Effects of Italian Ryegrass (IRG) Supplementation on Animal Performance, Gut Microbial Compositions and Odor Emission from Manure in Growing Pigs
Abstract
1. Introduction
2. Materials and Methods
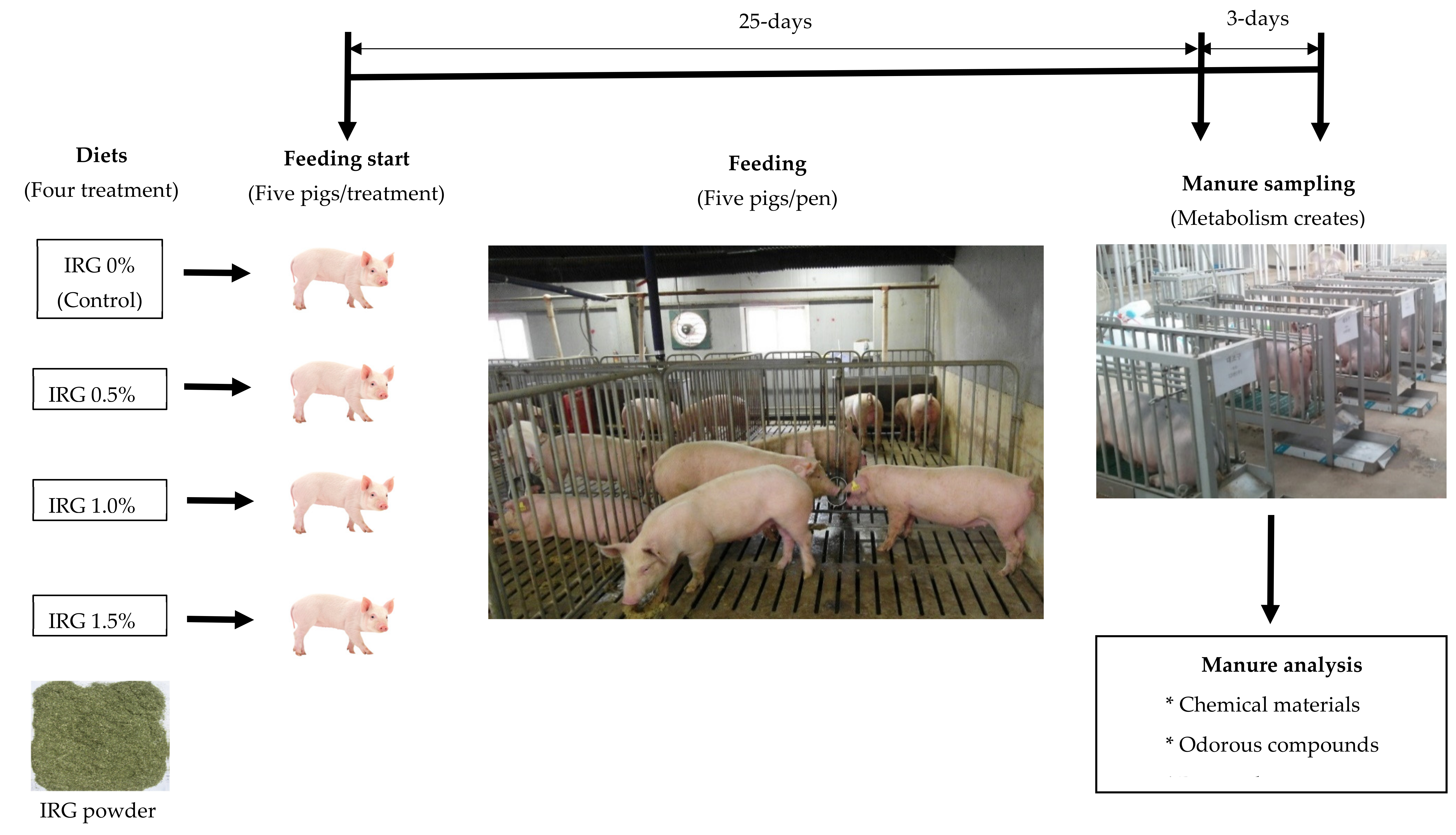
2.1. Animal and Manure Management
2.2. Manure Analysis
2.3. Bacterial Community Analysis
2.4. Statistical Analysis
3. Results & Discussion
3.1. Animal Performance
3.2. Chemical Materials and Odor Compounds of Manure
3.3. Bacterial Community Composition
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- MAFRA (Ministry of Agriculture, Food and Rural Affairs). A Case Study of the Farm to Improving Livestock Odor; MAFRA (Ministry of Agriculture, Food and Rural Affairs): Sejong, Korea, 2018; Available online: http://lib.mafra.go.kr/Search/Detail/43789?key (accessed on 3 March 2020).
- ACRC (Anti-Corruption and Civil Rights Commission). Census of Civil Complaints at 2017; ACRC (Anti-Corruption and Civil Rights Commission): Sejong, Korea, 2018. [Google Scholar]
- ME (Ministry of Environment). Law on Offensive Odor Control; ME (Ministry of Environment): Sejong, Korea, 2012. [Google Scholar]
- Maurer, D.L.; Koziel, J.A.; Harmon, J.D.; Hoff, S.J.; Rieck-Hinz, A.M.; Andersen, D.S. Summary of performance data for technologies to control gaseous, odor, and particulate emissions from livestock operations: Air management practices assessment tool (AMPAT). Data Brief 2016, 7, 1413–1429. [Google Scholar] [CrossRef]
- Statistics Korea. Agriculture, Forestry and Fisheries 2015; Statistics Korea: Daejeon, Korea, 2015. [Google Scholar]
- De Lange, C.; Pluske, J.; Gong, J.; Nyachoti, C. Strategic use of feed ingredients and feed additives to stimulate gut health and development in young pigs. Livest. Sci. 2010, 134, 124–134. [Google Scholar] [CrossRef]
- Wenk, C. The role of dietary fibre in the digestive physiology of the pig. Anim. Feed Sci. Tech. 2001, 90, 21–33. [Google Scholar] [CrossRef]
- De Leeuw, J.; Bolhuis, J.; Bosch, G.; Gerrits, W. Effects of dietary fibre on behaviour and satiety in pigs. Proc. Nutr. Soc. 2008, 67, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Oliviero, C.; Kokkonen, T.; Heinonen, M.; Sankari, S.; Peltoniemi, O. Feeding sows with high fibre diet around farrowing and early lactation: Impact on intestinal activity, energy balance related parameters and litter performance. Res. Vet. Sci. 2009, 86, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Calabro, S.; Moniello, G.; Piccolo, V.; Bovera, F.; Infascelli, F.; Tudisco, R.; Cutrignelli, M.I. Rumen fermentation and degradability in buffalo and cattle using the in vitro gas production technique. J. Anim. Physiol. Anim. Nutr. 2008, 92, 356–362. [Google Scholar] [CrossRef] [PubMed]
- NRC (National Research Council). Nutrient Requirements of Swine; National Academy Press: Washington, DC, USA, 2012. [Google Scholar]
- ME (Ministry of Environment). Standard methods for examination of water and wastewater; ME (Ministry of Environment): Sejong, Korea, 2017. [Google Scholar]
- Saez-Plaza, P.; Navas, M.J.; Wybraniec, S.; Michalowski, T.; Asuero, A.G. An overview of the kjeldahl method of nitrogen determination. Part II. Sample preparation, working scale, instrumental finish, and quality control. Crit. Rev. Anal. Chem. 2013, 43, 224–272. [Google Scholar] [CrossRef]
- Saha, U.K.; Sonon, L.; Kissel, D.E. Comparison of conductimetric and colorimetric methods with distillation titration method of analyzing ammonium nitrogen in total kjeldahl digests. Commun. Soil Sci. Plant Anal. 2012, 43, 2323–2341. [Google Scholar] [CrossRef]
- Hwang, O.H.; Cho, S.B.; Han, D.W.; Lee, S.R.; Kwag, J.H.; Park, S.K. Effect of storage period on the changes of odorous compound concentrations and bacterial ecology for identifying the cause of odor production from pig slurry. PLoS ONE 2016, 11, e0162714. [Google Scholar] [CrossRef]
- Choi, Y.; Lee, S.; Lee, H.; Lee, S.; Kim, S.; Lee, J.; Ha, J.; Oh, H.; Lee, Y.; Kim, Y.; et al. Rapid Detection of Escherichia coli in Fresh Foods Using a Combination of Enrichment and PCR Analysis. Korean J. Food Sci. Anim. Resour. 2018, 38, 829–834. [Google Scholar]
- Chun, J.; Kim, K.Y.; Lee, J.H.; Choi, Y. The analysis of oral microbial communities of wild-type and toll-like receptor 2-deficient mice using a 454 GS FLX Titanium pyrosequencer. BMC Microbiol. 2010, 10, 101. [Google Scholar] [CrossRef] [PubMed]
- Kim, O.S.; Cho, Y.J.; Lee, K.; Yoon, S.H.; Kim, M.; Na, H.; Park, S.C.; Jeon, Y.S.; Lee, J.H.; Yi, H.; et al. Introducing EzTaxon-e: A prokaryotic 16S rRNA gene sequence database with phylotypes that represent uncultured species. Int. J. Syst. Evol. Micr. 2012, 62, 716–721. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef] [PubMed]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef]
- SAS Institute Inc. SAS Enterprise Guide 7.13 HF4; SAS Institute Inc.: Cary, NC, USA, 2016. [Google Scholar]
- Gutierrez, N.A.; Kerr, B.J.; Patience, J.F. Effect of insoluble-low fermentable fiber from corn-ethanol distillation origin on energy, fiber, and amino acid digestibility, hindgut degradability of fiber, and growth performance of pigs. J. Anim. Sci. 2013, 91, 5314–5325. [Google Scholar] [CrossRef]
- Baird, D.M.; McCampbell, H.C.; Allison, J.R. Effects of levels of crude fiber, protein and bulk in diets for finishing hogs. J. Anim. Sci. 1975, 41, 1039–1047. [Google Scholar] [CrossRef]
- Szogi, A.A.; Loughrin, J.H.; Vanotti, M.B. Improved water quality and reduction of odorous compounds in anaerobic lagoon columns receiving pre-treated pig wastewater. Environ. Technol. 2017, 39, 2613–2621. [Google Scholar] [CrossRef]
- Trabue, S.; Kerr, B. Emissions of greenhouse gases, ammonia, and hydrogen sulfide from pigs fed standard diets and diets supplemented with dried distillers grains with solubles. J. Environ. Qual. 2013, 43, 1176–1186. [Google Scholar] [CrossRef]
- Wu, J.J.; Park, S.H.; Hengemuehle, S.M.; Yokoyama, M.T.; Person, H.L.; Masten, S.J. The effect of storage and ozonation on the physical, chemical, and biological characteristics of swine manure slurries. Ozone-Sci. Eng. 1998, 20, 35–50. [Google Scholar] [CrossRef]
- Cummings, J.; Macfarlane, G. The control and consequences of bacterial fermentation in the human colon. J. Appl. Bacteriol. 1991, 70, 443–459. [Google Scholar] [CrossRef]
- Van Loo, J.; Coussement, P.; De Leenheer, L.; Hoebregs, H.; Smits, G. On the presence of inulin and oligofructose as natural ingredients in the western diet. Crit. Rev. Food Sci. Nutr. 1995, 35, 525–552. [Google Scholar] [CrossRef] [PubMed]
- Delzenne, N.M.; Roberfroid, M. Physiological effects of non-digestible oligosaccharides. LWT-Food Sci. Technol. 1994, 27, 1–6. [Google Scholar] [CrossRef]
- Rideout, T.C.; Fan, M.Z.; Cant, J.P.; Wagner-Riddle, C.; Stonehouse, P. Excretion of major odor-causing and acidifying compounds in response to dietary supplementation of chicory inulin in growing pigs. J. Anim. Sci. 2004, 82, 1678–1684. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.F.; Zhu, Y.H.; Li, D.F.; Wang, Z.; Jensen, B.B. In vitro fermentation of various fiber and starch sources by pig fecal inocula. J. Anim. Sci. 2004, 82, 2615–2622. [Google Scholar] [CrossRef]
- De Camp, S.; Hill, B.; Hamkins, S.; Herr, C.; Richert, B.; Sutton, A.; Kelly, M.L.; Cobb, D.W.; Bundy, W.J. With added fat, soybean hulls may reduce odor without impairment. Feedstuff 2001, 10, 10–22. [Google Scholar]
- Iyayi, E.; Adeola, O. Quantification of short-chain fatty acids and energy production from hindgut fermentation in cannulated pigs fed graded levels of wheat bran. J. Anim. Sci. 2015, 93, 4781–4787. [Google Scholar] [CrossRef]
- Yen, J.; Nienaber, J.; Hill, D.; Pond, W. Potential contribution of absorbed volatile fatty acids to whole-animal energy requirement in conscious swine. J. Anim. Sci. 1991, 69, 2001–2012. [Google Scholar] [CrossRef]
- Bergman, E. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol. Rev. 1990, 70, 567–590. [Google Scholar] [CrossRef]
- Jensen, B.B.; Jørgensen, H. Effect of dietary fiber on microbial activity and microbial gas production in various regions of the gastrointestinal tract of pigs. Appl. Environ. Microbiol. 1994, 60, 1897–1904. [Google Scholar] [CrossRef]
- Knudsen, K.B. Nutritional and functional properties of fiber in swine diets. In Proceedings of the Midwest Swine Nutrition Conference, Indianapolis, IN, USA, 4 September 2014; p. 35. [Google Scholar]
- Willig, S.; Lösel, D.; Claus, R. Effects of resistant potato starch on odor emission from feces in swine production units. J. Agric. Food Chem. 2005, 53, 1173–1178. [Google Scholar] [CrossRef]
- Moughan, P.J. Amino acid digestibility and availability in food and feedstuffs. In Digestive Physiology in Pigs. Proc. 9th International Symposium; Ball, R.O., Ed.; University of Alberta: Edmonton, AB, Canada, 2003; Volume 1, pp. 199–221. [Google Scholar]
- Hawe, S.M.; Walker, N.; Moss, B.W. The effects of dietary fiber, lactose and antibiotic on the levels of skatole and indole in feces and subcutaneous fat in growing pigs. Anim. Sci. 1992, 54, 413–419. [Google Scholar] [CrossRef]
- Jensen, M.T.; Cox, R.P.; Jensen, B.B. 3-Methylindole (skatole) and indole production by mixed populations of pig fecal bacteria. Appl. Environ. Microbiol. 1995, 61, 3180–3184. [Google Scholar] [CrossRef] [PubMed]
- Overland, M.; Kjos, N.P.; Borg, M.; Skjerve, E.; Sorum, H. Organic acids in diets for entire male pigs: Effect on skatole level, microbiota in digesta, and growth performance. Livest. Sci. 2008, 115, 169–178. [Google Scholar] [CrossRef]
- Jha, R.; Berrocoso, J.F.D. Dietary fiber and protein fermentation in the intestine of swine and their interactive effects on gut health and on the environment: A review. Anim. Feed Sci. Tech. 2016, 212, 18–26. [Google Scholar] [CrossRef]
- Hass, R.; Busche, R.; Luciano, L.; Reale, E.; Engelhardt, W. Lack of butyrate is associated with induction of Bax and subsequent apoptosis in the proximal colon of guinea pig. Gastroenterology 1997, 112, 875–881. [Google Scholar] [CrossRef]
- Mentschel, J.; Claus, R. Increased butyrate formation in the pig colon by feeding raw potato starch leads to a reduction of colonocyte apoptosis and a shift to the stem cell compartment. Metabolism 2003, 52, 1400–1405. [Google Scholar] [CrossRef]
- Claus, R.; Lösel, D.; Lacorn, M.; Mentschel, J.; Schenkel, H. Effects of butyrate on apoptosis in the pig colon and its consequences for skatole formation and tissue accumulation. J. Anim. Sci. 2003, 81, 239–248. [Google Scholar] [CrossRef]
- Zamaratskaia, G.; Babol, J.; Andersson, H.; Andersson, K.; Lundström, K. Effect of live weight and dietary supplement of raw potato starch on the levels of skatole, androstenone, testosterone and oestrone sulphate in entire male pigs. Livest. Prod. Sci. 2005, 93, 235–243. [Google Scholar] [CrossRef]
- Westerman, P.W.; Zhang, R.H. Aeration of livestock manure slurry and lagoon liquid for odor control: A review. Appl. Eng. Agric. 1997, 13, 245–249. [Google Scholar] [CrossRef]
- Williams, A.G. Indicators of piggery slurry odour offensiveness. Agric. Wastes 1984, 10, 15–36. [Google Scholar] [CrossRef]
- Abdalla, K.Z.; Hammam, G. Correlation between biochemical oxygen demand and chemical oxygen demand for various wastewater treatment plants in Egypt to obtain the biodegradability indices. Int. J. Sci. Basic Appl. 2014, 13, 42–48. [Google Scholar]
- Zhu, J.; Ndegwa, P.M.; Luo, A. Effect of solid-liquid separation on BOD and VFA in swine manure. Environ. Technol. 2001, 22, 1237–1243. [Google Scholar] [CrossRef] [PubMed]
- Ndegwa, P.M.; Zhu, J.; Luo, A. Effects of bioreactor temperature and time on odor-related parameters in aerated swine manure slurries. Environ. Technol. 2003, 24, 1007–1016. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhu, J. Characteristics of solids, BOD and VFAs in liquid swine manure treated by short-term low-intensity aeration for long-term storage. Bioresour. Technol. 2006, 97, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Orupold, K.; Masirin, A.; Tenno, T. Estimation of biodegradation parameters of phenolic compounds on activated sludge by respirometry. Chemosphere 2001, 44, 1273–1280. [Google Scholar] [CrossRef]
- Razo-Flores, E.; Iniestra-Gonzalez, M.; Field, J.A.; Olguin-Lora, P.; Puig-Grajales, L. Biodegradation of mixtures of phenolic compounds in an upward-flow anaerobic sludge blanket reactor. Environ. Eng. 2003, 129, 999–1006. [Google Scholar] [CrossRef]
- Tassew, F.A.; Bergland, W.H.; Dinamarca, C.; Bakke, R. Effect of particulate disintegration on biomethane potential of particle rich substrates in batch anaerobic reactor. Appl. Sci. 2019, 9, 2880. [Google Scholar] [CrossRef]
- Birkett, A.; Muir, J.; Phillips, J.; Jones, G.; O’Dea, K. Resistant starch lowers fecal concentrations of ammonia and phenols in humans. Am. J. Clin. Nutr. 1996, 63, 766–772. [Google Scholar] [CrossRef]
- Mosenthin, R.; Hambrecht, E.; Sauer, W.; Garnsworthy, P.; Wiseman, J. Utilisation of Different Fibres in Piglet Feeds; Nottingham University Press: Nottingham, UK, 2001. [Google Scholar]
- Biddle, A.; Stewart, L.; Blanchard, J.; Leschine, S. Untangling the genetic basis of fibrolytic specialization by Lachnospiraceae and Ruminococcaceae in diverse gut communities. Diversity 2013, 5, 627–640. [Google Scholar] [CrossRef]
- Tap, J.; Mondot, S.; Levenez, F.; Pelletier, E.; Caron, C.; Furet, J.P.; Ugarte, E.; Munoz-Tamayo, R.; Paslier, D.L.E.; Nalin, R.; et al. Towards the human intestinal microbiota phylogenetic core. Environ. Microbiol. 2009, 11, 2574–2584. [Google Scholar] [CrossRef]
- Daly, K.; Proudman, C.J.; Duncan, S.H.; Flint, H.J.; Dyer, J.; Shirazi-Beechey, S.P. Alterations in microbiota and fermentation products in equine large intestine in response to dietary variation and intestinal disease. Br. J. Nutr. 2012, 107, 989–995. [Google Scholar] [CrossRef]
- Duncan, S.H.; Barcenilla, A.; Stewart, C.S.; Pryde, S.E.; Flint, H.J. Acetate utilization and butyryl coenzyme A (CoA): Acetate-CoA transferase in butyrate-producing bacteria from the human large intestine. Appl. Environ. Microbiol. 2002, 68, 5186–5190. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Martínez, I.; Walter, J.; Keshavarzian, A.; Rose, D.J. In vitro characterization of the impact of selected dietary fibers on fecal microbiota composition and short chain fatty acid production. Anaerobe 2013, 23, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Belenguer, A.; Duncan, S.H.; Holtrop, G.; Anderson, S.E.; Lobley, G.E.; Flint, H.J. Impact of pH on lactate formation and utilization by human fecal microbial communities. Appl. Environ. Microbiol. 2007, 73, 6526–6533. [Google Scholar] [CrossRef] [PubMed]
- Bourriaud, C.; Robins, R.; Martin, L.; Kozlowski, F.; Tenailleau, E.; Cherbut, C.; Michel, C. Lactate is mainly fermented to butyrate by human intestinal microfloras but inter-individual variation is evident. J. Appl. Microbiol. 2005, 99, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wright, A.D.; Liu, H.; Fan, Z.; Yang, F.; Zhang, Z.; Li, G. Response of the rumen microbiota of sika deer (Cervus nippon) fed different concentrations of tannin rich plants. PLoS ONE 2015, 10, e0123481. [Google Scholar] [CrossRef]
- Durmic, Z.; Pethick, D.W.; Mullan, B.P.; Accioly, J.M.; Schulze, H.; Hampson, D.J. Evaluation of large-intestinal parameters associated with dietary treatments designed to reduce the occurrence of swine dysentery. Br. J. Nutr. 2002, 88, 159–169. [Google Scholar] [CrossRef]
- Macfarlane, G.; Steed, H.; Macfarlane, S. Bacterial metabolism and health-related effects of galacto-oligosaccharides and other prebiotics. J. Appl. Microbiol. 2008, 104, 305–344. [Google Scholar] [CrossRef]
- Ritchie, L.E.; Sturino, J.M.; Carroll, R.J.; Rooney, L.W.; Azcarate-Peril, M.A.; Turner, N.D. Polyphenol-rich sorghum brans alter colon microbiota and impact species diversity and species richness after multiple bouts of dextran sodium sulfate-induced colitis. FEMS Microbiol. Ecol. 2015, 91, fiv008. [Google Scholar] [CrossRef]
- Wakshlag, J.J.; Simpson, K.W.; Struble, A.M.; Dowd, S.E. Negative fecal characteristics are associated with pH and fecal flora alterations during dietary change in dog. Int. J. Appl. Res. Vet. Med. 2011, 9, 278–283. [Google Scholar]
- Shinohara, K.; Ohashi, Y.; Kawasumi, K.; Terada, A.; Fujisawa, T. Effect of apple intake on fecal microbiota and metabolites in humans. Anaerobe 2010, 16, 510–515. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, T.S.; Lærke, H.N.; Theil, P.K.; Sørensen, J.F.; Saarinen, M.; Forssten, S.; Knudsen, K.E.B. Diets high in resistant starch and arabinoxylan modulate digestion processes and SCFA pool size in the large intestine and faecal microbial composition in pigs. Brit. J. Nutr. 2014, 112, 1837–1849. [Google Scholar] [CrossRef] [PubMed]
- Ben-Dov, E.; Shapiro, O.H.; Gruber, R.; Brenner, A.; Kushmaro, A. Changes in microbial diversity in industrial wastewater evaporation ponds following artificial salination. FEMS Microbiol. Ecol. 2008, 66, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Ritalahti, K.M.; Justicia-Leon, S.D.; Cusick, K.D.; Ramos-Hernandez, N.; Rubin, M.; Dornbush, J.; Loffler, F.E. Sphaerochaeta globosa gen. nov., sp. nov. and Sphaerochaeta pleomorpha sp. nov., free-living, spherical spirochaetes. Int. J. Syst. Evol. Microbiol. 2012, 62, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Park, J.H.; Kang, H.J.; Lee, Y.H.; Lee, T.J.; Park, H.D. Distribution and abundance of Spirochaetes in full-scale anaerobic digesters. Bioresour. Technol. 2013, 145, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Shivani, Y.; Subhash, Y.; Tushar, L.; Sasikala, C.; Ramana, C.V. Spirochaeta lutea sp. nov., isolated from marine habitats and emended description of the genus Spirochaeta. Syst. Appl. Microbiol. 2015, 38, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Bellgard, M.I.; Wanchanthuek, P.; La, T.; Ryan, K.; Moolhuijzen, P.; Albertyn, Z.; Babak, S.; Motro, Y.; Dunn, D.S.; Schibeci, D.; et al. Genome sequence of the pathogenic intestinal spirochete Brachyspira hyodysenteriae reveals adaptations to its lifestyle in the porcine large intestine. PLoS ONE 2009, 4, e4641. [Google Scholar] [CrossRef]
- Pohlschroeder, M.; Leschine, S.B.; Canale-Parola, E. Spirochaeta caldaria sp. nov., a thermophilic bacterium that enhances cellulose degradation by Clostridium thermocellum. Arch. Microbiol. 1994, 161, 17–24. [Google Scholar] [CrossRef]
- Madigou, C.; Poirier, S.; Bureau, C.; Chapleur, O. Acclimation strategy to increase phenol tolerance of an anaerobic microbiota. Bioresour. Technol. 2016, 216, 77–86. [Google Scholar] [CrossRef]
- Wang, W.; Wang, S.; Ren, X.; Hu, Z.; Yuan, S. Rapid establishment of phenol and quinolone-degrading consortia driven by the scoured cake layer in an anaerobic baffled ceramic membrane bioreactor. Environ. Sci. Pollut. Res. 2017, 24, 26125–26135. [Google Scholar] [CrossRef]
- Poirier, A.; Bize, A.; Bureau, C.; Bouchez, T.; Chapleur, O. Community shifts within anaerobic digestion microbiota facing phenol inhibition: Towards early warning microbial indicators? Water Res. 2016, 100, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.T.; Anderson, G.M.; Fulk, G.E. Formation of indoleacetic acid by intestinal anaerobes. J. Bacteriol. 1975, 124, 573–575. [Google Scholar] [CrossRef] [PubMed]



| Items | IRG 1 | ||||
|---|---|---|---|---|---|
| 0% | 0.5% | 1.0% | 1.5% | Raw 6 | |
| Ingredients (%; as fed basis) | |||||
| Corn, yellow | 72.34 | 69.74 | 66.91 | 64.26 | - |
| IRG 1, powder | 0.00 | 0.50 | 1.00 | 1.50 | - |
| Soybean oil | 0.67 | 1.30 | 1.97 | 2.62 | - |
| Molasses | 0.07 | 1.29 | 2.69 | 4.00 | - |
| Soy meal (CP 144%) | 24.07 | 24.34 | 24.64 | 24.85 | - |
| Limestone | 0.53 | 0.48 | 0.42 | 0.37 | - |
| DCP 1 | 1.52 | 1.55 | 1.57 | 1.60 | - |
| Mineral mixture 2 | 0.20 | 0.20 | 0.20 | 0.20 | - |
| Vitamin mixture 3 | 0.20 | 0.20 | 0.20 | 0.20 | - |
| Salt | 0.30 | 0.30 | 0.30 | 0.30 | - |
| Endopower 4 | 0.05 | 0.05 | 0.05 | 0.05 | - |
| BioPlus 2B 5 | 0.05 | 0.05 | 0.05 | 0.05 | - |
| Total | 100 | 100 | 100 | 100 | - |
| Analyzed chemical compositions | |||||
| Moisture (%) | 12.77 | 12.87 | 13.10 | 13.69 | 10.78 |
| Crude protein (%) | 15.44 | 16.23 | 14.98 | 15.52 | 13.48 |
| Crude fiber (%) | 1.99 | 1.75 | 2.08 | 2.19 | 23.62 |
| Crude ash (%) | 4.21 | 4.15 | 3.88 | 3.82 | 11.50 |
| DE 1 (kcal/kg) | 4438 | 4127 | 4110 | 4050 | 4103 |
| Ca (%) | 1.01 | 0.91 | 0.92 | 1.06 | 0.56 |
| P (%) | 0.66 | 0.64 | 0.63 | 0.71 | 0.32 |
| Lysine (%) | 0.70 | 0.75 | 0.78 | 0.71 | 0.61 |
| Methionine (%) | 0.22 | 0.22 | 0.24 | 0.21 | 0.17 |
| Threonine (%) | 0.69 | 0.58 | 0.58 | 0.56 | 0.63 |
| Tyrosine (%) | 0.54 | 0.61 | 0.57 | 0.57 | 0.46 |
| Items | IRG | SEM | Linear | Quadratic | |||
|---|---|---|---|---|---|---|---|
| 0% | 0.5% | 1.0% | 1.5% | ||||
| Initial body weight (kg) | 37.40 | 38.96 | 37.60 | 40.85 | 1.18 | 0.46 | 0.74 |
| Final body weight (kg) | 57.50 | 61.20 | 59.20 | 62.13 | 1.71 | 0.50 | 0.92 |
| Average daily gain (kg day−1) | 0.33 | 0.36 | 0.35 | 0.33 | 0.01 | 0.83 | 0.48 |
| Gain/feed | 0.19 | 0.20 | 0.19 | 0.18 | 0.01 | 0.69 | 0.48 |
| Items (mg L−1) | IRG | SEM | Linear | Quadratic | |||
|---|---|---|---|---|---|---|---|
| 0% | 0.5% | 1.0% | 1.5% | ||||
| pH | 9.07 | 9.16 | 9.13 | 8.84 | 0.06 | 0.16 | 0.13 |
| BOD | 30,184 | 29,580 | 32,681 | 36,774 | 1318 | 0.05 | 0.39 |
| COD | 13,845 | 14,607 | 16,717 | 19,099 | 642 | <0.01 | 0.45 |
| SS | 23,333 | 29,600 | 37,500 | 46,400 | 2881 | <0.01 | 0.79 |
| TN | 7908 | 8533 | 8457 | 8974 | 276 | 0.22 | 0.93 |
| NH4-N | 2174 | 2882 | 2937 | 2552 | 98 | 0.08 | <0.01 |
| Items (mg L−1) | IRG | SEM | Linear | Quadratic | |||
|---|---|---|---|---|---|---|---|
| 0% | 0.5% | 1.0% | 1.5% | ||||
| AA | 3604 | 2618 | 2745 | 3842 | 150.30 | 0.49 | <0.01 |
| PA | 488 | 415 | 487 | 673 | 25.86 | <0.01 | 0.01 |
| BA | 341 | 287 | 347 | 507 | 24.11 | 0.01 | 0.03 |
| VA | 86 | 75 | 83 | 138 | 7.85 | 0.01 | 0.04 |
| i-BA | 71 | 59 | 64 | 88 | 3.02 | 0.02 | <0.01 |
| i-VA | 189 | 143 | 154 | 207 | 7.95 | 0.33 | <0.01 |
| SCFA | 4520 | 3395 | 3662 | 5160 | 199.83 | 0.18 | <0.01 |
| BCFA | 260 | 203 | 219 | 295 | 10.77 | 0.18 | <0.01 |
| Phenol | 10.21 | 6.24 | 6.42 | 8.54 | 0.46 | 0.18 | <0.01 |
| p-Cresol | 143.32 | 156.00 | 132.32 | 95.43 | 4.56 | <0.01 | <0.01 |
| Indole | 6.83 | 4.29 | 3.27 | 4.45 | 0.44 | 0.02 | 0.03 |
| Skatole | 2.18 | 1.98 | 1.60 | 1.33 | 0.09 | <0.01 | 0.81 |
| Phenols | 153.53 | 162.24 | 138.74 | 103.97 | 4.48 | <0.01 | 0.01 |
| Indoles | 9.01 | 6.28 | 4.87 | 5.78 | 0.47 | <0.01 | 0.05 |
| Items | Treatment | |
|---|---|---|
| Control | IRG | |
| No. of total reads | 9344 | 6552 |
| No. of high-quality reads | 6589 | 4041 |
| Read length (bp) | 504 | 504 |
| OTUs | 1336 | 1100 |
| Shannon–Weaver index | 5.06 | 5.31 |
| Chao1 index | 2780 | 2230 |
| Goods library coverage | 0.92 | 0.90 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.; Cho, S.; Hwang, O. Effects of Italian Ryegrass (IRG) Supplementation on Animal Performance, Gut Microbial Compositions and Odor Emission from Manure in Growing Pigs. Agronomy 2020, 10, 647. https://doi.org/10.3390/agronomy10050647
Park S, Cho S, Hwang O. Effects of Italian Ryegrass (IRG) Supplementation on Animal Performance, Gut Microbial Compositions and Odor Emission from Manure in Growing Pigs. Agronomy. 2020; 10(5):647. https://doi.org/10.3390/agronomy10050647
Chicago/Turabian StylePark, Sungkwon, Sungback Cho, and Okhwa Hwang. 2020. "Effects of Italian Ryegrass (IRG) Supplementation on Animal Performance, Gut Microbial Compositions and Odor Emission from Manure in Growing Pigs" Agronomy 10, no. 5: 647. https://doi.org/10.3390/agronomy10050647
APA StylePark, S., Cho, S., & Hwang, O. (2020). Effects of Italian Ryegrass (IRG) Supplementation on Animal Performance, Gut Microbial Compositions and Odor Emission from Manure in Growing Pigs. Agronomy, 10(5), 647. https://doi.org/10.3390/agronomy10050647





