Beneficial Effects of Biochar and Chitosan on Antioxidative Capacity, Osmolytes Accumulation, and Anatomical Characters of Water-Stressed Barley Plants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Experiment Preparation
2.2. Growth Characters
2.3. Determination of Chlorophyll a and Chlorophyll b Concentrations as well as Relative Water Content (RWC%)
2.4. Determination of Electrolyte Leakage (EL%), Lipid Peroxidation and Proline Content
2.5. Determination of Soluble Sugar, Sucrose and Starch Contents
2.6. Antioxidant Enzymes Activity
2.7. Yield Characters
2.8. Anatomical Characters
2.9. Statistical Analysis
3. Results
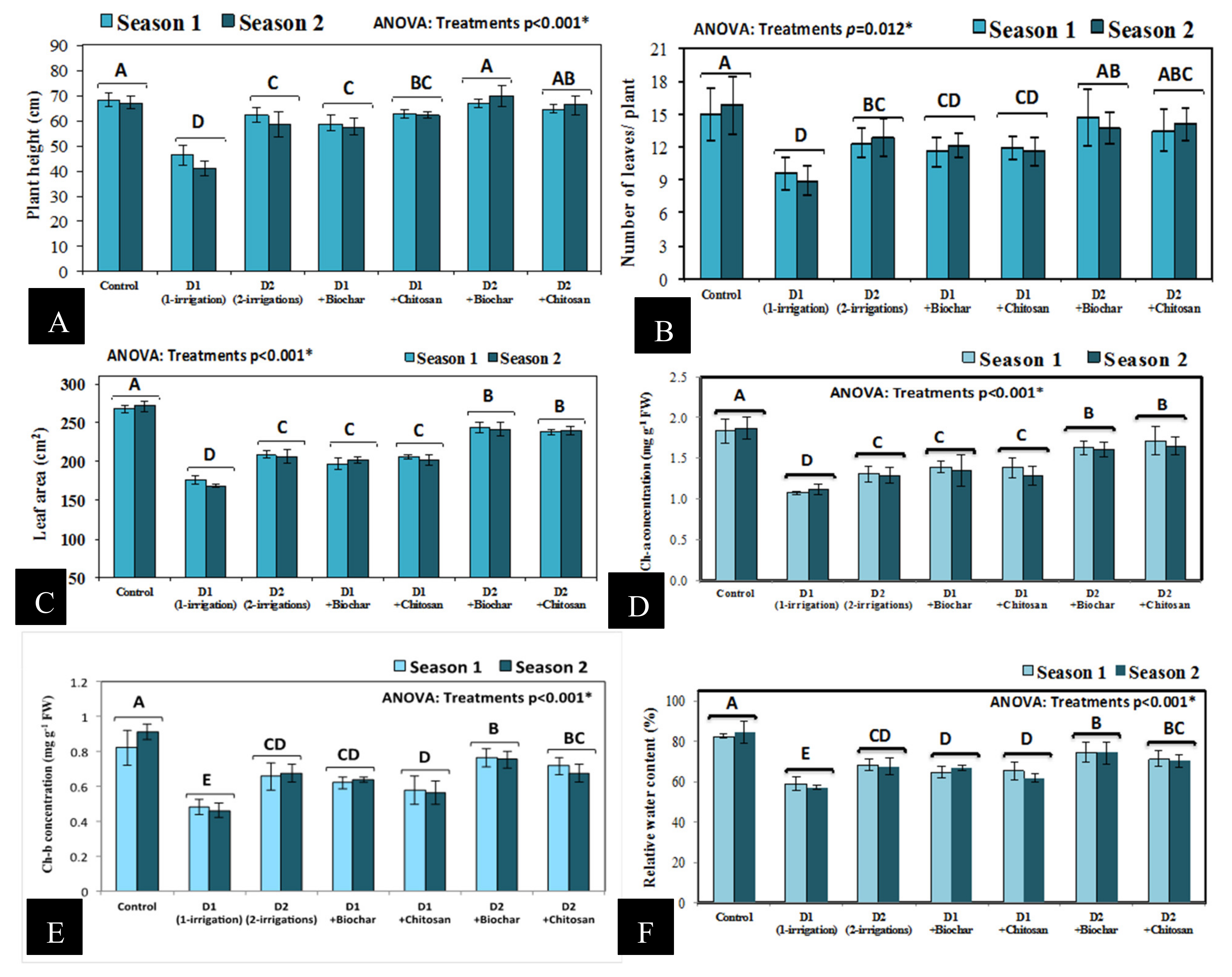
3.1. Plant Height, Number of Leaves, Leaf Area/Plant, Chlorophyll Concentrations, and Relative Water Content (RWC %)
3.2. Effect of Biochar and Chitosan on Electrolyte Leakage (EL%), Lipid Peroxidation and Proline Content
3.3. Soluble Sugars, Sucrose, and Starch Contents
3.4. Antioxidant Enzymes Activity of Stressed Barley Plants Affected by Biochar and Chitosan Treatments
3.5. Effect of Biochar and Chitosan on Yield Characters (1000 Grain Weight, Grains Yield/ha, and Biological Yield/ha)
3.6. Effect of Biochar and Chitosan on Anatomical Structure of Barley Leaves under Drought Conditions
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hafez, Y.M.; Abdelaal, K.A.A.; Eid, M.E.; Mehiar, F.F. Morpho-physiological and biochemical responses of barley plants (Hordeum vulgare L.) against barley net blotch disease with application of non-traditional compounds and fungicides. Egypt. J. Biol. Pest Control 2016, 26, 261–268. [Google Scholar]
- Hafez, Y.M.; Mourad, R.Y.; Mansour, M.; Abdelaal, K.A.A. Impact of non-traditional compounds and fungicides on physiological and biochemical characters of barely infected with Blumeria graminis f. sp hordei under field conditions. Egypt. J. Biol. Pest Control 2014, 24, 445–453. [Google Scholar]
- El-Nashaar, F.; Hafez, Y.M.; Abdelaal, K.A.A.; Abdelfatah, A.; Badr, M.; El-Kady, S.; Yousef, A. Assessment of host reaction and yield losses of commercial barley cultivars to Drechslera teres the causal agent of net blotch disease in Egypt. Fresenius Environ. Bull. 2020, 29, 2371–2377. [Google Scholar]
- Omara, R.I.; El-Kot, G.A.; Fadel, F.M.; Abdelaal, K.A.A.; Saleh, E.M. Efficacy of Certain Bioagents on patho-physiological characters of wheat plants under wheat leaf rust stress. Physiol. Mol. Plant Pathol. 2019, 106, 102–108. [Google Scholar] [CrossRef]
- Esmail, S.M.; Omara, R.I.; Abdelaal, K.A.A.; Hafez, M. Histological and biochemical aspects of compatible and incompatible wheat—Puccinia striiformis interactions. Physiol. Mol. Plant Pathol. 2019, 106, 120–128. [Google Scholar] [CrossRef]
- EL-Sabagh, A.; Abdelaal, K.A.A.; Barutcular, C. Impact of antioxidants supplementation on growth, yield and quality traits of canola (Brassica napus L.) under irrigation intervals in North Nile Delta of Egypt. J. Exp. Biol. Agric. Sci. 2017, 5, 163–172. [Google Scholar]
- Abdelaal, K.A.A.; EL-Maghraby, L.M.; Elansary, H.; Hafez, Y.M.; Ibrahim, E.I.; El-Banna, M.; El-Esawi, M.; Elkelish, A. Treatment of sweet pepper with stress tolerance-inducing compounds alleviates salinity stress oxidative damage by mediating the physio-biochemical activities and antioxidant systems. Agronomy 2020, 10, 26. [Google Scholar] [CrossRef] [Green Version]
- Abdelaal, K.A.A.; Attia, K.A.; Alamery, S.F.; El-Afry, M.M.; Ghazy, A.I.; Tantawy, D.S.; Al-Doss, A.A.; El-Shawy, E.E.; Abu-Elsaoud, A.M.; Hafez, Y.M. Exogenous application of proline and salicylic acid can mitigate the injurious impacts of drought stress on barley plants associated with physiological and histological characters. Sustainability 2020, 12, 1736. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Gao, M.; Ji, S.; Wang, S.; Meng, Y.; Zhou, Z. Carbon allocation, osmotic adjustment, antioxidant capacity and growth in cotton under long-term soil drought during flowering and boll-forming period. Plant Physiol. Biochem. 2016, 107, 137–146. [Google Scholar] [CrossRef]
- Abdelaal, K.A.A. Effect of Salicylic acid and Abscisic acid on morpho-physiological and anatomical characters of faba bean plants (Vicia faba L.) under drought stress. J. Plant Prod. Mansoura Univ. 2015, 6, 1771–1788. [Google Scholar] [CrossRef] [Green Version]
- Abdelaal, K.A.A.; Hafez, Y.M.; EL Sabagh, A.; Saneoka, H. Ameliorative effects of Abscisic acid and yeast on morpho-physiological and yield characteristics of maize plant (Zea mays L.) under drought conditions. Fresenius Environ. Bull. 2017, 26, 7372–7383. [Google Scholar]
- Samarah, N.H. Effects of drought stress on growth and yield of barley. Agron. Sustain. Dev. 2005, 25, 145–149. [Google Scholar] [CrossRef] [Green Version]
- Rashwan, E.A.A.; Abdelaal, K.A.A. Effect of Nano Zink-oxide foliar application on some flax cultivars under different irrigation treatments. Egypt. J. Plant Breed. 2019, 23, 119–145. [Google Scholar]
- McCue, K.F.; Hanson, A.D. Drought and salt tolerance: Towards understanding and application. Tibtechnology 1990, 8, 358. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, tolerance antioxidants and stress. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Helaly, M.N.; Mohammed, Z.; El-Shaeery, N.I.; Abdelaal, K.A.A.; Nofal, I.E. Cucumber grafting onto pumpkin can represent an interesting tool to minimize salinity stress. Physiological and anatomical studies. Middle E. J. Agric. Res. 2017, 6, 953–975. [Google Scholar]
- Abdelaal, K.A.A.; Omara, I.R.; Hafez, Y.M.; Esmail, S.M.; EL Sabagh, A. Anatomical, biochemical and physiological changes in some Egyptian wheat cultivars inoculated with Puccinia graminis f. sp. tritici. Fresenius Environ. Bull. 2018, 27, 296–305. [Google Scholar]
- Asada, K. Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol. 2006, 141, 391–396. [Google Scholar] [CrossRef] [Green Version]
- El-Banna, M.F.; Abdelaal, K.A.A. Response of strawberry plants grown in the hydroponic system to pretreatment with H2O2 before exposure to salinity stress. J. Plant Prod. Mansoura Univ. 2018, 9, 989–1001. [Google Scholar] [CrossRef]
- Huang, S.; Van Aken, O.; Schwarzlnder, M.; Belt, K.; Millar, A.H. The roles of mitochondrial reactive oxygen species in cellular signaling and stress responses in plants. Plant Physiol. 2016, 171, 1551–1559. [Google Scholar] [CrossRef] [Green Version]
- Hasan, M.K.; El Sabagh, A.; Sikdar, M.S.; Alam, M.J.; Ratnasekera, D.; Barutcular, C.; Abdelaal, K.A.; Islam, M.S. Comparative adaptable agronomic traits of blackgram and mungbean for saline lands. Plant Arch. 2017, 17, 589–593. [Google Scholar]
- Abdelaal, K.A.A.; Hafez, Y.M.; El-Afry, M.M.; Tantawy, D.S.; Alshaal, T. Effect of some osmoregulators on photosynthesis, lipid peroxidation, antioxidative capacity, and productivity of barley (Hordeum vulgare L.) under water deficit stress. Environ. Sci. Pollut. Res. 2018, 25, 30199–30211. [Google Scholar] [CrossRef] [PubMed]
- Omara, R.I.; Abdelaal, K.A.A. Biochemical, histopathological and genetic analysis associated with leaf rust infection in wheat plants (Triticum aestivum L.). Physiol. Mol. Plant Pathol. 2018, 104, 48–57. [Google Scholar] [CrossRef]
- Hafez, Y.M.; Mourad, R.Y.; Nasr, E.B.; Kotb, A.; Abdelaal, K.A.; Ghazy, A.I.; Al-Ateeq, T.K.; Ibrahim, E.I.; Mohammed, A.A. Biochemical and molecular characterization of non-host resistance keys in food crops. Saudi J. Biol. Sci. 2020, 27, 1091–1099. [Google Scholar] [CrossRef]
- Boussemart, J.P.; Leleu, H.; Ojo, O. The spread of pesticide practices among cost-efficient farmers. Environ. Model. Assess. 2013, 18, 523–532. [Google Scholar] [CrossRef]
- Sanchez-Reinoso, A.D.; Avila-Pedraza, E.A.; Restrepo-Diaz, H. Use of biochar in agriculture. Acta Biol. Colomb. 2020, 25. [Google Scholar] [CrossRef]
- Sanchez-Monedero, M.A.; Cayuela, M.L.; Roig, A.; Jindo, K.; Mondini, C.; Bolan, N. Role of biochar as an additive in organic waste composting. Bioresour. Technol. 2018, 247, 1155–1164. [Google Scholar] [CrossRef]
- Ali, S.; Rizwan, M.; Qayyum, M.F.; Ok, Y.S.; Ibrahim, M.; Riaz, M.; Arif, M.S.; Hafeez, F.; Al-Wabel, M.I.; Shahzad, A.N. Biochar soil amendment on alleviation of drought and salt stress in plants: A critical review. Environ. Sci. Pollut. Res. 2017, 24, 12700–12712. [Google Scholar] [CrossRef]
- Agegnehu, G.; Srivastava, A.K.; Bird, M.I. The role of biochar and biochar compost in improving soil quality and crop performance: A review. Appl. Soil Ecol. 2017, 119, 156–170. [Google Scholar] [CrossRef]
- Sorrenti, G.; Masiello, C.A.; Toselli, M. Biochar interferes with kiwifruit Fe-nutrition in calcareous soil. Geoderma 2016, 272, 10–19. [Google Scholar] [CrossRef] [Green Version]
- Chng, H.Y.; Ahmed, O.H.; Majid, N.M.A. Improving phosphorus availability, nutrient uptake and dry matter production of Zea mays L. on a tropical acid soil using poultry manure biochar and pineapple leaves compost. Exp. Agric. 2015, 52, 447–465. [Google Scholar] [CrossRef]
- Langeroodi, A.R.S.; Campiglia, E.; Mancinelli, R.; Radicetti, E. Can biochar improve pumpkin productivity and its physiological characteristics under reduced irrigation regimes? Sci. Hortic. 2019, 247, 195–204. [Google Scholar] [CrossRef]
- Xu, G.; Zhang, Y.; Sun, J.; Shao, H. Negative interactive effects between biochar and phosphorus fertilization on phosphorus availability and plant yield in saline sodic soil. Sci. Total Environ. 2016, 568, 910–915. [Google Scholar] [CrossRef] [PubMed]
- Haider, G.; Koyro, H.W.; Azam, F.; Steffens, D.; Muller, C.; Kammann, C. Biochar but not humic acid product amendment affected maize yields via improving plant-soil moisture relations. Plant Soil 2015, 395, 141–157. [Google Scholar] [CrossRef]
- Katiyar, D.; Hemantaranjan, A.; Singh, B.; Bhanu, A.N. A future perspective in crop protection: Chitosan and its oligosaccharides. Adv. Plants Agric. Res. 2014, 1, 1–8. [Google Scholar]
- Pongprayoon, W.; Roytrakul, S.; Pichayagkura, R.; Chadchawan, S. The role of hydrogen peroxide in chitosan-induced resistance to osmotic stress in rice (Oryza sativa L.). Plant Growth Regul. 2013, 70, 159–173. [Google Scholar] [CrossRef]
- Monirul, I.M.; Humayun, K.M.; Mamun, A.N.K.; Monirul, I.; Pronabananda, D. Studies on yield and yield attributes in tomato and chilli using foliar application of oligo-chitosan. GSC Biol. Pharm. Sci. 2018, 3, 020–028. [Google Scholar]
- Ahmed, K.B.M.; Khan, M.M.A.; Siddiqui, H.; Jahan, A. Chitosan and its oligosaccharides, a promising option for sustainable crop production—A review. Carbohydr. Polym. 2020, 227, 115331. [Google Scholar] [CrossRef]
- Zeng, D.; Luo, X.; Tu, R. Application of bioactive coatings based on chitosan for soybean seed protection. Int. J. Carbohydr. Chem. 2012. [Google Scholar] [CrossRef]
- Bistgani, Z.E.; Siadat, S.A.; Bakhshandeh, A.; Pirbalouti, A.G.; Hashemi, M. Morpho-physiological and phytochemical traits of (Thymus daenensis Celak.) in response to deficit irrigation and chitosan application. Acta Physiol. Plant. 2017, 39, 231. [Google Scholar] [CrossRef]
- Fahn, A.; Cutler, D.F. Xerophytes; Gebrüder Borntraeger: Berlin, Germany, 1992; pp. 1–107. [Google Scholar]
- Dickison, W.C. Integrative Plant Anatomy; Academic Press: San Diego, CA, USA, 2000. [Google Scholar]
- Nicotra, A.B.; Davidson, A. Adaptive phenotypic plasticity and plant water use. Funct. Plant Biol. 2010, 37, 117–127. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (A.O.A.C.). Official Methods of Analysis, 26th ed.; A.O.A.C. International: Washington, DC, USA, 2005. [Google Scholar]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic bio-membranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Sanchez, F.J.; de Andrés, E.F.; Tenorio, J.L.; Ayerbe, L. Growth of epicotyls, turgor maintenance and osmotic adjustment in pea plants (Pisum sativum L.) subjected to water stress. Field Crops Res. 2004, 86, 81–90. [Google Scholar] [CrossRef]
- Szalai, G.; Janda, T.; Padi, E.; Szigeti, Z. Role of light in post-chilling symptoms in maize. J. Plant Physiol. 1996, 148, 378–383. [Google Scholar] [CrossRef]
- Davenport, S.B.; Gallego, S.M.; Benavides, M.P.; Tomaro, M.L. Behavior of anti-oxidant defense system in the adaptive response to salt stress in (Helianthus annuus L.) cell. Plant Growth Reg. 2003, 40, 81–88. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Xu, W.; Cui, K.; Xu, A.; Nie, L.; Huang, J.; Peng, S. Drought stress condition increases root to shoot ratio via alteration of carbohydrate partitioning and enzymatic activity in rice seedlings. Acta Physiol. Plant. 2015, 37. [Google Scholar] [CrossRef]
- Kuai, J.; Liu, Z.; Wang, Y.; Meng, Y.; Chen, B.; Zhao, W.; Zhou, Z.; Oosterhuis, D.M. Waterlogging during flowering and boll forming stages affects sucrose metabolism in the leaves subtending the cotton boll and its relationship with boll weight. Plant Sci. 2014, 223, 79–98. [Google Scholar] [CrossRef]
- Aebi, H.E. Catalase. In Methods of Enzymatic Analysis, 3rd ed.; Verlag Chemie: Weinheim, Germany, 1983; pp. 273–286. [Google Scholar]
- Fryer, M.J.; Andrews, J.R.; Oxborough, K.; Blowers, D.A.; Baker, N.R. Relationship between CO2 assimilation, photosynthetic electron transport, and active O2 metabolism in leaves of maize in the field during periods of low temperature. Plant Physiol. 1998, 116, 571–580. [Google Scholar] [CrossRef] [Green Version]
- Hammerschmidt, R.; Nuckles, E.M.; Kuć, J. Association of enhanced peroxidase activity with induced systemic resistance of cucumber to Colletotrichum lagenarium. Physiol. Plant Pathol. 1982, 20, 73–82. [Google Scholar] [CrossRef]
- Nassar, M.A.; El-Sahhar, K.F. Botanical Preparations and Microscopy (Microtechnique); Academic Bookshop: Giza, Egypt, 1998; p. 219. [Google Scholar]
- Gomez, K.A.; Gomez, A.A. Statistical Procedures for Agricultural Research, 2nd ed.; Wiley Inter Science: New York, NY, USA, 1984; pp. 1–690. [Google Scholar]
- Duncan, B.D. Multiple ranges and multiple F-test. Biometrics 1955, 11, 1–42. [Google Scholar] [CrossRef]
- Boyer, J.S. Cell enlargement and growth — Induced water potentials. Physiol. Plant. 1988, 73, 311–316. [Google Scholar] [CrossRef]
- Wei, W.; Yang, H.; Fan, M.; Chen, H.; Guo, D.; Cao, J.; Kuzyakov, Y. Biochar effects on crop yields and nitrogen loss depending on fertilization. Sci. Total Environ. 2020, 702, 134423. [Google Scholar] [CrossRef]
- Hussain, M.; Malik, M.A.; Farooq, M.; Ashraf, M.Y.; Cheema, M.A. Improving drought tolerance by exogenous application of glycinebetaine and salicylic acid in sunflower. J. Agron. Crop Sci. 2008, 194, 193–199. [Google Scholar] [CrossRef]
- Ahmed, A.H.H.; Nesiem, M.R.A.E.; Allam, H.A.; El-Wakil, A.F. Effect of preharvest chitosan foliar application on growth, yield and chemical composition of Washington navel orange trees grown in two different regions. Afr. J. Biochem. Res. 2016, 10, 59–69. [Google Scholar] [CrossRef] [Green Version]
- Abdelaal, K.A.A.; Hafez, Y.M.; Badr, M.M.; Youseef, W.A.; Esmaeil, S.M. Biochemical, histological and molecular changes in susceptible and resistant wheat cultivars inoculated with stripe rust fungus Puccinia striiformis f. sp. tritici. Egypt. J. Biol. Pest Control 2014, 24, 421–429. [Google Scholar]
- Tardieu, F.; Reymond, M.; Hamard, P.; Granier, C.; Muller, B. Spatial distributions of expansion rate, cell division rate and cell size in maize leaves: A synthesis of the effects of soil water status, evaporative demand and temperature. J. Exp. Bot. 2000, 51, 1505–1514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Equiza, M.A.; Zheng, Q.; Tyree, M.T. Factors controlling plasticity of leaf morphology in Robinia pseudoacacia L. II: The impact of water stress on leaf morphology of seedlings grown in a controlled environment chamber. Ann. For. Sci. 2012, 69, 39–47. [Google Scholar] [CrossRef]





| Meteorological Data | Season | December | January | February | March | April | May |
|---|---|---|---|---|---|---|---|
| Precipitation (mm day−1) | 2017 | 8.36 | 7.78 | 7.85 | 0.58 | 47.42 | 0.03 |
| 2018 | 8.13 | 32.80 | 10.80 | 1.61 | 3.09 | 0.01 | |
| Relative humidity at 2 m (%) | 2017 | 67.35 | 68.84 | 67.23 | 63.81 | 59.35 | 53.37 |
| 2018 | 67.82 | 68.49 | 67.61 | 55.02 | 54.00 | 54.25 | |
| Maximum temperature at 2 m (°C) | 2017 | 21.88 | 17.16 | 18.86 | 21.86 | 25.25 | 29.92 |
| 2018 | 19.65 | 18.69 | 20.99 | 25.41 | 27.58 | 31.35 | |
| Minimum temperature at 2 m (°C) | 2017 | 14.21 | 9.70 | 10.37 | 12.50 | 14.21 | 18.37 |
| 2018 | 13.66 | 11.64 | 12.15 | 13.66 | 15.76 | 19.90 |
| Treatments | Thickness of Upper Epidermis (µm) | Thickness of Lower Epidermis (µm) | Thickness of Lamina (µm) | Thickness of Mesophyll Tissue (µm) | Diameter of Vascular Bundle (µm) |
|---|---|---|---|---|---|
| Control | 13. 65 a | 13.78 a | 136.95 a | 109.4 a | 67.56 b |
| D1 (one irrigation) | 7.95 d | 8.14 d | 105.45 d | 87.49 e | 61.42 e |
| D2 (two irrigations) | 10.64 c | 10.88 c | 116.04 c | 94.26 d | 66.07 c |
| D1 + Biochar | 10.05 c | 10.18 c | 119.95 b | 98.44 c | 64.76 d |
| D1 + Chitosan | 11.75 b | 11.24 b | 138.32 a | 108.45 ab | 68.84 b |
| D2 + Biochar | 12.75 a | 12.88 a | 137.86 a | 111.02 a | 68.47 b |
| D2 + Chitosan | 13.52 a | 13.63 a | 139.14 a | 112.19 a | 71.26 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hafez, Y.; Attia, K.; Alamery, S.; Ghazy, A.; Al-Doss, A.; Ibrahim, E.; Rashwan, E.; El-Maghraby, L.; Awad, A.; Abdelaal, K. Beneficial Effects of Biochar and Chitosan on Antioxidative Capacity, Osmolytes Accumulation, and Anatomical Characters of Water-Stressed Barley Plants. Agronomy 2020, 10, 630. https://doi.org/10.3390/agronomy10050630
Hafez Y, Attia K, Alamery S, Ghazy A, Al-Doss A, Ibrahim E, Rashwan E, El-Maghraby L, Awad A, Abdelaal K. Beneficial Effects of Biochar and Chitosan on Antioxidative Capacity, Osmolytes Accumulation, and Anatomical Characters of Water-Stressed Barley Plants. Agronomy. 2020; 10(5):630. https://doi.org/10.3390/agronomy10050630
Chicago/Turabian StyleHafez, Yaser, Kotb Attia, Salman Alamery, Abdelhalim Ghazy, Abdullah Al-Doss, Eid Ibrahim, Emad Rashwan, Lamiaa El-Maghraby, Ahmed Awad, and Khaled Abdelaal. 2020. "Beneficial Effects of Biochar and Chitosan on Antioxidative Capacity, Osmolytes Accumulation, and Anatomical Characters of Water-Stressed Barley Plants" Agronomy 10, no. 5: 630. https://doi.org/10.3390/agronomy10050630
APA StyleHafez, Y., Attia, K., Alamery, S., Ghazy, A., Al-Doss, A., Ibrahim, E., Rashwan, E., El-Maghraby, L., Awad, A., & Abdelaal, K. (2020). Beneficial Effects of Biochar and Chitosan on Antioxidative Capacity, Osmolytes Accumulation, and Anatomical Characters of Water-Stressed Barley Plants. Agronomy, 10(5), 630. https://doi.org/10.3390/agronomy10050630







