Interaction between Humic Substances and Plant Hormones for Phosphorous Acquisition
Abstract
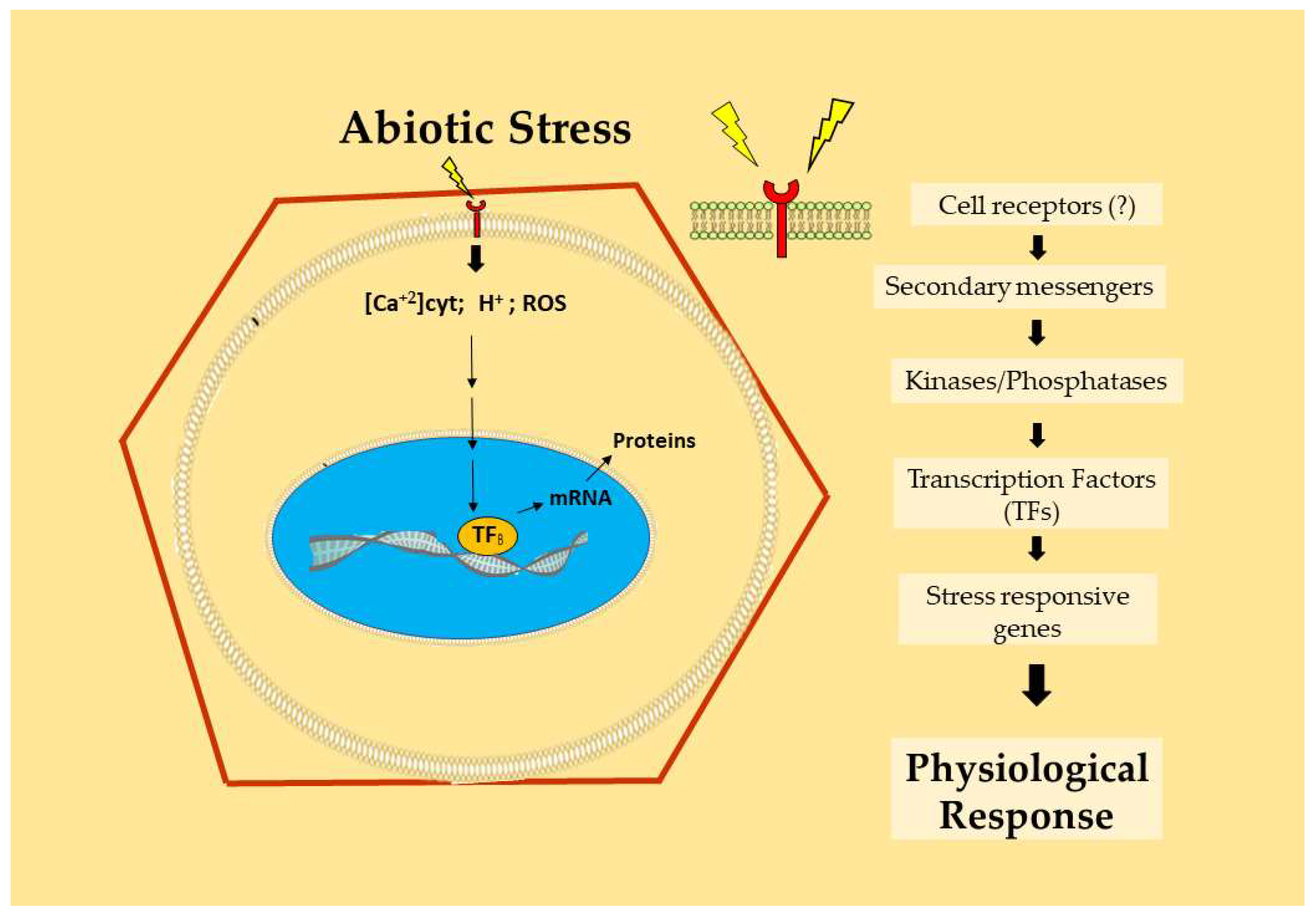
:1. Introduction
2. Hormonal-Like Actions of Humic Substances
2.1. Auxin (AUX)
2.2. Cytokinins (CK)
2.3. Gibberellin (GA)
2.4. Abscisic acid (ABA)
2.5. Ethylene (ET)
3. HS Modulation of Primary Plant Metabolism
4. P Uptake by HS Application Due to Phytohormonal Activity
5. Effect of HS on Secondary Metabolism under Abiotic Stress Conditions
6. Interaction between HS and P-Solubilizing Microorganisms
7. Interaction between HS and Mycorrhizal Fungi for P Uptake
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Magnone, D.; Bouwman, A.F.; Van Der Zee, S.E.; Sattari, S.Z.; Beusen, A.H.; Niasar, V.J. Efficiency of phosphorus resource use in Africa as defined by soil chemistry and the impact on crop production. Energy Procedia 2017, 123, 97–104. [Google Scholar] [CrossRef]
- Mogollón, J.; Beusen, A.; Van Grinsven, H.; Westhoek, H.; Bouwman, A. Future agricultural phosphorus demand according to the shared socioeconomic pathways. Glob. Environ. Chang. 2018, 50, 149–163. [Google Scholar] [CrossRef]
- Dawson, C.J.; Hilton, J. Fertiliser availability in a resource-limited world: Production and recycling of nitrogen and phosphorus. Food Policy 2011, 36, S14–S22. [Google Scholar] [CrossRef]
- Vance, C.P.; Uhde-Stone, C.; Allan, D.L. Phosphorus acquisition and use: Critical adaptations by plants for securing a nonrenewable resource. New Phytol. 2003, 157, 423–447. [Google Scholar] [CrossRef] [Green Version]
- Withers, P.J.; Rodrigues, M.; Soltangheisi, A.; De Carvalho, T.S.; Guilherme, L.R.; Benites, V.d.M.; Gatiboni, L.C.; De Sousa, D.M.; Nunes, R.d.S.; Rosolem, C.A. Transitions to sustainable management of phosphorus in Brazilian agriculture. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Nziguheba, G. Overcoming phosphorus deficiency in soils of Eastern Africa: Recent advances and challenges. In Advances in Integrated Soil Fertility Management in Sub-Saharan Africa: Challenges and Opportunities; Springer: Berlin, Germany, 2007; pp. 149–160. [Google Scholar]
- Schneider, K.D.; Thiessen Martens, J.R.; Zvomuya, F.; Reid, D.K.; Fraser, T.D.; Lynch, D.H.; O’Halloran, I.P.; Wilson, H.F. Options for improved phosphorus cycling and use in agriculture at the field and regional scales. J. Environ. Qual. 2019, 48, 1247–1264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Jiang, F.; Shen, Y.; Zhan, Q.; Bai, B.; Chen, W.; Chi, Y. Transcriptome analysis reveals candidate genes related to phosphorus starvation tolerance in sorghum. BMC Plant. Biol. 2019, 19, 306. [Google Scholar] [CrossRef]
- Baek, D.; Chun, H.J.; Yun, D.-J.; Kim, M.C.J.M. Cross-talk between phosphate starvation and other environmental stress signaling pathways in plants. Molecules 2017, 40, 697. [Google Scholar]
- Jiang, H.; Zhang, J.; Han, Z.; Yang, J.; Ge, C.; Wu, Q. Revealing new insights into different phosphorus-starving responses between two maize (Zea mays) inbred lines by transcriptomic and proteomic studies. Sci. Rep. 2017, 7, 44294. [Google Scholar] [CrossRef] [Green Version]
- Franco-Zorrilla, J.M.; Martin, A.C.; Solano, R.; Rubio, V.; Leyva, A.; Paz-Ares, J. Mutations at CRE1 impair cytokinin-induced repression of phosphate starvation responses in Arabidopsis. Plant. J. 2002, 32, 353–360. [Google Scholar] [CrossRef]
- Shen, C.; Yue, R.; Yang, Y.; Zhang, L.; Sun, T.; Tie, S.; Wang, H. OsARF16 is involved in cytokinin-mediated inhibition of phosphate transport and phosphate signaling in rice (Oryza sativa L.). PLoS ONE 2014, 9, e112906. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yi, K.; Tao, Y.; Wang, F.; Wu, Z.; Jiang, D.; Chen, X.; Zhu, L.; Wu, P. Cytokinin represses phosphate-starvation response through increasing of intracellular phosphate level. Plant. Cell Environ. 2006, 29, 1924–1935. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Gao, X.; Liao, L.; Harberd, N.P.; Fu, X. Phosphate starvation root architecture and anthocyanin accumulation responses are modulated by the gibberellin-DELLA signaling pathway in Arabidopsis. J. Plant. Physiol. 2007, 145, 1460–1470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, L.; Liu, D. Ethylene and plant responses to phosphate deficiency. Front. Plant. Sci. 2015, 6, 796. [Google Scholar] [CrossRef] [Green Version]
- Silva-Navas, J.; Conesa, C.M.; Saez, A.; Navarro-Neila, S.; Garcia-Mina, J.M.; Zamarreño, A.M.; Baigorri, R.; Swarup, R.; del Pozo, J.C. Role of cis-zeatin in root responses to phosphate starvation. New Phytol. 2019, 224, 242–257. [Google Scholar] [CrossRef]
- Vysotskaya, L.B.; Trekozova, A.W.; Kudoyarova, G.R. Effect of phosphorus starvation on hormone content and growth of barley plants. Acta Physiol. Plant. 2016, 38, 108. [Google Scholar] [CrossRef]
- Czarnecki, O.; Yang, J.; Weston, D.J.; Tuskan, G.A.; Chen, J.-G. A dual role of strigolactones in phosphate acquisition and utilization in plants. Int. J. Mol. Sci. 2013, 14, 7681–7701. [Google Scholar] [CrossRef] [Green Version]
- Ha, S.; Tran, L.-S. Understanding plant responses to phosphorus starvation for improvement of plant tolerance to phosphorus deficiency by biotechnological approaches. Crit. Rev. Biotechnol. 2014, 34, 16–30. [Google Scholar] [CrossRef]
- Torres-Vera, R.; García, J.M.; Pozo, M.J.; López-Ráez, J.A. Do strigolactones contribute to plant defence? Mol. Plant. Pathol. 2014, 15, 211–216. [Google Scholar] [CrossRef]
- Bezuglova, O.S.; Gorovtsov, A.V.; Polienko, E.A.; Zinchenko, V.E.; Grinko, A.V.; Lykhman, V.A.; Dubinina, M.N.; Demidov, A.J. Effect of humic preparation on winter wheat productivity and rhizosphere microbial community under herbicide-induced stress. J. Soils Sedim. 2019, 19, 2665–2675. [Google Scholar] [CrossRef]
- Giro, V.; Jindo, K.; Vittorazzi, C.; de Oliveira, R.; Conceição, G.; Canellas, L.; Olivares, F. Rock phosphate combined with phosphate-solubilizing microorganisms and humic substance for reduction of plant phosphorus demands from single superphosphate. In Proceedings of the III International Symposium on Organic Matter Management and Compost Use in Horticulture, Murcia, Spain, 20–24 April 2015; pp. 63–68. [Google Scholar]
- Olivares, F.L.; Busato, J.G.; de Paula, A.M.; da Silva Lima, L.; Aguiar, N.O.; Canellas, L.P. Plant growth promoting bacteria and humic substances: Crop promotion and mechanisms of action. Chem. Biol. Technol. Agric. 2017, 4, 30. [Google Scholar] [CrossRef] [Green Version]
- Rosa, S.D.; Silva, C.A.; Maluf, H.J.G.M. Wheat nutrition and growth as affected by humic acid-phosphate interaction. J. Plant. Nutr. Soil Sci. 2018, 181, 870–877. [Google Scholar] [CrossRef]
- Erro, J.; Urrutia, O.; Baigorri, R.; Fuentes, M.; Zamarreño, A.; Garcia-Mina, J. Incorporation of humic-derived active molecules into compound NPK granulated fertilizers: Main technical difficulties and potential solutions. Chem. Biol. Technol. Agric. 2016, 3, 18. [Google Scholar] [CrossRef] [Green Version]
- Gerke, J. Humic (organic matter)-Al (Fe)-phosphate complexes: An underestimated phosphate form in soils and source of plant-available phosphate. Soil Sci. 2010, 175, 417–425. [Google Scholar] [CrossRef]
- Zanin, L.; Tomasi, N.; Cesco, S.; Varanini, Z.; Pinton, R. Humic substances contribute to plant iron nutrition acting as chelators and biostimulants. Front. Plant. Sci. 2019, 10, 675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muscolo, A.; Sidari, M.; Nardi, S. Humic substance: Relationship between structure and activity. Deeper information suggests univocal findings. J. Geochem. Explor. 2013, 129, 57–63. [Google Scholar] [CrossRef]
- Polyakov, A.Y.; Lebedev, V.A.; Volkov, D.S.; Pankratov, D.A.; Veligzhanin, A.A.; Perminova, I.V.; Lucena, J.J. Eco-friendly iron-humic nanofertilizers synthesis for the prevention of iron chlorosis in soybean (Glycine max) grown in calcareous soil. Front. Plant. Sci. 2019, 10, 413. [Google Scholar] [CrossRef]
- Vaccaro, S.; Ertani, A.; Nebbioso, A.; Muscolo, A.; Quaggiotti, S.; Piccolo, A.; Nardi, S. Humic substances stimulate maize nitrogen assimilation and amino acid metabolism at physiological and molecular level. Chem. Biol. Technol. Agric. 2015, 2, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Stevenson, F.J. Humus Chemistry: Genesis, Composition, Reactions; John Wiley & Sons: Hoboken, NJ, USA, 1994. [Google Scholar]
- Piccolo, A. The supramolecular structure of humic substances: A novel understanding of humus chemistry and implications in soil science. Adv. Agron. 2002, 75, 57–134. [Google Scholar] [CrossRef]
- Canellas, L.; Dantas, D.; Aguiar, N.; Peres, L.; Zsögön, A.; Olivares, F.; Dobbss, L.; Façanha, A.; Nebbioso, A.; Piccolo, A.J. Probing the hormonal activity of fractionated molecular humic components in tomato auxin mutants. Ann. Appl. Biol. 2011, 159, 202–211. [Google Scholar] [CrossRef] [Green Version]
- Canellas, L.P.; Olivares, F.L. Physiological responses to humic substances as plant growth promoter. Chem. Biol. Technol. Agric. 2014, 1, 3. [Google Scholar] [CrossRef] [Green Version]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant. Soil 2014, 383, 3–41. [Google Scholar] [CrossRef] [Green Version]
- Canellas, L.P.; Olivares, F.L.; Aguiar, N.O.; Jones, D.L.; Nebbioso, A.; Mazzei, P.; Piccolo, A. Humic and fulvic acids as biostimulants in horticulture. Sci. Hortic. 2015, 196, 15–27. [Google Scholar] [CrossRef]
- García, A.C.; Olaetxea, M.; Santos, L.A.; Mora, V.; Baigorri, R.; Fuentes, M.; Zamarreño, A.M.; Berbara, R.L.L.; Garcia-Mina, J.M. Involvement of hormone-and ROS-signaling pathways in the beneficial action of humic substances on plants growing under normal and stressing conditions. Biomed. Res. Int. 2016, 2016, 13. [Google Scholar] [CrossRef]
- Mora, V.; Bacaicoa, E.; Zamarreno, A.-M.; Aguirre, E.; Garnica, M.; Fuentes, M.; García-Mina, J.-M. Action of humic acid on promotion of cucumber shoot growth involves nitrate-related changes associated with the root-to-shoot distribution of cytokinins, polyamines and mineral nutrients. J. Plant. Physiol. 2010, 167, 633–642. [Google Scholar] [CrossRef]
- Olaetxea, M.; De Hita, D.; Garcia, C.A.; Fuentes, M.; Baigorri, R.; Mora, V.; Garnica, M.; Urrutia, O.; Erro, J.; Zamarreño, A.M.J. Hypothetical framework integrating the main mechanisms involved in the promoting action of rhizospheric humic substances on plant root-and shoot-growth. Appl. Soil Ecol. 2018, 123, 521–537. [Google Scholar] [CrossRef]
- Rose, M.T.; Patti, A.F.; Little, K.R.; Brown, A.L.; Jackson, W.R.; Cavagnaro, T.R. A meta-analysis and review of plant-growth response to humic substances: Practical implications for agriculture. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2014; Volume 124, pp. 37–89. [Google Scholar]
- O’donnell, R. The auxin-like effects of humic preparations from leonardite. Soil Sci. 1973, 116, 106–112. [Google Scholar] [CrossRef]
- Canellas, L.P.; Olivares, F.L.; Okorokova-Façanha, A.L.; Façanha, A.R. Humic acids isolated from earthworm compost enhance root elongation, lateral root emergence, and plasma membrane H+-ATPase activity in maize roots. Plant. Physiol. 2002, 130, 1951–1957. [Google Scholar] [CrossRef] [Green Version]
- Shen, H.; Shen, J.Z.; Li, Y.; Lai, Y.L.; Jia, Z.H.; Yi, J.H. Promotion of lateral root growth and leaf quality of flue-cured tobacco by the combined application of humic acids and NPK chemical fertilizers. J. Exp. Agric. 2017, 53, 59–70. [Google Scholar] [CrossRef]
- Mora, V.; Baigorri, R.; Bacaicoa, E.; Zamarreno, A.M.; García-Mina, J.M. The humic acid-induced changes in the root concentration of nitric oxide, IAA and ethylene do not explain the changes in root architecture caused by humic acid in cucumber. Environ. Exp. Bot. 2012, 76, 24–32. [Google Scholar] [CrossRef]
- Zandonadi, D.B.; Santos, M.P.; Dobbss, L.B.; Olivares, F.L.; Canellas, L.P.; Binzel, M.L.; Okorokova-Façanha, A.L.; Façanha, A.R. Nitric oxide mediates humic acids-induced root development and plasma membrane H+-ATPase activation. Planta 2010, 231, 1025–1036. [Google Scholar] [CrossRef] [PubMed]
- Hager, A.; Debus, G.; Edel, H.G.; Stransky, H.; Serrano, R. Auxin induces exocytosis and the rapid synthesis of a high-turnover pool of plasma-membrane H+-ATPase. Planta 1991, 185, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Ramos, A.C.; Dobbss, L.B.; Santos, L.A.; Fernandes, M.S.; Olivares, F.L.; Aguiar, N.O.; Canellas, L.P. Humic matter elicits proton and calcium fluxes and signaling dependent on Ca2+-dependent protein kinase (CDPK) at early stages of lateral plant root development. Chem. Biol. Technol. Agric. 2015, 2, 3. [Google Scholar] [CrossRef] [Green Version]
- Quaggiotti, S.; Ruperti, B.; Pizzeghello, D.; Francioso, O.; Tugnoli, V.; Nardi, S. Effect of low molecular size humic substances on nitrate uptake and expression of genes involved in nitrate transport in maize (Zea mays L.). J. Exp. Bot. 2004, 55, 803–813. [Google Scholar] [CrossRef] [Green Version]
- Russell, L.; Stokes, A.R.; Macdonald, H.; Muscolo, A.; Nardi, S. Stomatal responses to humic substances and auxin are sensitive to inhibitors of phospholipase A 2. Plant. Soil 2006, 283, 175–185. [Google Scholar] [CrossRef]
- Albuzio, A.; Nardi, S.; Gulli, A.J. Plant growth regulator activity of small molecular size humic fractions. Sci. Total Environ. 1989, 81, 671–674. [Google Scholar] [CrossRef]
- Rivero, R.M.; Shulaev, V.; Blumwald, E. Cytokinin-dependent photorespiration and the protection of photosynthesis during water deficit. Plant. Physiol. 2009, 150, 1530–1540. [Google Scholar] [CrossRef] [Green Version]
- Pizzeghello, D.; Francioso, O.; Ertani, A.; Muscolo, A.; Nardi, S. Isopentenyladenosine and cytokinin-like activity of different humic substances. J. Geochem. Explor. 2013, 129, 70–75. [Google Scholar] [CrossRef]
- Jannin, L.; Arkoun, M.; Ourry, A.; Laîné, P.; Goux, D.; Garnica, M.; Fuentes, M.; San Francisco, S.; Baigorri, R.; Cruz, F. Microarray analysis of humic acid effects on Brassica napus growth: Involvement of N, C and S metabolisms. Plant. Soil 2012, 359, 297–319. [Google Scholar] [CrossRef]
- Rubio, V.; Bustos, R.; Irigoyen, M.L.; Cardona-López, X.; Rojas-Triana, M.; Paz-Ares, J. Plant hormones and nutrient signaling. Plant. Mol. Biol. 2009, 69, 361. [Google Scholar] [CrossRef]
- de Sanfilippo, E.C.; Argüello, J.; Abdala, G.; Orioli, G. Content of auxin-inhibitor-and gibberellin-like substances in humic acids. Biol. Plant. 1990, 32, 346. [Google Scholar] [CrossRef]
- Nardi, S.; Pizzeghello, D.; Reniero, F.; Muscolo, A. Biological activity of humic substances extracted from soils under different vegetation cover. Commun. Soil Sci. Plant. Anal. 1999, 30, 621–634. [Google Scholar] [CrossRef]
- Savy, D.; Canellas, L.; Vinci, G.; Cozzolino, V.; Piccolo, A. Humic-like water-soluble lignins from giant reed (Arundo donax L.) display hormone-like activity on plant growth. J. Plant. Growth Regul. 2017, 36, 995–1001. [Google Scholar] [CrossRef]
- Ertani, A.; Cavani, L.; Pizzeghello, D.; Brandellero, E.; Altissimo, A.; Ciavatta, C.; Nardi, S. Biostimulant activity of two protein hydrolyzates in the growth and nitrogen metabolism of maize seedlings. J. Plant. Nutr. Soil Sci. 2009, 172, 237–244. [Google Scholar] [CrossRef]
- Nakasha, J.J.; Sinniah, U.R.; Puteh, A.; Hassan, S.A. Potential regulatory role of gibberellic and humic acids in sprouting of Chlorophytum borivilianum tubers. Sci. World J. 2014, 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Pizzeghello, D.; Nicolini, G.; Nardi, S. Hormone-like activities of humic substances in different forest ecosystems. New Phytol. 2002, 155, 393–402. [Google Scholar] [CrossRef]
- Pizzeghello, D.; Nicolini, G.; Nardi, S. Hormone-like activity of humic substances in Fagus sylvaticae forests. New Phytol. 2001, 151, 647–657. [Google Scholar] [CrossRef]
- Ferrara, G.; Brunetti, G. Effects of the times of application of a soil humic acid on berry quality of table grape (Vitis vinifera L.) cv Italia. Span. J. Agric. Res. 2010, 8, 817–822. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, R.; Caldana, C.; Mueller-Roeber, B.; Schippers, J.H.M. The contribution of SERF1 to root-to-shoot signaling during salinity stress in rice. Plant. Signal. Behav. 2014, 9, e27540. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, N.; Rivero, R.M.; Shulaev, V.; Blumwald, E.; Mittler, R. Abiotic and biotic stress combinations. New Phytol. 2014, 203, 32–43. [Google Scholar] [CrossRef]
- García, A.C.; Santos, L.A.; Izquierdo, F.G.; Rumjanek, V.M.; Castro, R.N.; dos Santos, F.S.; de Souza, L.G.A.; Berbara, R.L.L. Potentialities of vermicompost humic acids to alleviate water stress in rice plants (Oryza sativa L.). J. Geochem. Explor. 2014, 136, 48–54. [Google Scholar] [CrossRef]
- Olaetxea, M.; Mora, V.; Bacaicoa, E.; Garnica, M.; Fuentes, M.; Casanova, E.; Zamarreño, A.M.; Iriarte, J.C.; Etayo, D.; Ederra, I. Abscisic acid regulation of root hydraulic conductivity and aquaporin gene expression is crucial to the plant shoot growth enhancement caused by rhizosphere humic acids. Plant. Physiol. 2015, 169, 2587–2596. [Google Scholar] [CrossRef] [PubMed]
- Mora, V.; Bacaicoa, E.; Baigorri, R.; Zamarreno, A.M.; Garcia-Mina, J.M. NO and IAA key regulators in the shoot growth promoting action of humic acid in Cucumis sativus L. J. Plant. Growth Regul. 2014, 33, 430–439. [Google Scholar] [CrossRef]
- Schmidt, W.; Santi, S.; Pinton, R.; Varanini, Z. Water-extractable humic substances alter root development and epidermal cell pattern in Arabidopsis. Plant. Soil 2007, 300, 259–267. [Google Scholar] [CrossRef]
- Mora, V.; Olaetxea, M.; Bacaicoa, E.; Baigorri, R.; Fuentes, M.; Zamarreño, A.; Garcia-Mina, J. Abiotic stress tolerance in plants: Exploring the role of nitric oxide and humic substances. In Nitric Oxide in Plants: Metabolism and Role in Stress Physiology; Springer: Berlin, Germany, 2014; pp. 243–264. [Google Scholar] [CrossRef]
- Roomi, S.; Masi, A.; Conselvan, G.B.; Trevisan, S.; Quaggiotti, S.; Pivato, M.; Arrigoni, G.; Yasmin, T.; Carletti, P. Protein profiling of Arabidopsis roots treated with humic substances: Insights into the metabolic and interactome networks. Front. Plant. Sci. 2018, 9, 1812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conselvan, G.B.; Fuentes, D.; Merchant, A.; Peggion, C.; Francioso, O.; Carletti, P. Effects of humic substances and indole-3-acetic acid on Arabidopsis sugar and amino acid metabolic profile. Plant. Soil 2018, 426, 17–32. [Google Scholar] [CrossRef]
- Sofi, A.; Ebrahimi, M.; Shirmohammadi, E. Effect of Humic Acid on Germination, Growth, and Photosynthetic Pigments of Medicago sativa L. under Salt Stress. ECOPERSIA 2018, 6, 21–30. [Google Scholar]
- Khan, A.; Khan, M.Z.; Hussain, F.; Akhtar, M.E.; Gurmani, A.R.; Khan, S. Effect of humic acid on the growth, yield, nutrient composition, photosynthetic pigment and total sugar contents of peas (Pisum sativum L). J. Chem. Soc. Pak. 2013, 35, 206–211. [Google Scholar]
- Liu, C.; Cooper, R.J.; Bowman, D.C. Humic acid application affects photosynthesis, root development, and nutrient content of creeping bentgrass. HortScience 1998, 33, 1023–1025. [Google Scholar] [CrossRef] [Green Version]
- Canellas, L.P.; Balmori, D.M.; Médici, L.O.; Aguiar, N.O.; Campostrini, E.; Rosa, R.C.; Façanha, A.R.; Olivares, F.L. A combination of humic substances and Herbaspirillum seropedicae inoculation enhances the growth of maize (Zea mays L.). Plant. Soil 2013, 366, 119–132. [Google Scholar] [CrossRef]
- Ertani, A.; Francioso, O.; Tugnoli, V.; Righi, V.; Nardi, S. Effect of commercial lignosulfonate-humate on Zea mays L. metabolism. J. Agric. Food Chem. 2011, 59, 11940–11948. [Google Scholar] [CrossRef] [PubMed]
- Delfine, S.; Tognetti, R.; Desiderio, E.; Alvino, A. Effect of foliar application of N and humic acids on growth and yield of durum wheat. Agron. Sustain. Dev. 2005, 25, 8. [Google Scholar] [CrossRef]
- El-Shabrawi, H.M.; Bakry, B.A.; Ahmed, M.A.; Abou-El-Lail, M.J.A.S. Humic and oxalic acid stimulates grain yield and induces accumulation of plastidial carbohydrate metabolism enzymes in wheat grown under sandy soil conditions. Agric. Sci. 2015, 6, 175. [Google Scholar] [CrossRef] [Green Version]
- Nardi, S.; Muscolo, A.; Vaccaro, S.; Baiano, S.; Spaccini, R.; Piccolo, A. Relationship between molecular characteristics of soil humic fractions and glycolytic pathway and krebs cycle in maize seedlings. Soil Biol. Biochem. 2007, 39, 3138–3146. [Google Scholar] [CrossRef]
- Trevisan, S.; Botton, A.; Vaccaro, S.; Vezzaro, A.; Quaggiotti, S.; Nardi, S. Humic substances affect Arabidopsis physiology by altering the expression of genes involved in primary metabolism, growth and development. Environ. Exp. Bot. 2011, 74, 45–55. [Google Scholar] [CrossRef]
- Carletti, P.; Masi, A.; Spolaore, B.; De Laureto, P.P.; De Zorzi, M.; Turetta, L.; Ferretti, M.; Nardi, S. Protein expression changes in maize roots in response to humic substances. J. Chem. Ecol. 2008, 34, 804. [Google Scholar] [CrossRef]
- Nikbakht, A.; Pessarakli, M.; Daneshvar-Hakimi-Maibodi, N.; Kafi, M.J.A.J. Perennial ryegrass growth responses to mycorrhizal infection and humic acid treatments. Agron. J. 2014, 106, 585–595. [Google Scholar] [CrossRef]
- Noroozisharaf, A.; Kaviani, M.J.P. Effect of soil application of humic acid on nutrients uptake, essential oil and chemical compositions of garden thyme (Thymus vulgaris L.) under greenhouse conditions. Physiol. Mol. Biol. Plants 2018, 24, 423–431. [Google Scholar] [CrossRef]
- Zancani, M.; Petrussa, E.; Krajňáková, J.; Casolo, V.; Spaccini, R.; Piccolo, A.; Macrì, F.; Vianello, A. Effect of humic acids on phosphate level and energetic metabolism of tobacco BY-2 suspension cell cultures. Environ. Exp. Bot. 2009, 65, 287–295. [Google Scholar] [CrossRef]
- Nikbakht, A.; Kafi, M.; Babalar, M.; Xia, Y.P.; Luo, A.; Etemadi, N.A. Effect of humic acid on plant growth, nutrient uptake, and postharvest life of gerbera. J. Plant. Nutr. 2008, 31, 2155–2167. [Google Scholar] [CrossRef]
- Zandonadi, D.B.; Canellas, L.P.; Façanha, A.R. Indolacetic and humic acids induce lateral root development through a concerted plasmalemma and tonoplast H+ pumps activation. Planta 2007, 225, 1583–1595. [Google Scholar] [CrossRef] [PubMed]
- Olaetxea, M.; Mora, V.; Bacaicoa, E.; Baigorri, R.; Garnica, M.; Fuentes, M.; Zamarreño, A.M.; Spíchal, L.; García-Mina, J.M. Root ABA and H+-ATPase are key players in the root and shoot growth-promoting action of humic acids. Plant. Direct 2019, 3, e00175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, Z.H.; Rehman, H.M.; Akhtar, T.; Alsamadany, H.; Hamooh, B.T.; Mujtaba, T.; Daur, I.; Al Zahrani, Y.; Alzahrani, H.A.; Ali, S. Humic substances: Determining potential molecular regulatory processes in plants. Front. Plant. Sci. 2018, 9, 263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olaetxea, M.; Mora, V.; García, A.C.; Santos, L.A.; Baigorri, R.; Fuentes, M.; Garnica, M.; Berbara, R.L.L.; Zamarreño, A.M.; Garcia-Mina, J.M.; et al. Root-Shoot Signaling crosstalk involved in the shoot growth promoting action of rhizospheric humic acids. Plant. Signal. 2016, 11, e1161878. [Google Scholar] [CrossRef] [Green Version]
- Robaglia, C.; Thomas, M.; Meyer, C. Sensing nutrient and energy status by SnRK1 and TOR kinases. Curr. Opin. Plant. Biol. 2012, 15, 301–307. [Google Scholar] [CrossRef]
- Deprost, D.; Yao, L.; Sormani, R.; Moreau, M.; Leterreux, G.; Nicolaï, M.; Bedu, M.; Robaglia, C.; Meyer, C. The Arabidopsis TOR kinase links plant growth, yield, stress resistance and mRNA translation. Embo Rep. 2007, 8, 864–870. [Google Scholar] [CrossRef] [Green Version]
- Canellas, L.P.; Canellas, N.O.; Soares, T.S.; Olivares, F.L. Humic Acids Interfere with Nutrient Sensing in Plants Owing to the Differential Expression of TOR. J. Plant. Growth Regul. 2019, 38, 216–224. [Google Scholar] [CrossRef]
- Aguiar, N.; Medici, L.; Olivares, F.; Dobbss, L.; Torres-Netto, A.; Silva, S.; Novotny, E.; Canellas, L. Metabolic profile and antioxidant responses during drought stress recovery in sugarcane treated with humic acids and endophytic diazotrophic bacteria. Ann. Appl. Biol. 2016, 168, 203–213. [Google Scholar] [CrossRef]
- Lotfi, R.; Kalaji, H.; Valizadeh, G.; Behrozyar, E.K.; Hemati, A.; Gharavi-Kochebagh, P.; Ghassemi, A. Effects of humic acid on photosynthetic efficiency of rapeseed plants growing under different watering conditions. Photosynthetica 2018, 56, 962–970. [Google Scholar] [CrossRef] [Green Version]
- Dixon, R.A.; Paiva, N.L. Stress-Induced Phenylpropanoid Metabolism. Plant. Cell 1995, 7, 1085–1097. [Google Scholar] [CrossRef]
- Malusà, E.; Russo, M.A.; Mozzetti, C.; Belligno, A. Modification of secondary metabolism and flavonoid biosynthesis under phosphate deficiency in bean roots. J. Plant. Nutr. 2006, 29, 245–258. [Google Scholar] [CrossRef]
- Ertani, A.; Pizzeghello, D.; Francioso, O.; Tinti, A.; Nardi, S. Biological activity of vegetal extracts containing phenols on plant metabolism. Molecules 2016, 21, 205. [Google Scholar] [CrossRef] [PubMed]
- Ertani, A.; Schiavon, M.; Altissimo, A.; Franceschi, C.; Nardi, S. Phenol-containing organic substances stimulate phenylpropanoid metabolism in Zea mays. J. Plant. Nutr. Soil Sci. 2011, 174, 496–503. [Google Scholar] [CrossRef]
- Schiavon, M.; Pizzeghello, D.; Muscolo, A.; Vaccaro, S.; Francioso, O.; Nardi, S. High molecular size humic substances enhance phenylpropanoid metabolism in maize (Zea mays L.). J. Chem. Ecol. 2010, 36, 662–669. [Google Scholar] [CrossRef]
- Aguiar, N.O.; Olivares, F.L.; Novotny, E.H.; Canellas, L.P. Changes in metabolic profiling of sugarcane leaves induced by endophytic diazotrophic bacteria and humic acids. PeerJ 2018, 6, e5445. [Google Scholar] [CrossRef] [PubMed]
- Canellas, N.O.A.; Olivares, F.L.; Canellas, L.P. Metabolite fingerprints of maize and sugarcane seedlings: Searching for markers after inoculation with plant growth-promoting bacteria in humic acids. Chem. Biol. Technol. Agric. 2019, 6, 14. [Google Scholar] [CrossRef]
- Zhang, X.; Ervin, E.J.C.s. Cytokinin-containing seaweed and humic acid extracts associated with creeping bentgrass leaf cytokinins and drought resistance. Crop. Sci. 2004, 44, 1737–1745. [Google Scholar] [CrossRef]
- Juszczuk, I.; Malusà, E.; Rychter, A.M. Oxidative stress during phosphate deficiency in roots of bean plants (Phaseolus vulgaris L.). J. Plant. Physiol. 2001, 158, 1299–1305. [Google Scholar] [CrossRef]
- Rai, A.K.; Takabe, T. Abiotic Stress Tolerance in Plants; Springer: Berlin, Germany, 2006; p. 265. [Google Scholar]
- Chen, S.; Zhao, H.; Ding, G.; Xu, F. Genotypic differences in antioxidant response to phosphorus deficiency in Brassica napus. Plant. Soil 2015, 391, 19–32. [Google Scholar] [CrossRef]
- Yu, Q.; Rengel, Z. Drought and salinity differentially influence activities of superoxide dismutases in narrow-leafed lupins. Plant. Sci. 1999, 142, 1–11. [Google Scholar] [CrossRef]
- Cordeiro, F.C.; Santa-Catarina, C.; Silveira, V.; de Souza, S.R. Humic acid effect on catalase activity and the generation of reactive oxygen species in corn (Zea mays). Biosci. Biotechnol. Biochem. 2011, 75, 70–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García, A.C.; Santos, L.A.; Izquierdo, F.G.; Sperandio, M.V.L.; Castro, R.N.; Berbara, R.L.L. Vermicompost humic acids as an ecological pathway to protect rice plant against oxidative stress. Ecol. Eng. 2012, 47, 203–208. [Google Scholar] [CrossRef]
- Nunes, R.O.; Domiciano, G.A.; Alves, W.S.; Melo, A.C.A.; Nogueira, F.C.S.; Canellas, L.P.; Olivares, F.L.; Zingali, R.B.; Soares, M.R. Evaluation of the effects of humic acids on maize root architecture by label-free proteomics analysis. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Knight, H.; Knight, M.R. Abiotic stress signalling pathways: Specificity and cross-talk. Trends Plant. Sci. 2001, 6, 262–267. [Google Scholar] [CrossRef]
- Salomon, D.; Bonshtien, A.; Sessa, G. A chemical-genetic approach for functional analysis of plant protein kinases. Plant. Signal. Behav. 2009, 4, 645–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canellas, L.P.C.; Natália, O.A.; Irineu, L.E.S.S.; Olivares, F.L.; Piccolo, A. Plant chemical priming by humic acids. Chem. Biol. Technol. Agric. 2020. accepted manuscript number. [Google Scholar]
- Mahajan, S.; Tuteja, N. Cold, salinity and drought stresses: An overview. Arch. Biochem. Biophys. 2005, 444, 139–158. [Google Scholar] [CrossRef]
- Crozet, P.; Margalha, L.; Confraria, A.; Rodrigues, A.; Martinho, C.; Adamo, M.; Elias, C.A.; Baena-González, E. Mechanisms of regulation of SNF1/AMPK/SnRK1 protein kinases. Front. Plant. Sci. 2014, 5, 190. [Google Scholar] [CrossRef]
- Ichimura, K.; Mizoguchi, T.; Yoshida, R.; Yuasa, T.; Shinozaki, K. Various abiotic stresses rapidly activate Arabidopsis MAP kinases ATMPK4 and ATMPK6. Plant. J. 2000, 24, 655–665. [Google Scholar] [CrossRef]
- Lata, C.; Prasad, M. Role of DREBs in regulation of abiotic stress responses in plants. J. Exp. Bot. 2011, 62, 4731–4748. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Q.Y.; Tian, A.G.; Zou, H.F.; Xie, Z.M.; Lei, G.; Huang, J.; Wang, C.M.; Wang, H.W.; Zhang, J.S.; Chen, S.Y. Soybean WRKY-type transcription factor genes, GmWRKY13, GmWRKY21, and GmWRKY54, confer differential tolerance to abiotic stresses in transgenic Arabidopsis plants. Plant. Biotechnol. J. 2008, 6, 486–503. [Google Scholar] [CrossRef] [PubMed]
- Naoumkina, M.A.; He, X.; Dixon, R.A. Elicitor-induced transcription factors for metabolic reprogramming of secondary metabolism in Medicago truncatula. BMC Plant. Biol. 2008, 8, 132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grunewald, W.; De Smet, I.; Lewis, D.R.; Löfke, C.; Jansen, L.; Goeminne, G.; Bossche, R.V.; Karimi, M.; De Rybel, B.; Vanholme, B. Transcription factor WRKY23 assists auxin distribution patterns during Arabidopsis root development through local control on flavonol biosynthesis. Proc. Natl. Acad. Sci. USA 2012, 109, 1554–1559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Gao, Y.; Xu, H.; Dai, Y.; Deng, D.; Chen, J. ZmWRKY33, a WRKY maize transcription factor conferring enhanced salt stress tolerances in Arabidopsis. Plant. Growth Regul. 2013, 70, 207–216. [Google Scholar] [CrossRef]
- Devaiah, B.N.; Karthikeyan, A.S.; Raghothama, K.G. WRKY75 transcription factor is a modulator of phosphate acquisition and root development in Arabidopsis. Plant. Physiol. 2007, 143, 1789–1801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jindo, K.; Soares, T.S.; Peres, L.E.P.; Azevedo, I.G.; Aguiar, N.O.; Mazzei, P.; Spaccini, R.; Piccolo, A.; Olivares, F.L.; Canellas, L.P. Phosphorus speciation and high-affinity transporters are influenced by humic substances. J. Plant. Nutr. Soil Sci. 2016, 179, 206–214. [Google Scholar] [CrossRef]
- Wang, H.; Xu, Q.; Kong, Y.-H.; Chen, Y.; Duan, J.-Y.; Wu, W.-H.; Chen, Y.-F. Arabidopsis WRKY45 Transcription Factor Activates PHOSPHATE TRANSPORTER 1;1 Expression in Response to Phosphate Starvation. J. Plant Physiol. 2014, 164, 2020–2029. [Google Scholar] [CrossRef] [Green Version]
- Graber, E.R.; Tsechansky, L.; Mayzlish-Gati, E.; Shema, R.; Koltai, H. A humic substances product extracted from biochar reduces Arabidopsis root hair density and length under P-sufficient and P-starvation conditions. Plant. Soil 2015, 395, 21–30. [Google Scholar] [CrossRef]
- Puglisi, E.; Pascazio, S.; Suciu, N.; Cattani, I.; Fait, G.; Spaccini, R.; Crecchio, C.; Piccolo, A.; Trevisan, M. Rhizosphere microbial diversity as influenced by humic substance amendments and chemical composition of rhizodeposits. J. Geochem. Explor. 2013, 129, 82–94. [Google Scholar] [CrossRef]
- Puglisi, E.; Fragoulis, G.; Ricciuti, P.; Cappa, F.; Spaccini, R.; Piccolo, A.; Trevisan, M.; Crecchio, C. Effects of a humic acid and its size-fractions on the bacterial community of soil rhizosphere under maize (Zea mays L.). Chemosphere 2009, 77, 829–837. [Google Scholar] [CrossRef]
- Farias, N.; Almeida, I.; Meneses, C. New bacterial phytase through metagenomic prospection. Molecules 2018, 23, 448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nahas, E. Microrganismos do solo produtores de fosfatases em diferentes sistemas agrícolas. Bragantia 2002, 61, 267–275. [Google Scholar] [CrossRef]
- Ekin, Z. Integrated use of humic acid and plant growth promoting rhizobacteria to ensure higher potato productivity in sustainable agriculture. Sustainability 2019, 11, 3417. [Google Scholar] [CrossRef] [Green Version]
- Hussein, K.A.; Joo, J.H. Isolation and characterization of rhizomicrobial isolates for phosphate solubilization and indole acetic acid production. J. Korean Soc. Appl. Biol. Chem. 2015, 58, 847–855. [Google Scholar] [CrossRef]
- Mulyatni, A.S.; Praptana, R.H.; Santoso, D. The effect of biostimulant in root and population of phosphate solubilizing bacteria: A study case in upland rice. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2018; p. 012016. [Google Scholar]
- Ponmurugan, P.; Gopi, C. In vitro production of growth regulators and phosphatase activity by phosphate solubilizing bacteria. Afr. J. Biotechnol. 2006, 5, 348–350. [Google Scholar]
- Kumar, V.; Narula, N. Solubilization of inorganic phosphates and growth emergence of wheat as affected by Azotobacter chroococcum mutants. Biol. Fertil. Soils 1999, 28, 301–305. [Google Scholar] [CrossRef]
- Dutton, M.V.; Evans, C.S. Oxalate production by fungi: Its role in pathogenicity and ecology in the soil environment. Can. J. Microbiol. 1996, 42, 881–895. [Google Scholar] [CrossRef]
- Marra, L.M.; Soares, C.R.F.S.; de Oliveira, S.M.; Ferreira, P.A.A.; Soares, B.L.; de Fráguas Carvalho, R.; de Lima, J.M.; de Souza Moreira, F.M. Biological nitrogen fixation and phosphate solubilization by bacteria isolated from tropical soils. Plant. Soil 2012, 357, 289–307. [Google Scholar] [CrossRef]
- Surange, S.; Wollum Ii, A.G.; Kumar, N.; Nautiyal, C.S. Characterization of Rhizobium from root nodules of leguminous trees growing in alkaline soils. Can. J. Microbiol. 1997, 43, 891–894. [Google Scholar] [CrossRef]
- Alori, E.T.; Glick, B.R.; Babalola, O.O. Microbial phosphorus solubilization and its potential for use in sustainable agriculture. Front. Microbiol. 2017, 8, 971. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.B.; Sayyed, R.Z.; Trivedi, M.H.; Gobi, T.A. Phosphate solubilizing microbes: Sustainable approach for managing phosphorus deficiency in agricultural soils. SpringerPlus 2013, 2, 587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Illmer, P.; Barbato, A.; Schinner, F. Solubilization of hardly-soluble AlPO4 with P-solubilizing microorganisms. Soil Biol. Biochem. 1995, 27, 265–270. [Google Scholar] [CrossRef]
- Whitelaw, M.A. Growth promotion of plants inoculated with phosphate-solubilizing fungi. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 1999; Volume 69, pp. 99–151. [Google Scholar]
- Winarso, S.; Sulistyanto, D.; Handayanto, E. Effects of humic compounds and phosphate solubilizing bacteria on phosphorus availability in an acid soil. J. Ecol. Nat. Environ. 2011, 3, 232–240. [Google Scholar]
- Taurian, T.; Anzuay, M.S.; Angelini, J.G.; Tonelli, M.L.; Ludueña, L.; Pena, D.; Ibáñez, F.; Fabra, A. Phosphate-solubilizing peanut associated bacteria: Screening for plant growth-promoting activities. Plant. Soil 2010, 329, 421–431. [Google Scholar] [CrossRef]
- Goldstein, A.H. Bacterial solubilization of mineral phosphates: Historical perspective and future prospects. Am. J. Altern. Agric. 1986, 1, 51–57. [Google Scholar] [CrossRef]
- da Silva Lima, L.; Olivares, F.L.; De Oliveira, R.R.; Vega, M.R.G.; Aguiar, N.O.; Canellas, L.P. Root exudate profiling of maize seedlings inoculated with Herbaspirillum seropedicae and humic acids. Chem. Biol. Technol. Agric. 2014, 1, 23. [Google Scholar] [CrossRef] [Green Version]
- Chaiharn, M.; Lumyong, S. Screening and optimization of indole-3-acetic acid production and phosphate solubilization from rhizobacteria aimed at improving plant growth. Curr. Microbiol. 2011, 62, 173–181. [Google Scholar] [CrossRef]
- Baldotto, M.A.; Baldotto, L.E.B. Ácidos húmicos. Rev. Ceres 2014, 61, 856–881. [Google Scholar] [CrossRef] [Green Version]
- Busato, J.G.; Lima, L.S.; Aguiar, N.O.; Canellas, L.P.; Olivares, F.L. Changes in labile phosphorus forms during maturation of vermicompost enriched with phosphorus-solubilizing and diazotrophic bacteria. Bioresour. Technol. 2012, 110, 390–395. [Google Scholar] [CrossRef] [Green Version]
- Baloach, N.; Yousaf, M.; Akhter, W.P.; Fahad, S.; Ullah, B.; Qadir, G.; Ahmed, Z.I. Integrated effect of phosphate solubilizing bacteria and humic acid on physiomorphic attributes of maize. Int J. Curr Microbiol. Appl. Sci. 2014, 3, 549–554. [Google Scholar]
- El-Sheshtawy, A.A.; Hager, M.A.; Shawer, S.S. Effect of bio-fertilizer, Phosphorus source and humic substances on yield, yield components and nutrients uptake by barley plant. J. Biol. Chem. Environ. Sci. 2019, 14, 279–300. [Google Scholar]
- Bashan, Y.; Kamnev, A.A.; de-Bashan, L.E. Tricalcium phosphate is inappropriate as a universal selection factor for isolating and testing phosphate-solubilizing bacteria that enhance plant growth: A proposal for an alternative procedure. Biol. Fertil. Soils 2013, 49, 465–479. [Google Scholar] [CrossRef]
- Pinos, N.Q.; Louro Berbara, R.L.; Elias, S.S.; van Tol de Castro, T.A.; García, A.C. Combination of humic substances and arbuscular mycorrhizal fungi affecting corn plant growth. J. Environ. Qual. 2019, 48, 1594–1604. [Google Scholar] [CrossRef]
- Vergara, C.; Araujo, K.E.C.; Souza, S.R.d.; Schultz, N.; Jaggin Júnior, O.J.; Sperandio, M.V.L.; Zilli, J.É. Plant-mycorrhizal fungi interaction and response to inoculation with different growth-promoting fungi. Pesqui. Agropecuária Bras. 2019, 54, 1–25. [Google Scholar] [CrossRef] [Green Version]
- Frey, S.D. Mycorrhizal Fungi as Mediators of Soil Organic Matter Dynamics. Annu. Rev. Ecol. Evol. Syst. 2019, 50, 237–259. [Google Scholar] [CrossRef]
- Naeeni, F.N.; Moghadam, A.R.L.; Moradi, P.; Rezaei, M.; Abdoosi, V. Quantitative and qualitative response of milk thistle (silybum marianum) to application of humic acid and mycorrhizal fungi. Pak. J. Bot. 2018, 50, 1615–1620. [Google Scholar]
- Padjung, R.; Saad, S.H.; Bahrun, A.H.; Ridwan, I. Growth and development of Theobroma cacao seedlings as a response to different dosages of vermicompost and arbuscular mycorrhizal fungi. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2019; p. 012017. [Google Scholar]
- Giovannetti, M.; Volpe, V.; Salvioli, A.; Bonfante, P. Fungal and plant tools for the uptake of nutrients in arbuscular mycorrhizas: A molecular view. In Mycorrhizal Mediation of Soil; Elsevier: Amsterdam, The Netherlands, 2017; pp. 107–128. [Google Scholar] [CrossRef]
- Solaiman, Z.M.; Yang, H.J.; Archdeacon, D.; Tippett, O.; Tibi, M.; Whiteley, A.S. Humus-rich compost increases lettuce growth, nutrient uptake, mycorrhizal colonisation, and soil fertility. Pedosphere 2019, 29, 170–179. [Google Scholar] [CrossRef]
- Cozzolino, V.; De Martino, A.; Nebbioso, A.; Di Meo, V.; Salluzzo, A.; Piccolo, A. Plant tolerance to mercury in a contaminated soil is enhanced by the combined effects of humic matter addition and inoculation with arbuscular mycorrhizal fungi. Environ. Sci. Pollut. Res. 2016, 23, 11312–11322. [Google Scholar] [CrossRef]
- Da Silva, M.B.; Oliver, F.C.; da Cruz, R.M.S.; Marchi, B.d.A.; das Almas, L.R.M.; Alberton, O. Response of arbuscular mycorrhizal fungal Rhizophagus clarus and the addition of humic substances in growth of tomato (Solanum lycopersicum L.). Sci. Agrar. 2017, 18, 123–130. [Google Scholar]
- Lermen, C.; da Cruz, R.M.S.; de Souza, J.S.; de Almeida Marchi, B.; Alberton, O. Growth of Lippia alba (Mill.) NE Brown inoculated with arbuscular mycorrhizal fungi with different levels of humic substances and phosphorus in the soil. J. Appl. Res. Med. Aromat. Plants 2017, 7, 48–53. [Google Scholar] [CrossRef]
- Fan, Y.; Lin, F.; Yang, L.; Zhong, X.; Wang, M.; Zhou, J.; Chen, Y.; Yang, Y. Decreased soil organic P fraction associated with ectomycorrhizal fungal activity to meet increased P demand under N application in a subtropical forest ecosystem. Biol. Fertil. Soils 2018, 54, 149–161. [Google Scholar] [CrossRef]
- Lindahl, B.D.; Ihrmark, K.; Boberg, J.; Trumbore, S.E.; Högberg, P.; Stenlid, J.; Finlay, R.D. Spatial separation of litter decomposition and mycorrhizal nitrogen uptake in a boreal forest. New Phytol. 2007, 173, 611–620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nehls, U.; Plassard, C. Nitrogen and phosphate metabolism in ectomycorrhizas. New Phytol. 2018, 220, 1047–1058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- George, T.S.; Giles, C.D.; Menezes-Blackburn, D.; Condron, L.M.; Gama-Rodrigues, A.C.; Jaisi, D.; Lang, F.; Neal, A.L.; Stutter, M.I.; Almeida, D.S. Organic phosphorus in the terrestrial environment: A perspective on the state of the art and future priorities. Plant. Soil 2018, 427, 191–208. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jindo, K.; Canellas, L.P.; Albacete, A.; Figueiredo dos Santos, L.; Frinhani Rocha, R.L.; Carvalho Baia, D.; Oliveira Aguiar Canellas, N.; Goron, T.L.; Olivares, F.L. Interaction between Humic Substances and Plant Hormones for Phosphorous Acquisition. Agronomy 2020, 10, 640. https://doi.org/10.3390/agronomy10050640
Jindo K, Canellas LP, Albacete A, Figueiredo dos Santos L, Frinhani Rocha RL, Carvalho Baia D, Oliveira Aguiar Canellas N, Goron TL, Olivares FL. Interaction between Humic Substances and Plant Hormones for Phosphorous Acquisition. Agronomy. 2020; 10(5):640. https://doi.org/10.3390/agronomy10050640
Chicago/Turabian StyleJindo, Keiji, Luciano Pasqualoto Canellas, Alfonso Albacete, Lidiane Figueiredo dos Santos, Rafael Luiz Frinhani Rocha, Daiane Carvalho Baia, Natália Oliveira Aguiar Canellas, Travis Luc Goron, and Fábio Lopes Olivares. 2020. "Interaction between Humic Substances and Plant Hormones for Phosphorous Acquisition" Agronomy 10, no. 5: 640. https://doi.org/10.3390/agronomy10050640
APA StyleJindo, K., Canellas, L. P., Albacete, A., Figueiredo dos Santos, L., Frinhani Rocha, R. L., Carvalho Baia, D., Oliveira Aguiar Canellas, N., Goron, T. L., & Olivares, F. L. (2020). Interaction between Humic Substances and Plant Hormones for Phosphorous Acquisition. Agronomy, 10(5), 640. https://doi.org/10.3390/agronomy10050640








