Ethyl Methyl Sulfonate-Induced Mutagenesis and Its Effects on Peanut Agronomic, Yield and Quality Traits
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. EMS Treatment
2.3. Generating Mutant Populations
2.4. Phenotype
2.5. Statistical Analysis
3. Results
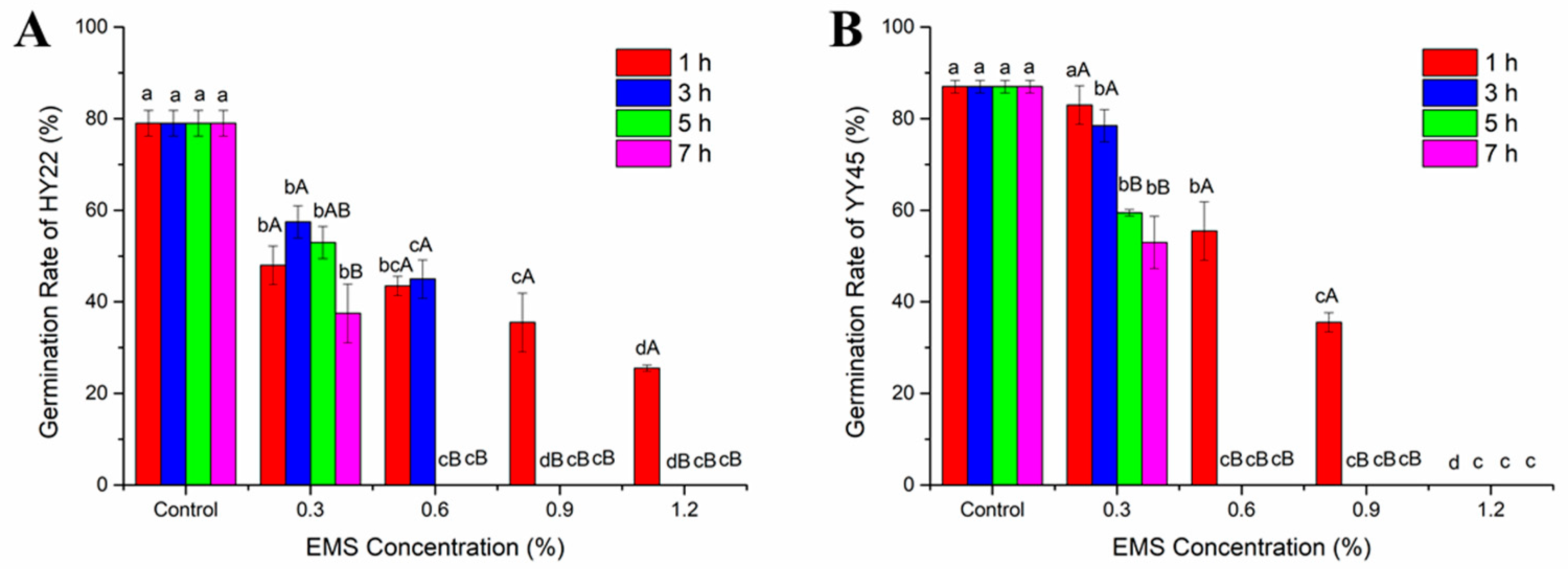
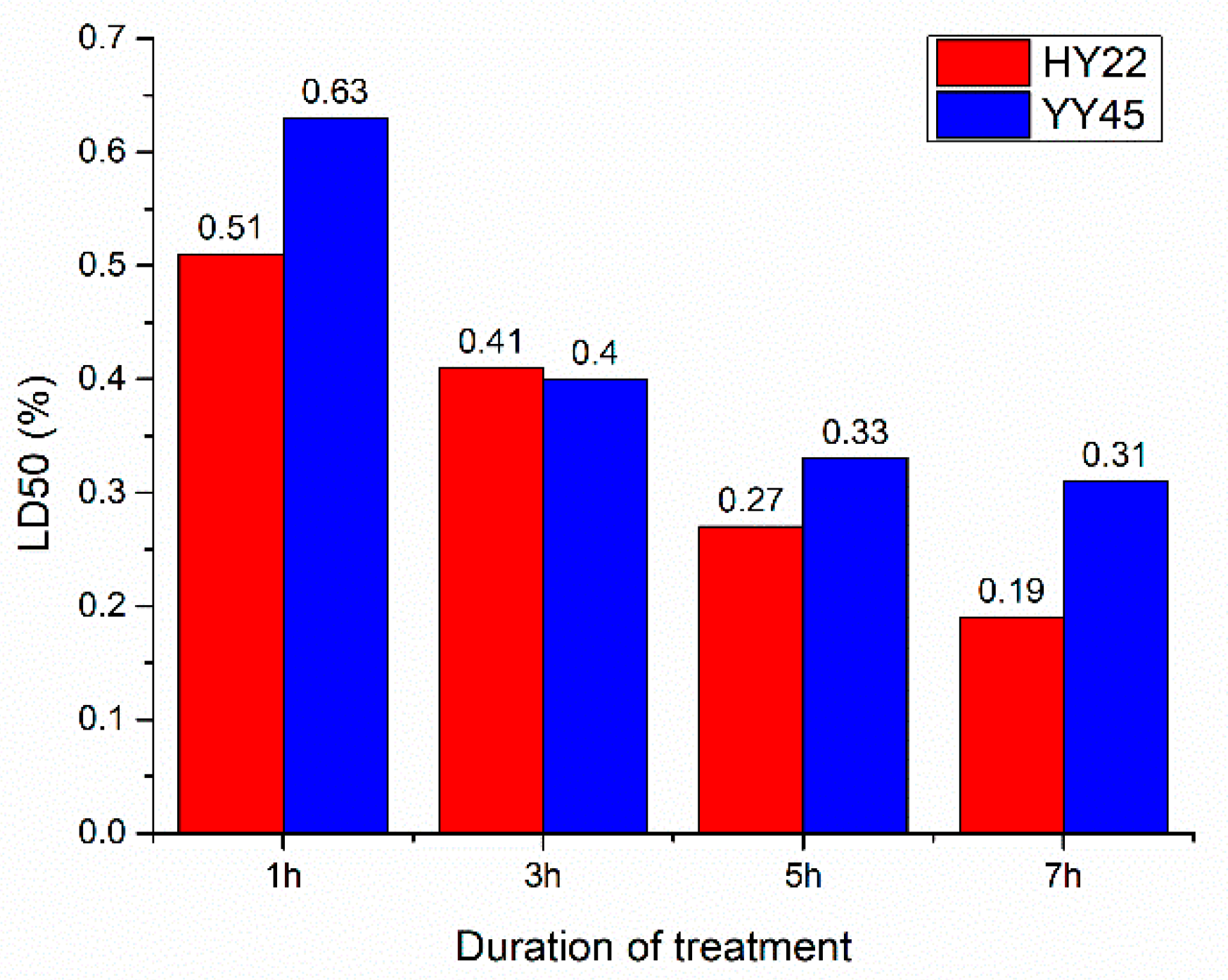
3.1. Germination Rate and LD50 in the M1 Generation
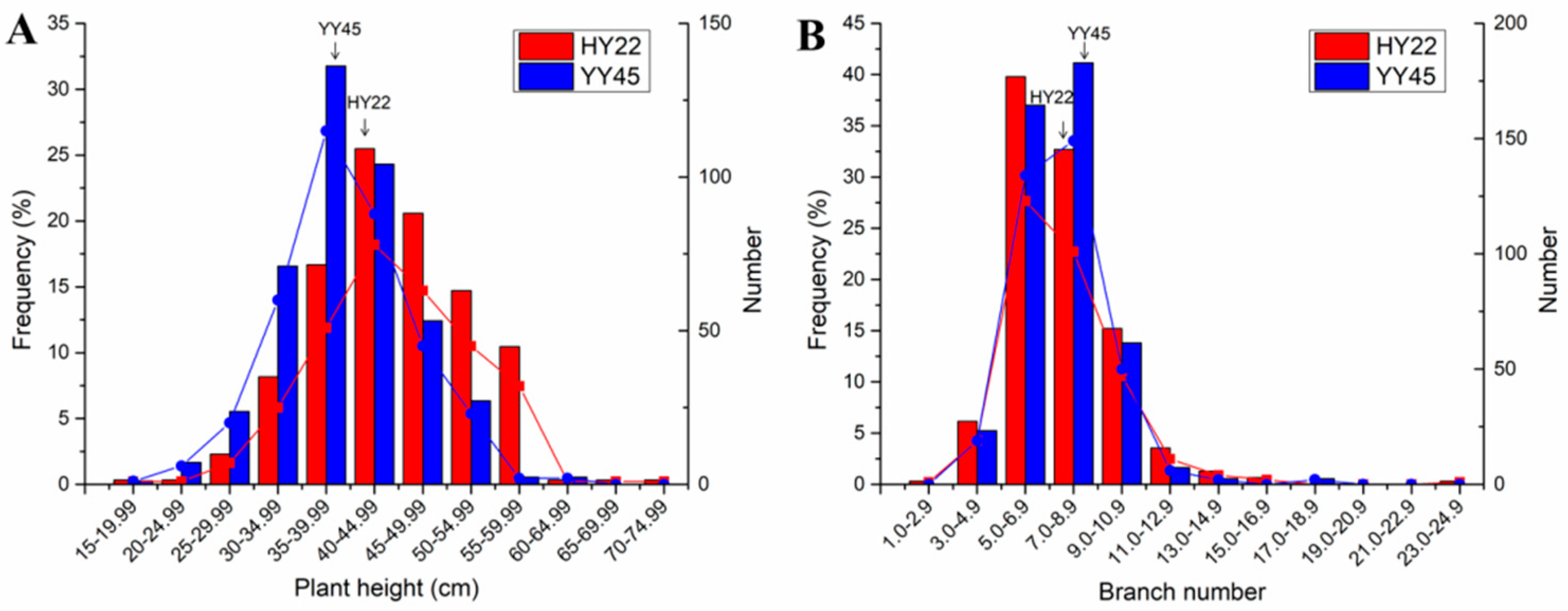
3.2. Plant Height and Branch Number in Population M2
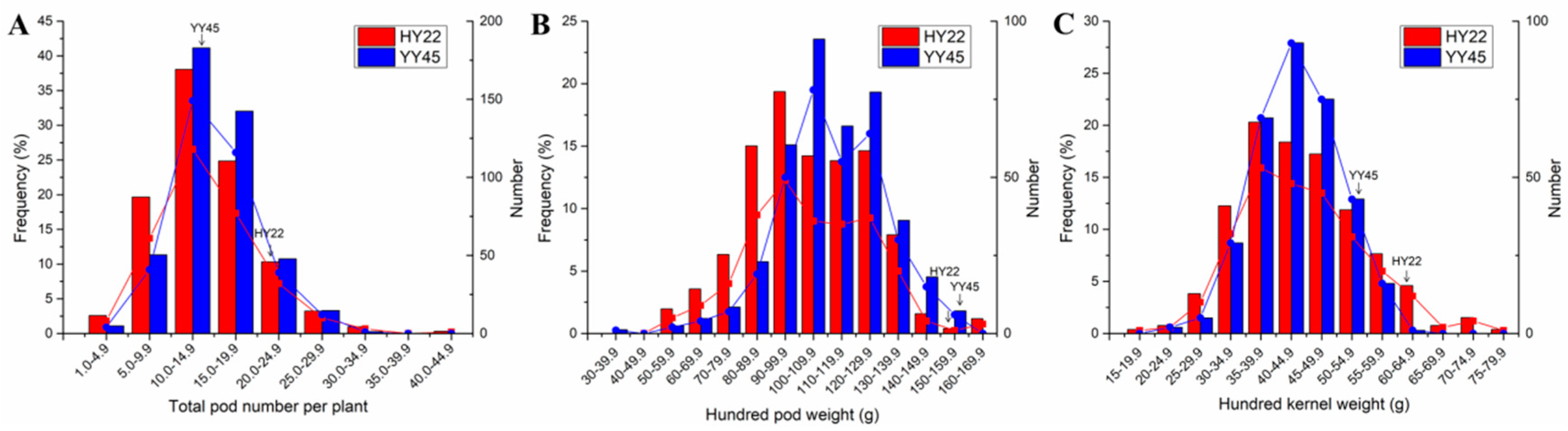
3.3. Plant Yield in the M2 Generation
3.4. Seed Quality in Plants of Generation M2
3.5. Mutants
3.5.1. Dwarf Mutants
3.5.2. Leaf Color and Shape Mutants
3.5.3. High oil and/or Protein Mutants
3.5.4. Seed Size and Testa Color Mutants
4. Discussion
4.1. Optimal Treatment Conditions Based on LD50
4.2. Potentially Useful Mutants Associated Important Traits
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. Food and Agriculture Organization of the United Nations. Available online: http://wwwfaoorg/ (accessed on 30 January 2020).
- Pandey, M.K.; Monyo, E.; Ozias-Akins, P.; Liang, X.; Guimarães, P.; Nigam, S.N.; Upadhyaya, H.D.; Janila, P.; Zhang, X.; Guo, B. Advances in Arachis genomics for peanut improvement. Biotechnol. Adv. 2012, 30, 639–651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Carvalho Moretzsohn, M.; Hopkins, M.S.; Mitchell, S.E.; Kresovich, S.; Valls, J.F.M.; Ferreira, M.E. Genetic diversity of peanut (Arachis hypogaea L.) and its wild relatives based on the analysis of hypervariable regions of the genome. BMC Plant Biol. 2004, 4, 11. [Google Scholar]
- Asif, A.; Khalil Ansari, M.Y. Generation of mutant lines of Nigella sativa L. by induced mutagenesis for improved seed yield. Ind. Crop. Prod. 2019, 139, 111552. [Google Scholar] [CrossRef]
- Menda, N.; Semel, Y.; Peled, D.; Eshed, Y.; Zamir, D. In silico screening of a saturated mutation library of tomato. Plant J. 2004, 38, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Galpaz, N.; Burger, Y.; Lavee, T.; Tzuri, G.; Sherman, A.; Melamed, T.; Eshed, R.; Meir, A.; Portnoy, V.; Bar, E. Genetic and chemical characterization of an EMS induced mutation in Cucumis melo CRTISO gene. Arch. Biochem. Biophys. 2013, 539, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, M.; Kaga, A.; Anai, T.; Shimizu, T.; Sayama, T.; Takagi, K.; Machita, K.; Watanabe, S.; Nishimura, M.; Yamada, N. Construction of a high-density mutant library in soybean and development of a mutant retrieval method using amplicon sequencing. BMC Genom. 2015, 16, 1014. [Google Scholar] [CrossRef] [Green Version]
- Henry, I.M.; Nagalakshmi, U.; Lieberman, M.C.; Ngo, K.J.; Krasileva, K.V.; Vasquez-Gross, H.; Akhunova, A.; Akhunov, E.; Dubcovsky, J.; Tai, T.H. Efficient genome-wide detection and cataloging of EMS-induced mutations using exome capture and next-generation sequencing. Plant Cell 2014, 26, 1382–1397. [Google Scholar] [CrossRef] [Green Version]
- Branch, W.D. Variability among advanced gamma-irradiation induced large-seeded mutant breeding lines in the ‘Georgia Browne’ peanut cultivar. Plant Breed. 2002, 121, 275–277. [Google Scholar] [CrossRef]
- Nadaf, H.; Kaveri, S.; Madhusudan, K.; Motagi, B. Induced genetic variability for yield and yield components in peanut (Arachis hypogaea L.). In Induced Plant Mutations in the Genomics Era; FAO: Rome, Italy, 2009; pp. 346–348. [Google Scholar]
- Wang, J.; Qiao, L.; Zhao, L.; Wang, P.; Guo, B.; Liu, L.; Sui, J. Performance of peanut mutants and their offspring generated from mixed high-energy particle field radiation and tissue culture. Genet. Mol. Res. 2015, 14, 10837–10848. [Google Scholar] [CrossRef]
- Bera, S.K.; Kamdar, J.H.; Kasundra, S.V.; Dash, P.; Maurya, A.K.; Jasani, M.D.; Chandrashekar, A.B.; Manivannan, N.; Vasanthi, R.P.; Dobariya, K.L.; et al. Improving oil quality by altering levels of fatty acids through marker-assisted selection of ahfad2 alleles in peanut (Arachis hypogaea L.). Euphytica 2018, 214, 162. [Google Scholar] [CrossRef]
- Mondal, S.; Badigannavar, A.M.; D’Souza, S.F. Induced variability for fatty acid profile and molecular characterization of high oleate mutant in cultivated groundnut (Arachis hypogaea L.). Plant Breed. 2011, 130, 242–247. [Google Scholar] [CrossRef]
- Patel, M.; Jung, S.; Moore, K.; Powell, G.; Ainsworth, C.; Abbott, A. High-oleate peanut mutants result from a MITE insertion into the FAD2 gene. Theor. Appl. Genet. 2004, 108, 1492–1502. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Wang, X.; Wu, Q.; Fang, C.; Guan, S.; Yang, W.; Wang, C.T.; Wang, P. Identification of differentially expressed genes from developing seeds of a normal oil peanut cultivar and its high oil EMS mutant. Res. Crop. 2013, 14, 511–516. [Google Scholar]
- Wan, L.; Li, B.; Pandey, M.K.; Wu, Y.; Lei, Y.; Yan, L.; Dai, X.; Jiang, H.; Zhang, J.; Wei, G. Transcriptome analysis of a new peanut seed coat mutant for the physiological regulatory mechanism involved in seed coat cracking and pigmentation. Front. Plant Sci. 2016, 7, 1491. [Google Scholar] [CrossRef] [Green Version]
- Alberte, R.S.; Hesketh, J.D.; Kirby, J.S. Comparisons of photosynthetic activity and lamellar characteristics of virescent and normal green peanut leaves. Z. Für Pflanzenphysiol. 1976, 77, 152–159. [Google Scholar] [CrossRef]
- Sui, J.; Jiang, D.; Zhang, D.; Song, X.; Wang, J.; Zhao, M.; Qiao, L. The salinity responsive mechanism of a hydroxyproline-tolerant mutant of peanut based on digital gene expression profiling analysis. PLoS ONE 2016, 11, e0162556. [Google Scholar] [CrossRef]
- Gowda, M.; Bhat, R.; Motagi, B.; Sujay, V.; Kumari, V.; Sujatha, B. Association of high-frequency origin of late leaf spot resistant mutants with AhMITE1 transposition in peanut. Plant Breed. 2010, 129, 567–569. [Google Scholar]
- Wan, L.; Li, B.; Lei, Y.; Yan, L.; Ren, X.; Chen, Y.; Dai, X.; Jiang, H.; Zhang, J.; Guo, W. Mutant transcriptome sequencing provides insights into pod development in peanut (Arachis hypogaea L.). Front. Plant Sci. 2017, 8, 1900. [Google Scholar] [CrossRef] [Green Version]
- Maluszynski, M.; Szarejko, I.; Barriga, P.; Balcerzyk, A. Heterosis in crop mutant crosses and production of high yielding lines using doubled haploid systems. Euphytica 2001, 120, 387–398. [Google Scholar] [CrossRef]
- Greene, E.A.; Codomo, C.A.; Taylor, N.E.; Henikoff, J.G.; Till, B.J.; Reynolds, S.H.; Enns, L.C.; Burtner, C.; Johnson, J.E.; Odden, A.R. Spectrum of chemically induced mutations from a large-scale reverse-genetic screen in Arabidopsis. Genetics 2003, 164, 731–740. [Google Scholar]
- Till, B.J.; Cooper, J.; Tai, T.H.; Colowit, P.; Greene, E.A.; Henikoff, S.; Comai, L. Discovery of chemically induced mutations in rice by TILLING. BMC Plant Biol. 2007, 7, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sevanthi, A.; Kandwal, P.; Kale, P.B.; Prakash, C.; Ramkumar, M.; Yadav, N.; Mahato, A.K.; Sureshkumar, V.; Behera, M.; Deshmukh, R.K. Whole genome characterization of a few EMS-induced mutants of upland rice variety Nagina 22 reveals a staggeringly high frequency of SNPs which show high phenotypic plasticity towards the wild-type. Front. Plant Sci. 2018, 9, 1179. [Google Scholar] [CrossRef]
- Roychowdhury, R.; Tah, J. Genetic variability study for yield and associated quantitative characters in mutant genotypes of Dianthus caryophyllus L. Int. J. Biosci. 2011, 1, 38–44. [Google Scholar]
- Kodym, A.; Afza, R. Physical and chemical mutagenesis. In Plant Functional Genomics; Springer: Berlin/Heidelberg, Germany, 2003; pp. 189–203. [Google Scholar]
- Zheng, Y.; Zheng, Y.; Wu, Z.; Sun, X.; Wang, C. Study on physiological characteristics and supporting techniques of Huayu 22 peanut. Asian Agric. Res. 2015, 7, 74–82. [Google Scholar]
- Li, S.; Liang, X.; Zhou, G. Breeding of a new peanut variety Yueyou 45. Guangdong Agric. Sci. 2010, 11, 19–20. [Google Scholar]
- Arisha, M.H.; Shah, S.N.; Gong, Z.; Jing, H.; Li, C.; Zhang, H. Ethyl methane sulfonate induced mutations in M2 generation and physiological variations in M1 generation of peppers (Capsicum annuum L.). Front. Plant Sci. 2015, 6, 399. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Zhang, B.; Li, J.; Yang, X.; Ren, Z. Ethyl methanesulfonate (EMS)-mediated mutagenesis of cucumber (Cucumis sativus L.). Agric. Sci. 2014, 2014, 48085. [Google Scholar]
- Weng, Y.; Shi, A.; Ravelombola, W.S.; Yang, W.; Qin, J.; Motes, D.; Moseley, D.O.; Chen, P. A rapid method for measuring seed protein content in cowpea (Vigna unguiculata (L.) Walp). Am. J. Plant Sci. 2017, 8, 2387. [Google Scholar] [CrossRef] [Green Version]
- Arisha, M.; Liang, B.; Shah, S.M.; Gong, Z.; Li, D. Kill curve analysis and response of first generation Capsicum annuum L. B12 cultivar to ethyl methane sulfonate. Genet. Mol. Res. 2014, 13, 10049–10061. [Google Scholar] [CrossRef]
- Shah, S.; Gong, Z.; Arisha, M.; Khan, A.; Tian, S. Effect of ethyl methyl sulfonate concentration and different treatment conditions on germination and seedling growth of the cucumber cultivar Chinese long (9930). Genet. Mol. Res. 2015, 14, 2440–2449. [Google Scholar] [CrossRef]
- Gnanamurthy, S.; Dhanavel, D. Effect of EMS on induced morphological mutants and chromosomal variation in Cowpea (Vigna unguiculata (L.) Walp). Int. Lett. Nat. Sci. 2014, 17, 33–43. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Kumar, V.; Lal, S.K.; Jolly, M.; Sachdev, A. Influence of gamma rays and ethyl methane sulphonate (EMS) on the levels of phytic acid, raffinose family oligosaccharides and antioxidants in soybean seeds of different genotypes. J. Plant Biochem. Biotechnol. 2015, 24, 204–209. [Google Scholar] [CrossRef]
- Ali, H.; Shah, T.M.; Iqbal, N.; Atta, B.M.; Haq, M.A. Mutagenic induction of double-podding trait in different genotypes of chickpea and their characterization by STMS marker. Plant Breed. 2010, 129, 116–119. [Google Scholar] [CrossRef]
- Devi, A.S.; Mullainathan, L. Physical and chemical mutagenesis for improvement of chilli (Capsicum annuum L.). World Appl. Sci. J. 2011, 15, 108–113. [Google Scholar]
- Borovsky, Y.; Tadmor, Y.; Bar, E.; Meir, A.; Lewinsohn, E.; Paran, I. Induced mutation in β-CAROTENE HYDROXYLASE results in accumulation of β-carotene and conversion of red to orange color in pepper fruit. Theor. Appl. Genet. 2013, 126, 557–565. [Google Scholar] [CrossRef]
- Kumar, A.P.; Boualem, A.; Bhattacharya, A.; Parikh, S.; Desai, N.; Zambelli, A.; Leon, A.; Chatterjee, M.; Bendahmane, A. SMART–sunflower mutant population and reverse genetic tool for crop improvement. BMC Plant Biol. 2013, 13, 38. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.; Goyal, S. Improvement of mungbean varieties through induced mutations. Afr. J. Plant Sci. 2009, 3, 174–180. [Google Scholar]
- Wang, T.L.; Uauy, C.; Robson, F.; Till, B. TILLING in extremis. Plant Biotechnol. J. 2012, 10, 761–772. [Google Scholar] [CrossRef]
- Uauy, C.; Wulff, B.B.; Dubcovsky, J. Combining traditional mutagenesis with new high-throughput sequencing and genome editing to reveal hidden variation in polyploid wheat. Annu. Rev. Genet. 2017, 51, 435–454. [Google Scholar] [CrossRef] [Green Version]
- Bertioli, D.J.; Jenkins, J.; Clevenger, J.; Dudchenko, O.; Gao, D.; Seijo, G.; Leal-Bertioli, S.C.; Ren, L.; Farmer, A.D.; Pandey, M.K. The genome sequence of segmental allotetraploid peanut Arachis hypogaea. Nat. Genet. 2019, 51, 877–884. [Google Scholar] [CrossRef] [Green Version]
- Shirasawa, K.; Hirakawa, H.; Nunome, T.; Tabata, S.; Isobe, S. Genome-wide survey of artificial mutations induced by ethyl methanesulfonate and gamma rays in tomato. Plant Biotechnol. J. 2016, 14, 51–60. [Google Scholar] [CrossRef] [PubMed]
- McCallum, C.M.; Comai, L.; Greene, E.A.; Henikoff, S. Targeted screening for induced mutations. Nat. Biotechnol. 2000, 18, 455–457. [Google Scholar] [CrossRef] [PubMed]
- Krasileva, K.V.; Vasquez-Gross, H.A.; Howell, T.; Bailey, P.; Paraiso, F.; Clissold, L.; Simmonds, J.; Ramirez-Gonzalez, R.H.; Wang, X.; Borrill, P. Uncovering hidden variation in polyploid wheat. Proc. Natl. Acad. Sci. USA 2017, 114, E913–E921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winfield, M.O.; Wilkinson, P.A.; Allen, A.M.; Barker, G.L.; Coghill, J.A.; Burridge, A.; Hall, A.; Brenchley, R.C.; D’Amore, R.; Hall, N. Targeted re-sequencing of the allohexaploid wheat exome. Plant Biotechnol. J. 2012, 10, 733–742. [Google Scholar] [CrossRef] [PubMed]
- King, R.; Bird, N.; Ramirez-Gonzalez, R.; Coghill, J.A.; Patil, A.; Hassani-Pak, K.; Uauy, C.; Phillips, A.L. Mutation scanning in wheat by exon capture and next-generation sequencing. PLoS ONE 2015, 10, e0137549. [Google Scholar] [CrossRef] [Green Version]
- Ashikari, M.; Wu, J.; Yano, M.; Sasaki, T.; Yoshimura, A. Rice gibberellin-insensitive dwarf mutant gene Dwarf 1 encodes the α-subunit of GTP-binding protein. Proc. Natl. Acad. Sci. USA 1999, 96, 10284–10289. [Google Scholar] [CrossRef] [Green Version]
- Ueguchi-Tanaka, M.; Fujisawa, Y.; Kobayashi, M.; Ashikari, M.; Iwasaki, Y.; Kitano, H.; Matsuoka, M. Rice dwarf mutant d1, which is defective in the α subunit of the heterotrimeric G protein, affects gibberellin signal transduction. Proc. Natl. Acad. Sci. USA 2000, 97, 11638–11643. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Wang, J.; Huang, T.; Wang, F.; Yuan, F.; Cheng, X.; Zhang, Y.; Shi, S.; Wu, J.; Liu, K. A missense mutation in the VHYNP motif of a DELLA protein causes a semi-dwarf mutant phenotype in Brassica napus. Theor. Appl. Genet. 2010, 121, 249–258. [Google Scholar] [CrossRef]
- Sazuka, T.; Kamiya, N.; Nishimura, T.; Ohmae, K.; Sato, Y.; Imamura, K.; Nagato, Y.; Koshiba, T.; Nagamura, Y.; Ashikari, M. A rice tryptophan deficient dwarf mutant, tdd1, contains a reduced level of indole acetic acid and develops abnormal flowers and organless embryos. Plant J. 2009, 60, 227–241. [Google Scholar] [CrossRef]
- Bishop, G.J. Brassinosteroid mutants of crops. J. Plant Growth Regul. 2003, 22, 325–335. [Google Scholar] [CrossRef]
- Kwon, M.; Choe, S. Brassinosteroid biosynthesis anddwarf mutants. J. Plant Biol. 2005, 48, 1. [Google Scholar] [CrossRef]
- Bennett, T.; Leyser, O. Strigolactone signalling: Standing on the shoulders of DWARFs. Curr. Opin. Plant Biol. 2014, 22, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, N.; Matsuoka, M.; Kitano, H.; Asano, T.; Kaku, H.; Komatsu, S. gid1, a gibberellin-insensitive dwarf mutant, shows altered regulation of probenazole-inducible protein (PBZ1) in response to cold stress and pathogen attack. Plant Cell Environ. 2006, 29, 619–631. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Nakazawa, M.; Shibata, K.; Yokota, T.; Ishikawa, A.; Suzuki, K.; Kawashima, M.; Ichikawa, T.; Shimada, H.; Matsui, M. shk1-D, a dwarf Arabidopsis mutant caused by activation of the CYP72C1 gene, has altered brassinosteroid levels. Plant J. 2005, 42, 13–22. [Google Scholar] [CrossRef]
- Tanabe, S.; Ashikari, M.; Fujioka, S.; Takatsuto, S.; Yoshida, S.; Yano, M.; Yoshimura, A.; Kitano, H.; Matsuoka, M.; Fujisawa, Y. A novel cytochrome P450 is implicated in brassinosteroid biosynthesis via the characterization of a rice dwarf mutant, dwarf11, with reduced seed length. Plant Cell 2005, 17, 776–790. [Google Scholar] [CrossRef] [Green Version]
- Jiang, L.; Liu, X.; Xiong, G.; Liu, H.; Chen, F.; Wang, L.; Meng, X.; Liu, G.; Yu, H.; Yuan, Y. DWARF 53 acts as a repressor of strigolactone signalling in rice. Nature 2013, 504, 401–405. [Google Scholar] [CrossRef] [Green Version]
- Hooda, M.; Dhillon, R.; Bangarwa, K. Albinism in jojoba (Simmondsia chinensis). Natl. J. Plant Improv. 2004, 1, 69–70. [Google Scholar]
- Chen, T.; Zhang, Y.; Zhao, L.; Zhu, Z.; Lin, J.; Zhang, S.; Wang, C. Physiological character and gene mapping in a new green-revertible albino mutant in rice. J. Genet. Genom. 2007, 34, 331–338. [Google Scholar] [CrossRef]
- Pawar, N.; Pai, S.; Nimbalkar, M.; Kolar, F.; Dixit, G. Induction of chlorophyll mutants in zingiber officinale roscoe by gamma rays and EMS. Emir. J. Food Agric. 2010, 22, 406–411. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Huang, Y.; Miao, Z.; Hu, Z.; Song, X.; Liu, L. Identification and characterization of BGL11 (t), a novel gene regulating leaf-color mutation in rice (Oryza sativa L.). Genes Genom. 2013, 35, 491–499. [Google Scholar] [CrossRef]
- Mochizuki, N.; Brusslan, J.A.; Larkin, R.; Nagatani, A.; Chory, J. Arabidopsis genomes uncoupled 5 (GUN5) mutant reveals the involvement of Mg-chelatase H subunit in plastid-to-nucleus signal transduction. Proc. Natl. Acad. Sci. USA 2001, 98, 2053–2058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rissler, H.M.; Collakova, E.; DellaPenna, D.; Whelan, J.; Pogson, B.J. Chlorophyll biosynthesis. Expression of a second chl I gene of magnesium chelatase in Arabidopsis supports only limited chlorophyll synthesis. Plant Physiol. 2002, 128, 770–779. [Google Scholar] [CrossRef] [PubMed]
- Jensen, P.E.; Petersen, B.L.; Stummann, B.; Henningsen, K.; Willows, R.; Vothknecht, U.; Kannangara, C.; von Wettstein, D. Structural genes for Mg-chelatase subunits in barley: Xantha-f,-g and-h. Mol. Gen. Genet. MGG 1996, 250, 383–394. [Google Scholar] [PubMed]
- Zhang, H.; Li, J.; Yoo, J.H.; Yoo, S.C.; Cho, S.H.; Koh, H.J.; Seo, H.S.; Paek, N.C. Rice Chlorina-1 and Chlorina-9 encode ChlD and ChlI subunits of Mg-chelatase, a key enzyme for chlorophyll synthesis and chloroplast development. Plant Mol. Biol. 2006, 62, 325–337. [Google Scholar] [CrossRef]
- Jung, K.; Hur, J.; Ryu, C.; Choi, Y.; Chung, Y.; Miyao, A.; Hirochika, H.; An, G. Characterization of a rice chlorophyll-deficient mutant using the T-DNA gene-trap system. Plant Cell Physiol. 2003, 44, 463–472. [Google Scholar] [CrossRef] [Green Version]
- Papenbrock, J.; Pfündel, E.; Mock, H.P.; Grimm, B. Decreased and increased expression of the subunit CHL I diminishes Mg chelatase activity and reduces chlorophyll synthesis in transgenic tobacco plants. Plant J. 2000, 22, 155–164. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, X.; He, B.; Diao, L.; Sheng, S.; Wang, J.; Guo, X.; Su, N.; Wang, L.; Jiang, L.; et al. A chlorophyll-deficient rice mutant with impaired chlorophyllide Esterification in chlorophyll biosynthesis. Plant Physiol. 2007, 145, 29–40. [Google Scholar] [CrossRef] [Green Version]
- Zhou, K.; Ren, Y.; Lv, J.; Wang, Y.; Liu, F.; Zhou, F.; Zhao, S.; Chen, S.; Peng, C.; Zhang, X.; et al. Young Leaf Chlorosis 1, a chloroplast-localized gene required for chlorophyll and lutein accumulation during early leaf development in rice. Planta 2013, 237, 279–292. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, M.L.; Barkley, N.A.; Pittman, R.N. A simple allele-specific PCR assay for detecting FAD2 alleles in both A and B genomes of the cultivated peanut for high-oleate trait selection. Plant Mol. Biol. Rep. 2010, 28, 542–548. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.; Zhao, Y.; Prakash, C.S.; He, G.; Yin, D. Insights into the novel members of the FAD2 gene family involved in high-oleate fluxes in peanut. Genome 2015, 58, 375–383. [Google Scholar] [CrossRef]




| Mutant ID | Phenotype | Oil Content (%) | Protein Content (%) | Plant Height (cm) | ||
|---|---|---|---|---|---|---|
| HY22 mutants | HY93 | High oil | Dwarf | 64.58 | 23.67 | 30.6 |
| HY100 | High protein | Dwarf | 51.82 | 32.95 | 34.2 | |
| HY104 | High protein | Dwarf | 40.33 | 30.06 | 36.7 | |
| HY106 | High protein | Dwarf | 44.68 | 31.77 | 37.1 | |
| HY123 | High protein | Dwarf | 44.96 | 32.03 | 30.3 | |
| HY125 | High protein | Dwarf | 50.20 | 32.40 | 34.3 | |
| HY126 | High protein | Dwarf | 47.06 | 32.13 | 35.0 | |
| HY131 | High protein | Yellow leaves | 45.01 | 30.72 | 40.2 | |
| YY45 mutants | YY37 | High oil | Small leaves | 59.70 | 21.06 | 45.0 |
| YY38 | High oil and protein | Dwarf | 61.93 | 32.42 | 32.0 | |
| YY47 | High oil and protein | Dwarf | 61.17 | 30.12 | 25.6 | |
| YY62 | High protein | Dwarf | 44.86 | 30.66 | 34.8 | |
| YY64 | High protein | Dwarf | 51.79 | 30.07 | 27.0 | |
| YY66 | High protein | Big leaves | 45.74 | 30.54 | 41.9 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, T.; Huang, L.; Wang, M.; Huang, Y.; Zeng, R.; Wang, X.; Wang, L.; Wan, S.; Zhang, L. Ethyl Methyl Sulfonate-Induced Mutagenesis and Its Effects on Peanut Agronomic, Yield and Quality Traits. Agronomy 2020, 10, 655. https://doi.org/10.3390/agronomy10050655
Chen T, Huang L, Wang M, Huang Y, Zeng R, Wang X, Wang L, Wan S, Zhang L. Ethyl Methyl Sulfonate-Induced Mutagenesis and Its Effects on Peanut Agronomic, Yield and Quality Traits. Agronomy. 2020; 10(5):655. https://doi.org/10.3390/agronomy10050655
Chicago/Turabian StyleChen, Tingting, Luping Huang, Miaomiao Wang, Yang Huang, Ruier Zeng, Xinyue Wang, Leidi Wang, Shubo Wan, and Lei Zhang. 2020. "Ethyl Methyl Sulfonate-Induced Mutagenesis and Its Effects on Peanut Agronomic, Yield and Quality Traits" Agronomy 10, no. 5: 655. https://doi.org/10.3390/agronomy10050655
APA StyleChen, T., Huang, L., Wang, M., Huang, Y., Zeng, R., Wang, X., Wang, L., Wan, S., & Zhang, L. (2020). Ethyl Methyl Sulfonate-Induced Mutagenesis and Its Effects on Peanut Agronomic, Yield and Quality Traits. Agronomy, 10(5), 655. https://doi.org/10.3390/agronomy10050655






