef]
- Johansen, C.; Merkley, K.E.; Dolby, G.R. Critical phosphorus concentrations in parts of Macroptilium atropurpureum cv. Siratro and Desmodium intortum cv. Greenleaf as affected by plant age. Aust. J. Agric. Res. 1980, 31, 693–702. [Google Scholar] [CrossRef]
- Stribley, D.P.; Tinker, P.B.; Rayner, J.H. Relation of internal phosphorus concentration and plant weight in plants infected by vesicular-arbuscular mycorrhizas. New Phytol. 1980, 86, 261–266. [Google Scholar] [CrossRef]
- Haneklaus, S.H.; Schnug, E. Assessing the plant phosphorus status. In Phosphorus in Agriculture: 100% Zero; Schnug, E., De Kok, L., Eds.; Springer: Dordrecht, The Netherlands, 2016; pp. 95–125. ISBN 978-94-017-7612-7. [Google Scholar]
- Jones, J.B., Jr. Phosphorus toxicity in tomato plants: When and how does it occur? Commun. Soil Sci. Plant Anal. 1998, 29, 1779–1784. [Google Scholar] [CrossRef]
- Fageria, N.K.; Wright, R.J.; Baligar, V.C. Rice cultivar evaluation for phosphorus use efficiency. Plant Soil 1988, 111, 105–109. [Google Scholar] [CrossRef]
- Wang, X.; Yan, X.; Liao, H. Genetic improvement for phosphorus efficiency in soybean: A radical approach. Ann. Bot. 2010, 106, 215–222. [Google Scholar] [CrossRef]
- Costa, J.M.; Csizinszky, A.A.; Dorais, M.A.; Jones, J.B.; Huevelink, E.; Lindhout, P.; Peet, M.M.; Saltveit, M.E.; Schuster, D.J.; van Lenteren, J.C.; et al. Tomatoes; CABI: Cambridge, MA, USA, 2005; ISBN 0-85199-396-6. [Google Scholar]
- Castner, J.L. Solanaceae. In Photographic Atlas of Botany and Guide to Plant Identification; Feline Press: Gainesville, FL, USA, 2004; pp. 214–215. ISBN 0-9625150-0-0. [Google Scholar]
- Kelley, W.T.; Boyhan, G.E.; Harrison, K.A.; Sumner, P.E.; Langston, D.B.; Sparks, A.N.; Culpepper, S.; Hurst, W.C.; Fonsah, E.G. Commercial Tomato Production Handbook; University of Georgia Extension: Athens, GA, USA, 2010. [Google Scholar]
- Davies, J.N.; Hobson, G.E.; McGlasson, W.B. The constituents of tomato fruit: The influence of environment, nutrition, and genotype. Crit. Rev. Food Sci. Nutr. 1981, 15, 205–280. [Google Scholar] [CrossRef]
- Bhowmik, D.; Kumar, K.S.; Paswan, S.; Srivastava, S. Tomato—A natural medicine and its health benefits. J. Pharmacogn. Phytochem. 2012, 1, 33–43. [Google Scholar]
- Rao, A.V.; Waseem, Z.; Agarwal, S. Lycopene content of tomatoes and tomato products and their contribution to dietary lycopene. Food Res. Int. 1998, 31, 737–741. [Google Scholar] [CrossRef]
- Gerszberg, A.; Hnatuszko-Konka, K. Tomato tolerance to abiotic stress: A review of most often engineered target sequences. Plant Growth Regul. 2017, 83, 175–198. [Google Scholar] [CrossRef] [Green Version]
- Food and Agriculture Organization (FAO) of the United Nations. Crops, Tomatoes; Food and Agriculture Organization (FAO) of the United Nations: Rome, Italy, 2019. [Google Scholar]
- Bai, Y.; Lindhout, P. Domestication and breeding of tomatoes: What have we gained and what can we gain in the future? Ann. Bot. 2007, 100, 1085–1094. [Google Scholar] [CrossRef] [PubMed]
- Coltman, R.; Gerloff, G.; Gabelman, W. Intraspecific variation in growth, phosphorus acquisition and phosphorus utilization in tomatoes under phosphorus-deficiency stress. In Proceedings of the 9th International Plant Nutrition Colloquium, Coventry, UK, 22–27 August 1982. [Google Scholar]
- Soderlund, R.; Svensson, B.H. The global nitrogen cycle. Ecol. Bull. 1976, 7, 23–73. [Google Scholar]
- Fuentes-Lara, L.O.; Medrano-Macías, J.; Pérez-Labrada, F.; Rivas-Martínez, E.N.; García-Enciso, E.L.; González-Morales, S.; Juárez-Maldonado, A.; Rincón-Sánchez, F.; Benavides-Mendoza, A. From elemental sulfur to hydrogen sulfide in agricultural soils and plants. Molecules 2019, 24, 2282. [Google Scholar] [CrossRef] [Green Version]
- Tang, H.; Liu, Y.; Li, X.; Muhammad, A.; Huang, G. Carbon sequestration of cropland and paddy soils in China: Potential, driving factors, and mechanisms. Greenh. Gases Sci. Technol. 2019, 9, 872–885. [Google Scholar] [CrossRef]
- Liu, G.D.; Simonne, E.H.; Morgan, K.T.; Hochmuth, G.J.; Agehara, S.; Mylavarapu, R. Fertilizer management for vegetable production in Florida. In Vegetable Production Handbook of Florida, 23rd ed.; Chapter 2; Dittmar, P., Paret, M., Freeman, J., Smith, H., Eds.; University of Florida Institute of Food and Agricultural Sciences: Gainesville, FL, USA, 2019; pp. 3–9. [Google Scholar]
- Kaya, C.; Kirnak, H.; Higgs, D. Enhancement of growth and normal growth parameters by foliar application of potassium and phosphorus in tomato cultivars grown at high (NaCl) salinity. J. Plant Nutr. 2001, 24, 357–367. [Google Scholar] [CrossRef]
- Rady, M.M.; El-Shewy, A.A.; El-Yazal, M.A.S.; Abdelaal, K.E.S. Response of salt-stressed common bean plant performances to foliar application of phosphorus (MAP). Int. Lett. Nat. Sci. 2018, 72, 7–20. [Google Scholar] [CrossRef]
- Mosali, J.; Desta, K.; Teal, R.K.; Freeman, K.W.; Martin, K.L.; Lawles, J.W.; Raun, W.R. Effect of foliar application of phosphorus on winter wheat grain yield, phosphorus uptake, and use efficiency. J. Plant Nutr. 2006, 29, 2147–2163. [Google Scholar] [CrossRef]
- Ticconi, C.A.; Abel, S. Short on phosphate: Plant surveillance and countermeasures. Trends Plant Sci. 2004, 9, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Poehlein, A.; Daniel, R.; Schink, B.; Simeonova, D.D. Life based on phosphite: A genome-guided analysis of Desulfotignum phosphitoxidans. BMC Genom. 2013, 14, 753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rolando, C.; Gaskin, R.; Horgan, D.; Williams, N.; Bader, M.K.F. The use of adjuvants to improve uptake of phosphorous acid applied to Pinus radiata needles for control of foliar Phytophthora diseases. N. Z. J. Sci. 2014, 44, 8. [Google Scholar] [CrossRef] [Green Version]
- Bertsch, F.; Ramírez, F.; Henríquez, C. Evaluación del fosfito como fuente fertilizante de fósforo vía radical y foliar. Agron. Costarric. 2009, 33, 249–265. [Google Scholar]
- Gómez-Merino, F.C.; Trejo-Téllez, L.I. Biostimulant activity of phosphite in horticulture. Sci. Hortic. 2015, 196, 82–90. [Google Scholar] [CrossRef] [Green Version]
- Ozturk, L.; Eker, S.; Torun, B.; Cakmak, I. Variation in phosphorus efficiency among 73 bread and durum wheat genotypes grown in a phosphorus-deficient calcareous soil. Plant Soil 2005, 269, 69–80. [Google Scholar] [CrossRef]
- Cordell, D.; Schmid-Neset, T.; White, S.; Drangert, J.-O. Preferred future phosphorus scenarios: A framework for meeting long-term phosphorus needs for global food demand. In International Conference on Nutrient Recovery from Wastewater Streams; Ashlet, K., Mavinic, D., Koch, F., Eds.; IWA Publishing: London, UK, 2009; pp. 23–43. ISBN 9781843392323. [Google Scholar]
- Harris, D.C.; Lucy, C.A. Quantitataive Chemical Analysis, 9th ed.; Schultz, L., Murphy, B., Bristow, A., Eds.; W.H. Freeman and Company: New York, NY, USA, 2016; ISBN 978-1-4641-3538-5. [Google Scholar]
- Edwards, C.L.; Maguire, R.O.; Whitehurst, G.B.; Thomason, W.E.; Alley, M.M. Using synthetic chelating agents to decrease phosphorus binding in soils. Soil Sci. 2016, 181, 377–385. [Google Scholar] [CrossRef]
- Qin, Z.; Shober, A.L.; Scheckel, K.G.; Penn, C.J.; Turner, K.C. Mechanisms of phosphorus removal by phosphorus sorbing materials. J. Environ. Qual. 2018, 47, 1232–1241. [Google Scholar] [CrossRef]
- Fang, H.; Cui, Z.; He, G.; Huang, L.; Chen, M. Phosphorus adsorption onto clay minerals and iron oxide with consideration of heterogenous particle morphology. Sci. Total Environ. 2017, 605–606, 357–367. [Google Scholar] [CrossRef]
- Li, M.; Liu, J.; Xu, Y.; Guangren, Q. Phosphate adsorption on metal oxides and metal hydroxides: A comparative review. Environ. Rev. 2016, 24, 319–332. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, Y.Q.; Babatunde, A.O.; Wang, L.; Ren, Y.X.; Han, Y. Characteristics and mechanisms of phosphate adsorption on dewatered alum sludge. Sep. Purif. Technol. 2006, 51, 193–200. [Google Scholar] [CrossRef] [Green Version]
- Freeman, J.S.; Rowell, D.L. The adsorption and precipitation of phosphate onto calcite. J. Soil Sci. 1981, 32, 75–84. [Google Scholar] [CrossRef]
- Péret, B.; Clément, M.; Nussaume, L.; Desnos, T. Root developmental adaptation to phosphate starvation: Better safe than sorry. Trends Plant Sci. 2011, 16, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Roberts, T.L. Improving nutrient use efficiency. Turk. J. Agr. 2008, 32, 177–182. [Google Scholar]
- Chacon, N.; Silver, W.L.; Dubinsky, E.A.; Cusack, D.F. Iron reduction and soil phosphorus solubilization in humid tropical forests soils: The roles of labile carbon pools and an electron shuttle compounds. Biogeochemistry 2006, 78, 67–84. [Google Scholar] [CrossRef]
- Wang, E.; Bell, M.; Luo, Z.; Moody, P.; Probert, M.E. Modelling crop response to phosphorus inputs and phosphorus use efficiency in a crop rotation. Field Crops Res. 2014, 155, 120–132. [Google Scholar] [CrossRef]
- Schröder, J.J.; Smit, A.L.; Cordell, D.; Rosemarin, A. Improved phosphorus use efficiency in agriculture: A key requirement for its sustainable use. Chemosphere 2011, 84, 822–831. [Google Scholar] [CrossRef]
- Odom, H.T.; Kangas, P.; Best, G.R.; Rushton, B.T.; Leibowitz, S.; Butner, J.R. Studies on Phosphate Mining, Reclamation, and Energy; Center of Wetlands, University of Florida: Gainesville, FL, USA, 1981. [Google Scholar]
- Ballard, R.; Fiskell, J.G.A. Phosphorus retention in coastal plain forest soils: I. Relationship to soil properties. Soil Sci. Soc. Am. J. 1974, 38, 250–255. [Google Scholar] [CrossRef]
- Wilhelm, R.G.; Beam, P.; Krupka, K.M.; Kaplan, D.I.; Whelan, G.; Serne, R.J.; Mattigod, S.V. Understanding Variation in Partition Coefficient, Kd, Values: The Kd Model, Methods of Measurement, and Application of Chemical Reaction Codes; EPA 402-R-99-004A; United States Environmental Protection Agency and Office of Air and Radiation: Washington, DC, USA, 1999.
- Shaheen, S.; Tsadilas, C. Phosphorus sorption and availability to canola grown in an Alfisol amended with various soil amendments. Commun. Soil Sci. Plant Anal. 2013, 44, 89–103. [Google Scholar] [CrossRef]
- Ditzler, C.A. Soil properties and classification (soil taxonomy). In The Soils of the USA; West, L.T., Singer, M.J., Hartemink, A.E., Eds.; Springer International Publishing: Gewerbestrasse/Cham, Switzerland, 2017; pp. 29–42. ISBN 978-3-319-41868-1. [Google Scholar]
- United States Department of Agriculture (USDA) Natural Resource Conservation Service (NRCS). Global Soil Regions Map. 2005. Available online: https://www.nrcs.usda.gov/wps/portal/nrcs/detail/soils/use/worldsoils/?cid=nrcs142p2_054013 (accessed on 10 April 2020).
- Ahmed, A.W.A.M.; Elsheikh, M.A.; El Mahi, Y.E.G. Relationship between phosphorus fractions of some selected Sudanese soil orders to phosphate availability. Eurasian J. Soil Sci. 2018, 7, 224–229. [Google Scholar] [CrossRef]
- Bayuelo-Jiménez, J.S.; Muraoka, T.; de la Cruz-Torres, E.; Quintero-Ponce, E.; Paredes-Gutiérrez, L.C.; Zaman, M. Phosphorus fractions and dynamics as affected by land—Use changes in the Central Mexican highlands. Soil Use Manag. 2020, 36, 240–249. [Google Scholar] [CrossRef]
- Alovisi, A.M.T.; Alovisi, A.A.; Serra, A.P.; Tokura, L.K.; Davide, L.M.C.; Lournte, E.R.P.; da Silva, R.S.; Tokura, W.I.; de Souza, D.A.; do Mar, G.D. Phosphorus fractions and their transformation in Entisol. J. Agric. Sci. 2019, 11, 485–493. [Google Scholar] [CrossRef] [Green Version]
- Esmail, A.O.; Sheikh-Abdullah, S.M.; Maruf, M.T. Phosphorus availability in Entisols, Inceptisols, and Mollisols of Iraqi Kurdistan. Soil Sci. 2019, 184, 95–100. [Google Scholar] [CrossRef]
- Kolka, R.; Bridgham, S.D.; Ping, C.-L. Soils of Peatlands: Histosols and Gelisols. In Wetland Soils: Genesis, Hydrology, Landscapes, and Classification, 2nd ed.; Vepraskas, M.J., Craft, C.B., Eds.; CRC Press: Boca Raton, FL, USA, 2016; pp. 277–310. ISBN 978-1-4398-9800-0. [Google Scholar]
- Haus, N.W.; Wilhelm, K.R.; Bockheim, J.G.; Fournelle, J.; Miller, M. A case for chemical weathering soils of Hurd Peninsula, Livingston Island, South Shetland Islands, Antarctica. Geoderma 2016, 263, 185–194. [Google Scholar] [CrossRef] [Green Version]
- Mora, E.; Toro, M.; Flores, E.; Lopez-Hernandez, D. Plant growth promoting abilities of phosphate solubilizing bacteria native from a high P-sorbing Ultisol. Ann. Adv. Agric. Sci. 2017, 1. [Google Scholar] [CrossRef]
- Camargo, L.A.; Marques, J., Jr.; Pereira, G.T.; Alleoni, L.R.F.; Bahia, A.D.S.; Teixeira, D.D.B. Pedotransfer functions to assess adsorbed phosphate using iron oxide content and magnetic susceptibility in an Oxisol. Soil Use Manag. 2016, 32, 172–182. [Google Scholar] [CrossRef]
- Rausch, C.; Bucher, M. Molecular mechanisms of phosphate transport in plants. Planta 2002, 216, 23–37. [Google Scholar] [CrossRef]
- Duby, G.; Boutry, M. The plasma membrane proton pump ATPase: A highly regulated P-type ATPase with multiple physiological roles. Pflug. Arch. Eur. J. Physiol. 2009, 457, 645–655. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E.; Moller, I.M.; Murphy, A. Translocation in the Phloem. In Plant Physiology and Development, 6th ed.; Sinauer Associates Inc.: Sunderland, MA, USA, 2015; pp. 285–316. ISBN 978-1-60535-255-8. [Google Scholar]
- Nussaume, L.; Kanno, S.; Javot, H.; Marin, E.; Nakanishi, T.M.; Thibaud, M.-C. Phosphate import in plants: Focus on the PHT1 transporters. Front. Plant Sci. 2011, 2, 83. [Google Scholar] [CrossRef] [Green Version]
- Parra-Almuna, L.; Pontigo, S.; Larama, G.; Cumming, J.R.; Perez-Tienda, J.; Ferrol, N.; de la Luz Mora, M. Expression analysis and functional characterization of two PHT1 family phosphate transporters in ryegrass. Planta 2020, 251, 6. [Google Scholar] [CrossRef]
- Versaw, W.K.; Metzenberg, R.L. Repressible cation-phosphate symporters in Neurospora crassa. Proc. Natl. Acad. Sci. USA 1995, 92, 3884–3887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakano, K. Proton/Phosphate stoichiometry in uptake of inorganic phosphate by cultured cells of Catharanthus roseus (L.) G. Don. Plant Physiol. 1990, 93, 479–483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luan, M.; Tang, R.; Tang, Y.; Tian, W.; Hou, C.; Zhao, F.; Lan, W.; Luan, S. Transport and homeostasis of potassium and phosphate limiting factors for sustainable crop production. J. Exp. Bot. 2017, 68, 3091–3105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ham, B.-K.; Chen, J.; Yan, Y.; Lucas, W.J. Insights into plant phosphate sensing and signaling. Curr. Opin. Biotechnol. 2018, 49, 1–9. [Google Scholar] [CrossRef]
- Versaw, W.K.; Harrison, M.J. A chloroplast phosphate transporter, PHT2; 1, influences allocation of phosphate within the plant and phosphate-starvation responses. Plant Cell 2002, 14, 1751–1766. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Meng, S.; Li, M.; Zhao, Z. Genomic identification and expression analysis of the phosphate transporter gene family in poplar. Front. Plant Sci. 2016, 7, 1398. [Google Scholar] [CrossRef] [Green Version]
- Guo, B.; Jin, Y.; Wussler, C.; Blancaflor, E.B.; Motes, C.M.; Versaw, W.K. Functional analysis of the Arabidopsis PHT4 family of intracellular phosphate transporters. New Phytol. 2008, 177, 889–898. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.-Y.; Huang, T.-K.; Yang, S.-Y.; Hong, Y.-T.; Huang, S.-M.; Wang, F.-N.; Chiang, S.-F.; Tsai, S.-Y.; Lu, W.-C.; Chiou, T.J. Identification of plant vacuolar transporters mediating phosphate storage. Natl. Commun. 2016, 7, 11095. [Google Scholar] [CrossRef] [Green Version]
- Teng, W.; Zhao, Y.-Y.; Zhao, X.-Q.; He, X.; Ma, W.-Y.; Deng, Y.; Chen, X.P.; Tong, Y.-P. Genome-wide identification, characterization, and expression analysis of PHT1 phosphate transporters in wheat. Front. Plant Sci. 2017, 8, 543. [Google Scholar] [CrossRef] [Green Version]
- Rae, A.L.; Cybinski, D.H.; Jarmey, J.M.; Smith, F.W. Characterization of two phosphate transporters from barley; Evidence for diverse function and kinetic properties among members of the Pht1 family. Plant Mol. Biol. 2003, 53, 27–36. [Google Scholar] [CrossRef]
- Chen, A.; Chen, X.; Wang, H.; Liao, D.; Gu, M.; Qu, H.; Sun, S.; Xu, G. Genome-wide investigation and expression analysis suggest diverse roles and genetic redundancy of Pht1 family genes in response to Pi deficiency in tomato. BMC Plant Biol. 2014, 14, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mudge, S.R.; Rae, A.L.; Diatloff, E.; Smith, F.W. Expression analysis suggests novel roles for members of the Pht1 family of phosphate transporters in Arabidopsis. Plant J. 2002, 31, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.; Shin, H.S.; Dewbre, G.R.; Harrison, M.J. Phosphate transport in Arabidopsis: Pht1;1 and Pht1;4 play a major role in phosphate acquisition from both low- and high-phosphate environments. Plant J. 2004, 39, 629–642. [Google Scholar] [CrossRef] [PubMed]
- Weiss, M.; Haimovich, G.A.L.; Pick, U.R.I. Phosphate and sulfate uptake in the halotolerant alga Dunaliella are driven by Na+-symport mechanism. J. Plant Physiol. 2001, 158, 1519–1525. [Google Scholar] [CrossRef]
- Hürlimann, H.C.; Stadler-Waibel, M.; Werner, T.P.; Freimoser, F.M. Pho91 is a vacuolar phosphate transporter that regulates phosphate and polyphosphate metabolism in Saccharomyces cerevisiae. Mol. Biol. Cell 2007, 18, 4438–4445. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Yue, W.; Ying, Y.; Wang, S.; Secco, D.; Liu, Y.; Whelan, J.; Tyerman, S.D.; Shou, H. Rice SPX-Major Facility Superfamily 3, A vacuolar phosphate efflux transporter, is involved in maintaining phosphate homeostasis in rice. Plant Physiol. 2015, 169, 2822–2831. [Google Scholar]
- Liu, C.; Muchhal, U.S.; Uthappa, M.; Kononowicz, A.K.; Ragothama, K.G. Tomato phosphate transporter genes are differently regulated in plant tissues by phosphorus. Plant Physiol. 1998, 116, 91–99. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Li, G.; Li, Y.; Min, J.; Kronzucker, H.J.; Shi, W. Tomato plants ectopically expressing Arabidopsis GRF9 show enhanced resistance to phosphate deficiency and improved fruit production in the field. J. Plant Physiol. 2018, 226, 31–39. [Google Scholar] [CrossRef]
- Leggewie, G.; Willmitzer, L.; Riesmeier, J.W. Two cDNAs from potato are able to complement a phosphate uptake-deficient yeast mutant: Identification of phosphate transporters from higher plants. Plant Cell 1997, 9, 381–392. [Google Scholar]
- Schünmann, P.H.D.; Richardson, A.E.; Smith, F.W.; Delhaize, E. Characterization of promoter expression patterns derived from the Pht1 phosphate transporter genes of barley (Hordeum vulgare L.). J. Exp. Bot. 2004, 55, 855–865. [Google Scholar] [CrossRef] [Green Version]
- Preuss, C.P.; Huang, C.Y.; Tyerman, S.D. Proton-coupled high-affinity phosphate transport revealed from heterologous characterization in Xenopus of barley-root plasma membrane transporter, HvPHT1;1. Plant Cell Environ. 2011, 34, 681–689. [Google Scholar] [CrossRef]
- Huang, C.Y.; Shirley, N.; Genc, Y.; Shi, B.; Langridge, P. Phosphate utilization efficiency correlates with expression of low-affinity phosphate transporters and noncoding RNA, IPS1, in barley. Plant Physiol. 2011, 156, 1217–1229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukatira, U.T.; Liu, C.; Varadarajan, D.K.; Raghothama, K.G. Negative regulation of phosphate starvation-induced genes. Plant Physiol. 2001, 127, 1854–1862. [Google Scholar] [CrossRef]
- Tittarelli, A.; Milla, L.; Vargas, F.; Morales, A.; Neupert, C.; Meisel, L.; Salvo-G, H.; Penaloza, E.; Munoz, G.; Corcuera, L.J. Isolation and comparative analysis of the wheat TaPT2 promoter: Identification in silico of new putative regulatory motifs conserved between monocots and dicots. J. Exp. Bot. 2007, 58, 2573–2582. [Google Scholar] [CrossRef] [PubMed]
- Kisko, M.; Bouain, N.; Rouached, A.; Choudhary, S.P.; Rouched, H. Molecular mechanisms of phosphate and zinc signaling crosstalk in plants: Phosphate and zinc loading into root xylem in Arabidopsis. Environ. Exp. Bot. 2015, 114, 57–64. [Google Scholar] [CrossRef]
- Xie, X.; Hu, W.; Fan, X.; Chen, H.; Tang, M. Interactions between phosphorus, zinc, and iron homeostasis in nonmycorrhizal and mycorrhizal plants. Front. Plant Sci. 2019, 10, 1172. [Google Scholar] [CrossRef]
- Vance, C.P.; Uhde-Stone, C.; Allan, D.L. Phosphorus acquisition and use: Critical adaptations by plants for securing a nonrenewable resource. New Phytol. 2003, 157, 423–447. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Calderón, L.; Chacon-López, A.; Pérez-Torres, C.-A.; Herrera-Estrella, L. Phosphorus: Plant strategies to cope with its scarcity. In Cell Biology of Metals and Nutrients; Springer: Berlin/Heidelberg, Germany, 2010; pp. 173–198. ISBN 978-3-642-10613-2. [Google Scholar]
- Rouached, H.; Arpat, A.B.; Poirier, Y. Regulation of phosphate starvation responses in plants: Signaling players and cross-talks. Mol. Plant 2010, 3, 288–299. [Google Scholar] [CrossRef]
- Hammond, J.P.; Broadley, M.R.; White, P.J. Genetic responses to phosphorus deficiency. Ann. Bot. 2004, 94, 323–332. [Google Scholar] [CrossRef]
- Rubio, V.; Linhares, F.; Solano, R.; Martín, A.C.; Iglesias, J.; Leyva, A.; Paz-Ares, J. A conserved MYB transcription factor involved in phosphate starvation signaling both in vascular plants and in unicellular algae. Genes Dev. 2001, 15, 2122–2133. [Google Scholar] [CrossRef] [Green Version]
- Shin, R.; Berg, R.H.; Schachtman, D.P. Reactive oxygen species and root hairs in Arabidopsis root response to nitrogen, phosphorus and potassium deficiency. Plant Cell Physiol. 2005, 46, 1350–1357. [Google Scholar] [CrossRef]
- Robinson, W.D.; Carson, I.; Ying, S.; Ellis, K.; Plaxton, W.C. Eliminating the purple acid phosphatase AtPAP26 in Arabidopsis thaliana delays leaf senescence and impairs phosphorus remobilization. New Phytol. 2012, 196, 1024–1029. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, J.; Zhang, X.; Fan, H.; Gu, M.; Qu, H.; Xu, G. Phosphate transporter OsPht1;8 in rice plays in important role in phosphorus redistribution from source to sink organs and allocation between embryo and endosperm of seeds. Plant Sci. 2015, 230, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Denton, M.D.; Veneklaas, E.J.; Freimoser, F.M.; Lambers, H. Banksia species (Proteaceae) from severely phosphorus-impoverished soils exhibit extreme efficiency in the use and re-mobilization of phosphorus. Plant Cell Environ. 2007, 30, 1557–1565. [Google Scholar] [CrossRef]
- Lambers, H.; Clode, P.L.; Hawkins, H.J.; Laliberté, E.; Oliveira, R.S.; Reddell, P.; Shane, M.W.; Stitt, M.; Weston, P. Metabolic adaptations of the non-mycotrophic Proteaceae to soils with low phosphorus availability. Annu. Plant Rev. 2018, 15, 289–335. [Google Scholar] [CrossRef]
- Besford, R. Uptake and distribution of phosphorus in tomato plants. Plant Soil 1979, 51, 331–340. [Google Scholar] [CrossRef]
- Irshad, M.; Gill, M.A.; Aziz, T.; Ahmed, I. Growth response of cotton cultivars to zinc deficiency stress in chelator-buffered nutrient solution. Pak. J. Bot. 2004, 36, 373–380. [Google Scholar]
- Bozzo, G.G.; Dunn, E.L.; Plaxton, W.C. Differential synthesis of phosphate—Starvation inducible purple acid phosphatase isozymes in tomato (Lycopersicon esculentum) suspension cells and seedlings. Plant Cell Environ. 2005, 29, 303–313. [Google Scholar] [CrossRef]
- Ha, S.; Tran, L.-S. Understanding plant responses to phosphorus starvation for improvement of plant tolerance to phosphorus deficiency by biotechnological approaches. Crit. Rev. Biotechnol. 2014, 34, 16–30. [Google Scholar] [CrossRef]
- Puga, M.I.; Rojas-Triana, M.; de Lorenzo, L.; Leyva, A.; Rubio, V.; Paz-Ares, J. Novel signals in the regulation of Pi starvation responses in plants: Facts and promises. Curr. Opin. Plant Biol. 2017, 39, 40–49. [Google Scholar] [CrossRef]
- Hackenberg, M.; Shi, B.-J.; Gustafson, P.; Langridge, P. Characterization of phosphorus-regulated miR399 and miR827 and their isomirs in barley under phosphorus-sufficient and phosphorus-deficient conditions. BMC Plant Biol. 2013, 13, 214. [Google Scholar] [CrossRef] [Green Version]
- Veneklaas, E.J.; Lambers, H.; Bragg, J.; Finnegan, P.M.; Lovelock, C.E.; Plaxton, W.C.; Price, C.A.; Scheible, W.R.; Shane, M.W.; White, P.J. Opportunities for improving phosphorus--use efficiency in crop plants. New Phytol. 2012, 195, 306–320. [Google Scholar] [CrossRef]
- Haran, S.; Logendra, S.; Seskar, M.; Bratanova, M.; Raskin, I. Characterization of Arabidopsis acid phosphatase promoter and regulation of acid phosphatase expression. Plant Physiol. 2000, 124, 615–626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poirier, Y.; Bucher, M. Phosphate transport and homeostasis in Arabidopsis. Arabidopsis Book 2002, 1, e0024. [Google Scholar] [CrossRef] [Green Version]
- Mimura, T.; Dietz, K.J.; Kaiser, W.; Schramm, M.; Kaiser, G.; Heber, U. Phosphate transport across biomembranes and cytosolic phosphate homeostasis in barley leaves. Planta 1990, 180, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhang, T.; Wang, M.; Liu, Y.; Brestic, M.; Chen, T.H.H.; Yang, X. Genetic engineering of the biosynthesis of glycine betaine modulates phosphate homeostasis by regulating phosphate acquisition in tomato. Front. Plant Sci. 2019, 9, 1995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Groot, C.C.; van den Boogaard, R.; Marcelis, L.F.M.; Harbinson, J.; Lambers, H. Contrasting effects of N and P deprivation on the regulation of photosynthesis in tomato plants in relation to feedback limitation. J. Exp. Bot. 2003, 54, 1957–1967. [Google Scholar] [CrossRef]
- Wu, P.; Ma, L.; Hou, X.; Wang, M.; Wu, Y.; Liu, F.; Deng, X.W. Phosphate starvation triggers distinct alterations of genome expression in arabidopsis roots and leaves. Plant Physiol. 2003, 132, 1260–1271. [Google Scholar] [CrossRef] [Green Version]
- Ogawa, N.; DeRisi, J.; Brown, P.O. New components of a system for phosphate accumulation and polyphosphate metabolism in Saccharomyces cerevisiae revealed by genomic expression analysis. Mol. Biol. Cell 2000, 11, 4309–4321. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Jin, Y.; Du, Y.-L.; Wang, T.; Turner, N.C.; Yang, R.P.; Siddique, K.H.M.; Li, F.M. Genotypic variation in yield, yield components, root morphology and architecture, in soybean in relation to water and phosphorus supply. Front. Plant Sci. 2017, 8, 1499. [Google Scholar] [CrossRef] [Green Version]
- Lynch, J.P.; Brown, K.M. Topsoil foraging—An architectural adaptation of plants to low phosphorus availability. Plant Soil 2001, 237, 225–237. [Google Scholar] [CrossRef]
- Raven, J.A.; Lambers, H.; Smith, S.E.; Westoby, M. Costs of acquiring phosphorus by vascular land plants: Patterns and implications for plant coexistence. New Phytol. 2018, 217, 1420–1427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williamson, L.C.; Ribrioux, S.P.; Fitter, A.H.; Leyser, H.O. Phosphate availability regulates root system architecture in Arabidopsis. Plant Physiol. 2001, 126, 875–882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, C.R.; Ochoa, I.; Nielsen, K.L.; Beck, D.; Lynch, J.P. Genetic variation for adventitious rooting in response to low phosphorus availability: Potential utility for phosphorus acquisition from stratified soils. Funct. Plant Biol. 2003, 30, 973–985. [Google Scholar] [CrossRef] [Green Version]
- Walk, T.C.; Jaramillo, R.; Lynch, J.P. Architectural tradeoffs between adventitious and basal roots for phosphorus acquisition. Plant Soil 2006, 279, 347–366. [Google Scholar] [CrossRef]
- Madlung, A.; Behringer, F.J.; Lomax, T.L. Ethylene plays multiple nonprimary roles in modulating the gravitropic response in tomato. Plant Physiol. 1999, 120, 897–906. [Google Scholar] [CrossRef] [Green Version]
- Ma, Q.; Rengel, Z.; Siddique, K.H. Wheat and white lupin differ in root proliferation and phosphorus use efficiency under heterogeneous soil P supply. Crop Pasture Sci. 2011, 62, 467–473. [Google Scholar] [CrossRef]
- Jing, J.; Rui, Y.; Zhang, F.; Rengel, Z.; Shen, J. Localized application of phosphorus and ammonium improves growth of maize seedlings by stimulating root proliferation and rhizosphere acidification. Field Crops Res. 2010, 119, 355–364. [Google Scholar] [CrossRef]
- Kumar, A.; Shahbaz, M.; Koirala, M.; Blagodatskaya, E.; Seidel, S.J.; Kuzyakov, Y.; Pausch, J. Root trait plasticity and plant nutrient acquisition in phosphorus limited soil. J. Plant Nutr. Soil Sci. 2019, 182, 945–952. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.; Chen, W.L.; Xu, C.X.; Zhu, H.H.; Yao, Q. Influences of arbuscular mycorrhizal fungus and phosphorus level on the lateral root formation of tomato seedlings. J. Appl. Ecol. 2015, 26, 1186–1192. [Google Scholar]
- Garcia, M.; Ascencio, J. Root morphology and acid phosphatase activity in tomato plants during development of and recovery from phosphorus stress. J. Plant Nutr. 1992, 15, 2491–2503. [Google Scholar] [CrossRef]
- Hammond, J.P.; Broadley, M.R.; White, P.J.; King, G.J.; Bowen, H.C.; Hayden, R.; Meacham, M.C.; Mead, A.; Overs, T.; Spracklen, W.P. Shoot yield drives phosphorus use efficiency in Brassica oleracea and correlates with root architecture traits. J. Exp. Bot. 2009, 60, 1953–1968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Postma, J.A.; Dathe, A.; Lynch, J.P. The optimal lateral root branching density for maize depends on nitrogen and phosphorus availability. Plant Physiol. 2014, 166, 590–602. [Google Scholar] [CrossRef] [Green Version]
- Chevalier, F.; Pata, M.; Nacry, P.; Doumas, P.; Rossignol, M. Effects of phosphate availability on the root system architecture: Large-scale analysis of the natural variation between Arabidopsis accessions. Plant Cell Environ. 2003, 26, 1839–1850. [Google Scholar] [CrossRef] [Green Version]
- Reymond, M.; Svistoonoff, S.; Loudet, O.; Nussaume, L.; Desnos, T. Identification of QTL controlling root growth response to phosphate starvation in Arabidopsis thaliana. Plant Cell Environ. 2006, 29, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Kaeppler, S.M.; Lynch, J.P. Mapping of QTLs for lateral root branching and length in maize (Zea mays L.) under differential phosphorus supply. Theor. Appl. Genet. 2005, 111, 688–695. [Google Scholar] [CrossRef]
- Gilroy, S.; Jones, D.L. Through form to function: Root hair development and nutrient uptake. Trends Plant Sci. 2000, 5, 56–60. [Google Scholar] [CrossRef]
- Gahoonia, T.S.; Nielsen, N.E. Root traits as tools for creating phosphorus efficient crop varieties. Plant Soil 2004, 260, 47–57. [Google Scholar] [CrossRef]
- Wissuwa, M. How do plants achieve tolerance to phosphorus deficiency? Small causes with big effects. Plant Physiol. 2003, 133, 1947–1958. [Google Scholar] [CrossRef] [Green Version]
- Giri, J.; Bhosale, R.; Huang, G.; Pandey, B.K.; Parker, H.; Zappala, S.; Yang, J.; Dievart, A.; Bureau, C.; Ljung, K.; et al. Rice auxin influx carrier OsAUX1 facilitates root hair elongation in response to low external phosphate. Nat. Commun. 2018, 9, 1408. [Google Scholar] [CrossRef]
- Tominaga-Wada, R.; Nukumizu, Y.; Sato, S.; Wada, T. Control of plant trichome and root-hair development by a tomato (Solanum lycopersicum) R3 MYB transcription factor. PLoS ONE 2013, 8, e54019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foehse, D.; Jungk, A. Influence of phosphate and nitrate supply on root hair formation of rape, spinach and tomato plants. Plant Soil 1983, 74, 359–368. [Google Scholar] [CrossRef]
- Bayuelo-Jiménez, J.S.; Gallardo-Valdéz, M.; Pérez-Decelis, V.A.; Magdaleno-Armas, L.; Ochoa, I.; Lynch, J.P. Genotypic variation for root traits of maize (Zea mays L.) from the Purhepecha Plateau under contrasting phosphorus availability. Field Crops Res. 2011, 121, 350–362. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Lynch, J.P.; Brown, K.M. Ethylene and phosphorus availability have interacting yet distinct effects on root hair development. J. Exp. Bot. 2003, 54, 2351–2361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Z.; Walk, T.C.; Marcus, A.; Lynch, J.P. Morphological synergism in root hair length, density, initiation and geometry for phosphorus acquisition in Arabidopsis thaliana: A modeling approach. Plant Soil 2001, 236, 221–235. [Google Scholar] [CrossRef]
- Genc, Y.; Huang, C.Y.; Langridge, P. A study of the role of root morphological traits in growth of barley in zinc-deficient soil. J. Exp. Bot. 2007, 58, 2775–2784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bates, T.R.; Lynch, J.P. Plant growth and phosphorus accumulation of wild type and two root hair mutants of Arabidopsis thaliana (Brassicaceae). Am. J. Bot. 2000, 87, 958–963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lambers, H.; Shane, M.W.; Cramer, M.D.; Pearse, S.J.; Veneklaas, E.J. Root structure and functioning for efficient acquisition of phosphorus: Matching morphological and physiological traits. Ann. Bot. 2006, 98, 693–713. [Google Scholar] [CrossRef] [Green Version]
- Brown, L.; George, T.; Thompson, J.; Wright, G.; Lyon, J.; Dupuy, L.; Hubbard, S.; White, P. What are the implications of variation in root hair length on tolerance to phosphorus deficiency in combination with water stress in barley (Hordeum vulgare)? Ann. Bot. 2012, 110, 319–328. [Google Scholar] [CrossRef]
- Bates, T.R.; Lynch, J.P. The efficiency of Arabidopsis thaliana (Brassicaceae) root hairs in phosphorus acquisition. Am. J. Bot. 2000, 87, 964–970. [Google Scholar] [CrossRef] [Green Version]
- Narang, R.A.; Bruene, A.; Altmann, T. Analysis of phosphate acquisition efficiency in different Arabidopsis accessions. Plant Physiol. 2000, 124, 1786–1799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Z.; Bielenberg, D.; Brown, K.M.; Lynch, J.P. Regulation of root hair density by phosphorus availability in Arabidopsis thaliana. Plant Cell Environ. 2001, 24, 459–467. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Lin, G.; Zhang, X.; Chen, Y.; Zhang, S.; Chen, B. Relative importance of an arbuscular mycorrhizal fungus (Rhizophagus intraradices) and root hairs in plant drought tolerance. Mycorrhiza 2014, 24, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Masucci, J.D.; Schiefelbein, J.W. Hormones act downstream of TTG and GL2 to promote root hair outgrowth during epidermis development in the Arabidopsis root. Plant Cell. 1996, 8, 1505–1517. [Google Scholar] [CrossRef] [Green Version]
- Rahman, A.; Hosokawa, S.; Oono, Y.; Amakawa, T.; Goto, N.; Tsurumi, S. Auxin and ethylene response interactions during Arabidopsis root hair development dissected by auxin influx modulators. Plant Physiol. 2002, 130, 1908–1917. [Google Scholar] [CrossRef] [Green Version]
- Dakora, F.D.; Phillips, D.A. Root exudates as mediators of mineral acquisition in low-nutrient environments. In Food Security in Nutrient-Stressed Environments: Exploiting Plants’ Genetic Capabilities; Adu-Gyamfi, J.J., Ed.; Springer: Dordrecht, The Netherlands, 2002; pp. 201–213. ISBN 978-94-017-1570-6. [Google Scholar]
- Perez, M.J.; Smyth, T.J.; Israel, D.W. Comparative effects of two forage species on ehizospehre acidification and solubilization of phosphate rocks of different reactivity. J. Plant Nutr. 2007, 30, 1421–1439. [Google Scholar] [CrossRef]
- Hinsinger, P. Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: A review. Plant Soil 2001, 237, 173–195. [Google Scholar] [CrossRef]
- Calba, H.; Firdaus, C.P.; Thee, C.; Poss, R.; Jaillard, B. The dynamics of protons, aluminum, and calcium in the rhizosphere of maize cultivated in tropical acid soils: Experimental study and modelling. Plant Soil 2004, 260, 33–46. [Google Scholar] [CrossRef]
- Wang, L.; Nancollas, G.H. Calcium orthophosphates: Crystallization and dissolution. Chem. Rev. 2008, 108, 4628–4669. [Google Scholar] [CrossRef] [Green Version]
- Chow, L.C.; Eanes, E. Solubility of calcium phosphates. Monogr. Oral Sci. 2001, 18, 94–111. [Google Scholar]
- Houmani, H.; Rabhi, M.; Abdelly, C.; Debez, A. Implication of rhizosphere acidification in nutrient uptake by plants: Cases of potassium (K), phosphorus (P), and iron (Fe). In Crop Production and Global Environmental Issues; Springer: Dordrecht, The Netherlands, 2015; pp. 103–122. [Google Scholar]
- Shen, H.; Chen, J.; Wang, Z.; Yang, C.; Sasaki, T.; Yamamoto, Y.; Matsumoto, H.; Yan, X. Root plasma membrane H+-ATPase is involved in the adaptation of soybean to phosphorus starvation. J. Exp. Bot. 2006, 57, 1353–1362. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.; Shi, W.; Jia, L.; Liang, J.; Zhang, J. TFT6 and TFT7, two different members of tomato 14-3-3 gene family, play distinct roles in plant adaption to low phosphorus stress. Plant Cell Environ. 2012, 35, 1393–1406. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Zhang, D.; Song, T.; Xu, F.; Lin, S.; Xu, W.; Li, Q.; Zhu, Y.; Liang, J. Arabidopsis plasma membrane H+-ATPase genes AHA2 and AHA7 have distinct and overlapping roles in the modulation of root tip H+ efflux in response to low-phosphorus stress. J. Exp. Bot. 2017, 68, 1731–1741. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Drevon, J.; Jaillard, B.; Souche, G.; Hinsinger, P. Proton release of two genotypes of bean (Phaseolus vulgaris L.) as affected by N nutrition and P deficiency. Plant Soil 2004, 260, 59–68. [Google Scholar] [CrossRef]
- Yan, F.; Zhu, Y.; Muller, C.; Zorb, C.; Schubert, S. Adaptation of H+-Pumping and plasma membrane H+ ATPase activity in proteoid roots of white lupin under phosphate deficiency. Plant Physiol. 2002, 129, 50–63. [Google Scholar] [CrossRef] [Green Version]
- Zoysa, A.; Loganathan, P.; Hedley, M. Phosphorus utilization efficiency and depletion of phosphate fractions in the rhizosphere of three tea (Camellia sinensis L.) clones. Nutr. Cycl. Agroecosyst. 1999, 53, 189–201. [Google Scholar] [CrossRef]
- Canellas, L.P.; Olivares, F.L.; Okorokova-Facanha, A.L. Humic acids isolated from eartheorm compost enhance root elongation, lateral root emergence, and plasma membrance H+-ATPase activity in maize roots. Plant Physiol. 2002, 130, 1951–1957. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, K.; Hayashi, K.; Kinoshita, T. Auxin activates the plasma membrane H+-ATPase by phosphorylation during hypocotyl elongation in Arabidopsis. Cell Biol. Signal Transduct. 2012, 159, 632–641. [Google Scholar] [CrossRef] [Green Version]
- Jakkeral, S.A.; Kajjidoni, S. Root exudation of organic acids in selected genotypes under phosphorus deficient condition in blackgram (Vigna mungo L. Hepper). Karnataka J. Agr. Sci. 2011, 24, 316–319. [Google Scholar]
- Minemba, D.; Gleeson, D.B.; Veneklaas, E.; Ryan, M.H. Variation in morphological and physiological root traits and organic acid exudation of three sweet potato (Ipomoea batatas) cultivars under seven phosphorus levels. Sci. Hortic. 2019, 256, 108572. [Google Scholar] [CrossRef]
- Yu, W.; Kan, Q.; Zhang, J.; Zeng, B.; Chen, Q. Role of the plasma membrane H+-ATPase in the regulation of organic acid exudation under aluminum toxicity and phosphorus deficiency. Plant Signal. Behav. 2016, 11, e1106660. [Google Scholar] [CrossRef] [Green Version]
- Lambers, H.; Clements, J.C.; Nelson, M.N. How a phosphorus-acquisition strategy based on carboxylate exudation powers the success and agronomic potential of lupines (Lupinus, Fabaceae). Am. J. Bot. 2013, 100, 263–288. [Google Scholar] [CrossRef] [PubMed]
- Neumann, G.; Römheld, V. Root excretion of carboxylic acids and protons in phosphorus-deficient plants. Plant Soil 1999, 211, 121–130. [Google Scholar] [CrossRef]
- Luo, H.M.; Watanabe, T.; Shinano, T.; Tadano, T. Comparison of aluminum tolerance and phosphate absorption between rape (Brassica napus L.) and Tomato (Lycopersicum esculentum Mill.) in relation to organic acid exudation. Soil Sci. Plant Nutr. 1999, 45, 897–907. [Google Scholar] [CrossRef]
- Imas, P.; Bar-Yosef, B.; Kadkafi, U.; Ganmore-Neumann, R. Phosphate induced carboxylate and proton release by tomato roots. Plant Soil 1997, 191, 35–39. [Google Scholar] [CrossRef]
- De Souza, M.F.; Soares, E.M.B.; Silva, I.R.D.; Novais, R.F.; Silva, M.F.D.O. Competitive sorption and desorption of phosphate and citrate in clayey and sandy loam soils. Rev. Bras. Ciênc. 2014, 38, 1153–1161. [Google Scholar] [CrossRef] [Green Version]
- Duputel, M.; Devau, N.; Brossard, M.; Jaillard, B.; Jones, D.L.; Hinsinger, P.; Gérard, F. Citrate adsorption can decrease soluble phosphate concentration in soils: Results of theoretical modeling. Appl. Geochem. 2013, 35, 120–131. [Google Scholar] [CrossRef]
- Jayachandran, K.; Hetrick, B.A.D.; Schwab, A.P. Mycorrhizal mediation of phosphorus availability: Synthetic iron chelate effects on phosphorus solubilization. Soil Sci. Soc. Am. J. 1989, 53, 1701–1706. [Google Scholar] [CrossRef]
- Teixeira, R.D.S.; Ribeiro da Silva, I.; Nogueira de Sousa, R.; Márcio Mattiello, E.; Barros Soares, E.M. Organic acid coated-slow-release phosphorus fertilizers improve P availability and maize growth in a tropical soil. J. Soil Sci. Plant Nutr. 2016, 16, 1097–1112. [Google Scholar] [CrossRef] [Green Version]
- Imas, P.; Bar-Yosef, B.; Kafkafi, U.; Ganmore-Neumann, R. Release of carboxylic anions and protons by tomato roots in response to ammonium nitrate ratio and pH in nutrient solution. Plant Soil 1997, 191, 27–34. [Google Scholar] [CrossRef]
- Dixon, M.; Webb, E.C. Enzymes, 2nd ed.; Academic Press: New York, NY, USA, 1964. [Google Scholar]
- Sardans, J.; Peñuelas, J.; Estiarte, M. Warming and drought alter soil phosphatase activity and soil P availability in a Mediterranean shrubland. Plant Soil 2006, 289, 227–238. [Google Scholar] [CrossRef]
- Li, S.M.; Li, L.; Zhang, F.; Tang, C. Acid phosphatase role in chickpea/maize intercropping. Ann. Bot. 2004, 94, 297–303. [Google Scholar] [CrossRef] [Green Version]
- Wasaki, J.; Yamamura, T.; Shinano, T.; Osaki, M. Secreted acid phosphatase is expressed in cluster roots of lupin in response to phosphorus deficiency. Plant Soil 2003, 248, 129–136. [Google Scholar] [CrossRef]
- Playsted, C.W.S.; Johnston, M.E.; Ramage, C.M.; Edwards, D.G.; Cawthray, G.R.; Lambers, H. Functional significance of dauciform roots: Exudation of carboxylates and acid phosphatase under phosphorus deficiency in Caustis blakei (Cyperaceae). New Phytol. 2006, 170, 491–500. [Google Scholar] [CrossRef]
- McComb, R.B.; Bowers, G.N., Jr.; Posen, S. Alkaline Phosphatase; Springer Science and Business Media: New York, NY, USA, 2013; ISBN 978-1-4613-2972-5. [Google Scholar]
- Kim, K.Y.; Jordan, D.; McDonald, G.A. Effect of phosphate-solubilizing bacteria and vesicular-arbuscular mycorrhizae on tomato growth and soil microbial activity. Biol. Fertil. Soils 1997, 26, 79–87. [Google Scholar] [CrossRef]
- Sharma, S.B.; Sayyed, R.Z.; Trivedi, M.H.; Gobi, T.A. Phosphate solubilizing microbes: Sustainable approach for managing phosphorus deficiency in agricultural soils. SpringerPlus 2013, 2, 587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagy, R.; Drissner, D.; Amrhein, N.; Jakobsen, I.; Bucher, M. Mycorrhizal phosphate uptake pathway in tomato is phosphorus-repressible and transcriptionally regulated. New Phytol. 2009, 181, 950–959. [Google Scholar] [CrossRef]
- Nagy, R.; Karandashov, V.; Chague, V.; Kalinkevich, K.; Tamasloukht, M.; Xu, G.; Jakpbsen, I.; Levy, A.A.; Amrhein, N.; Bucher, M. The characterization of novel mycorrhiza-specific phosphate transporters from Lycopersicon esculentum and Solanum tuberosum uncovers functional redundancy in symbiotic phosphate transport in solanaceous species. Plant J. 2005, 42, 236–250. [Google Scholar] [CrossRef]
- Gomez-Ariza, J.; Balestrini, R.; Novero, M.; Bonfante, P. Cell-specific gene expression of phosphate transporters in mycorrhizal tomato roots. Biol. Fertil. Soils 2009, 45, 845–853. [Google Scholar] [CrossRef]
- Poulsen, K.H.; Nagy, R.; Gao, L.-L.; Smith, S.E.; Bucher, M.; Smith, F.A.; Jakobsen, I. Physiological and molecular evidence for Pi uptake via the symbiotic pathway in a reduced mycorrhizal colonization mutant in tomato associated with a compatible fungus. New Phytol. 2005, 168, 445–454. [Google Scholar] [CrossRef]
- Goldstein, A.; Krishnaraj, P. Phosphate solubilizing microorganisms vs. phosphate mobilizing microorganisms: What separates a phenotype from a trait? In First International Meeting on Microbial Phosphate Solubilization; Velazquez, E., Rodriguez-Barrueco, C., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 203–213. [Google Scholar]
- Alori, E.T.; Glick, B.R.; Babalola, O.O. Microbial phosphorus solubilization and its potential for use in sustainable agriculture. Front. Microbiol. 2017, 8, 971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Wang, L.; Ma, F.; Zhang, X.; Fu, D. Arbuscular mycorrhiza improved phosphorus efficiency in paddy fields. Ecol. Eng. 2016, 95, 64–72. [Google Scholar] [CrossRef]
- Rengel, Z. Breeding for better symbiosis. In Food Security in Nutrient-Stressed Environments: Exploiting Plants’ Genetic Capabilities; Adu-Gyamfi, J.J., Ed.; Springer: Dordrecht, The Netherlands, 2002; pp. 245–260. [Google Scholar]
- Besserer, A.; Puech-Pagès, V.; Kiefer, P.; Gomez-Roldan, V.; Jauneau, A.; Roy, S.; Portais, J.C.; Roux, C.; Bécard, G.; Séjalon-Delmas, N. Strigolactones stimulate arbuscular mycorrhizal fungi by activating mitochondria. PLoS Biol. 2006, 4, e226. [Google Scholar] [CrossRef] [PubMed]
- Rezacova, V.; Konvalinkova, T.; Jansa, J. Carbon Fluxes in Mycorrhizal Plants. In Mycorrhiza—Eco-Physiology, Secondary Metabolites, Nanomaterials; Varma, A., Prasad, R., Tuteja, N., Eds.; Springer: Cham, Switzerland, 2017; ISBN 978-3-319-57848-4. [Google Scholar]
| Family | Phosphate Transporter Family 1 (PHT1) | Phosphate Transporter Family 2 (PHT2) | Phosphate Transporter Family 3 (PHT3) | Phosphate Transporter Family 4 (PHT4) | Phosphate Transporter Family 5 (PHT5) |
|---|---|---|---|---|---|
| Affinity | High and low | High and low | High and low | High and low | High and low |
| Symport agent | H+ | H+ | H+ | H+, Na+ | H+ |
| Location | Plasma membrane | Inner plastid membrane of chloroplast | Mitochondrial membrane | Golgi apparatus | Vacuole |
| Representative homo-paralog | LePT1 to LePT5 (from tomato) | Pht2;1 (from Arabidopsis green tissue) | PtrPHT3.1a (from poplar (Populus trichocarpa L.)) | Pht4;6-1 (from yeast) | OsSPX-MFS1 (from rice) |
| Notes | Acquires P in both high- and low-P soils | Moderates P translocation | Regulates P distribution | Regulates P transport between cytosol | Also named vacuolar phosphate transporter |
| Sources | [75,76,77,78] | [71,75,79] | [72,75] | [73,75,80] | [74,81,82] |
| Soil Order | Characteristic Impacting Phosphorus Bioavailability | Potential Strategy to Cope with Phosphorus Availability | Relevant Source |
|---|---|---|---|
| Alfisol | Common fixation to Ca-phosphates | Microbial symbiosis | [54] |
| Andisol | High P sorption Al-compounds | Dense root hair growth on lateral roots | [52] |
| Aridisol | High calcium carbonate concentration | Rhizosphere acidification from proton exudation | [54] |
| Entisol | Predominate Ca-phosphate compounds | Rhizosphere acidification from proton exudation | [57] |
| Gelisol | Inorganic phosphate immobilization | Phosphatase exudation | [58] |
| Histisol | Inorganic phosphate immobilization | Phosphatase exudation | [52] |
| Inceptisol | High calcium carbonate concentration | Rhizosphere acidification from proton exudation | [57] |
| Mollisol | Inorganic phosphate immobilization | Microbial symbiosis | [57] |
| Oxisol | Spatially dependent high Fe-oxide content | Root plasticity with fine root proliferation | [61] |
| Spodosol | High Al and Fe content with high P fixation | Orgnic acid exudation | [52] |
| Ultisol | Common P retention with Fe-oxides | Use of native bacteria as biofertilizer to release sorbed P | [60] |
| Vertisol | Predominant Ca-phosphate compounds | Rhizosphere acidification from proton exudation | [54] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dixon, M.; Simonne, E.; Obreza, T.; Liu, G. Crop Response to Low Phosphorus Bioavailability with a Focus on Tomato. Agronomy 2020, 10, 617. https://doi.org/10.3390/agronomy10050617
Dixon M, Simonne E, Obreza T, Liu G. Crop Response to Low Phosphorus Bioavailability with a Focus on Tomato. Agronomy. 2020; 10(5):617. https://doi.org/10.3390/agronomy10050617
Chicago/Turabian StyleDixon, Mary, Eric Simonne, Thomas Obreza, and Guodong Liu. 2020. "Crop Response to Low Phosphorus Bioavailability with a Focus on Tomato" Agronomy 10, no. 5: 617. https://doi.org/10.3390/agronomy10050617
APA StyleDixon, M., Simonne, E., Obreza, T., & Liu, G. (2020). Crop Response to Low Phosphorus Bioavailability with a Focus on Tomato. Agronomy, 10(5), 617. https://doi.org/10.3390/agronomy10050617





