Fermented Alfalfa Brown Juice Significantly Stimulates the Growth and Development of Sweet Basil (Ocimum basilicum L.) Plants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sources of Brown Juice and Plant Materials
2.2. Experimental Study
2.2.1. Analysis of Biometric Features of Basil Plants
2.2.2. Determination of Chlorophyll Contents
2.2.3. Anatomical Analysis
2.3. Statistical Analysis
3. Results
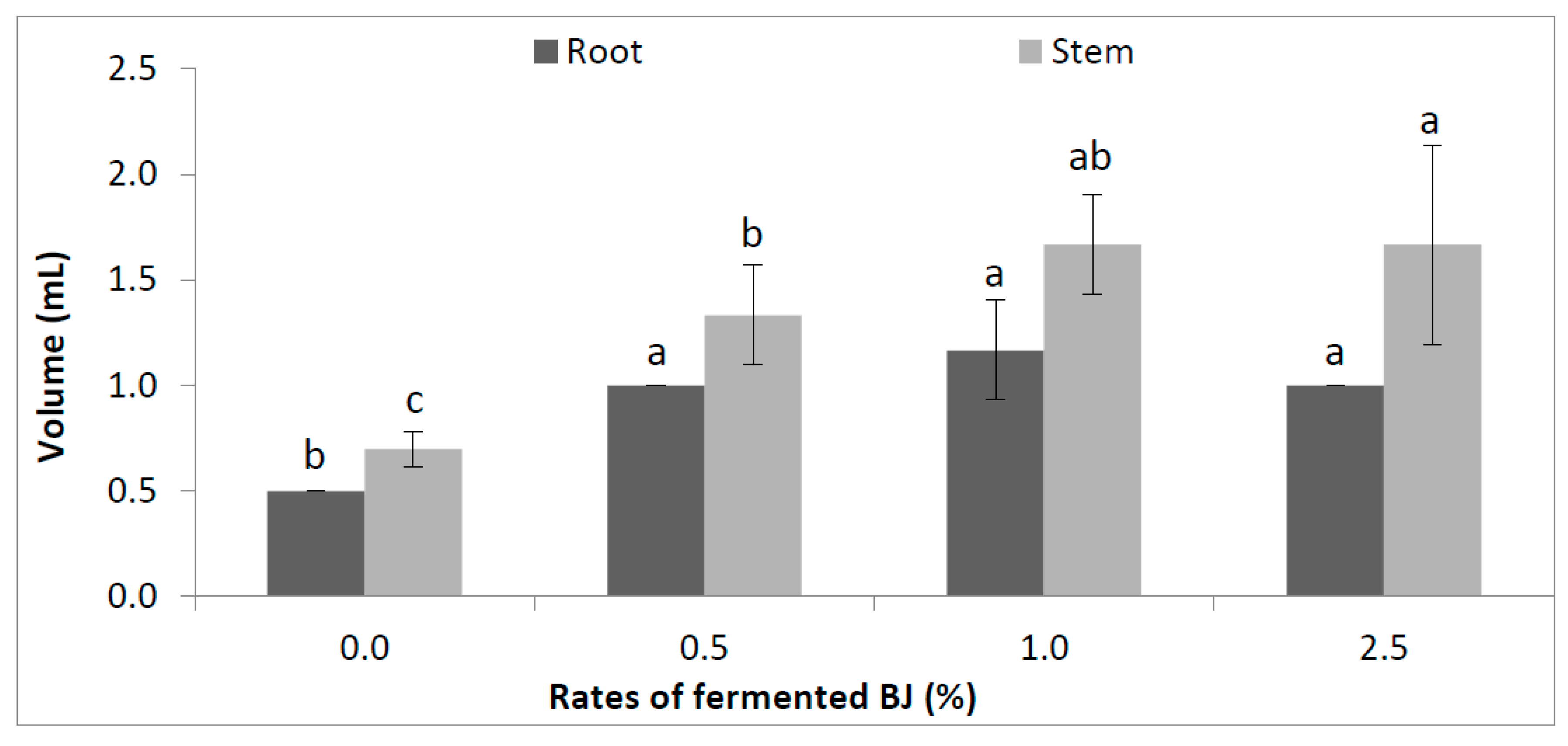
3.1. Plant Biometric Features
3.2. Contents of Photosynthetic Pigment
3.3. Anatomical Traits of Sweet Basil
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- de Boer, J.; Aiking, H. Strategies towards healthy and sustainable protein consumption: A transition framework at the levels of diets, dishes, and dish ingredients. Food Qual. Prefer. 2019, 73, 171–181. [Google Scholar] [CrossRef]
- Tenerio, A.T.; Kyriakopoulou, K.E.; Suarez-Garcia, E.; van den Berg, C.; van der Goot, A.J. Understanding differences in protein fractionation from conventional crops, and herbaceous and aquatic biomass—Consequences for industrial use. Trends Food Sci. Technol. 2018, 71, 235–245. [Google Scholar] [CrossRef]
- Fári, M.G.; Domokos-Szabolcsy, E. Method for Producing Plant Protein Coagulum. Hun Patent WO2019150144, 8 August 2019. [Google Scholar]
- Gaweł, E.; Grzelak, M.; Janyszek, M. Lucerne (Medicago sativa L.) in the human diet—Case reports and short reports. J. Herb. Med. 2017, 10, 8–16. [Google Scholar] [CrossRef]
- Tripathi, A.D.; Mishra, R.; Maurya, K.K.; Singh, R.B.; Wilson, D.W. Estimates for world population and global food availability for global health. Role Funct. Food Secur. Glob. Health 2019, 3–24. [Google Scholar] [CrossRef]
- Pirie, N.W. Leaf Protein and Other Aspects of Fodder Fractionation; Cambridge University Press: Cambridge, UK, 1987; p. 209. [Google Scholar]
- Bákonyi, N.; Kisvarga, S.; Barna, D.; Tóth, I.O.; El-Ramady, H.; Abdalla, N.; Kovács, S.; Rozbach, M.; Fehér, C.; Elhawat, N.; et al. Chemical Traits of Fermented Alfalfa Brown Juice: Its Implications on Physiological, Biochemical, Anatomical, and Growth Parameters of Celosia. Agronomy 2020, 10, 247. [Google Scholar] [CrossRef] [Green Version]
- Jadhav, R.K.; Chhaya, G. Influence of deproteinised foliage fluid liquid biofertilizer on nitrate reductase activity of Eleusine coracana plants. Curr. Bot. 2018, 33–36. [Google Scholar] [CrossRef]
- Thomsen, M.H.; Bech, D.; Kiel, P. Manufacturing of Stabilised Brown Juice for L-lysine Production—From University Lab Scale over Pilot Scale to Industrial Production. Chem. Biochem. Eng. Q. 2003, 18, 37–46. [Google Scholar]
- Bákonyi, N.; Tóth, I.O.; Barna, D.; Fári, M.G. Preliminary Experiments on Preserving Alfalfa Brown Juice for Bio-Industrial Use (Előkísérletek a Lucerna Barnalé Tartósítására Bioipari Hasznosítás Céljából); XXXVII, Óvári Tudományos Napok; Széchenyi István University: Mosonmagyaróvár, Hungary, 2018. [Google Scholar]
- Manwatkar, W.G.; Gogle, D.P. The Effect of Deproteinised Juice (DPJ) on Seed Germination and Seedling Growth of Different Plants (by Paper Towel Method). Int. J. Life Sci. 2014, A2, 65–68. [Google Scholar]
- Lamont, J.R.; Wilkins, O.; Bywater-Ekegärd, M.; Smith, D.L. From yogurt to yield: Potential applications of lactic acid bacteria in plant production. Soil Biol. Biochem. 2017, 111, 1–9. [Google Scholar] [CrossRef]
- Cheynier, V.; Comte, G.; Davies, K.M.; Lattanzio, V.; Martens, S. Plant phenolics: Recent advances on their biosynthesis, genetics, and ecophysiology. Plant Physiol. Biochem. 2013, 72, 1–20. [Google Scholar] [CrossRef]
- Shende, G.C.; Gogle, D.P. To study the effect of various concentration of deproteinized leaf juice (DPJ) of selected plants on growth of Aspergillus niger. Int. J. Life Sci. 2016, A6, 186–188. [Google Scholar]
- Jadhav, R.K.; Kadam, R.; Joshi, D. Physiology of Gramineae Crops by Deproteinised Foliage Extract and its Influence on Photosynthetic Chemistry. J. Phytochem. 2019, I, 149–163. [Google Scholar]
- Zanin, V. A New Nutritional Idea for Man: Lucerne Leaf Concentrate; APEF, Association Pour la Promotion des Extraits Foliaires en Nutrition: Paris, France, 1998. [Google Scholar]
- Smith, D.L.; Subramanian, S.; Lamont, J.R.; Bywater-Ekegärd, M. Signaling in the phytomicrobiome: Breadth and potential. Front. Plant Sci. 2015, 6, 921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onofrei, V.; Burducea, M.; Lobiuc, A.; Teliban, G.-C.; Ranghiuc, G.; Robu, T. Influence of Organic Foliar Fertilization on Antioxidant Activity and Content of Polyphenols in Ocimum Basilicum L. Acta Pol. Pharm. 2017, 74, 611–615. [Google Scholar]
- Alishah, H.M.; Heidari, R.; Hassani, H.; Dizaji, A.A. Effect of Water Stress on Some Morphological and Biochemical Characteristics of Purple Basil (Ocimum basilicum). J. Biol. Sci. 2006, 6, 763–767. [Google Scholar] [CrossRef] [Green Version]
- Nurzyńska-Wierdak, R.; Rożek, E.; Dzida, K.; Borowski, B. Growth response to nitrogen and potassium fertilization of common basil (Ocimum basilicum L.) plants. Hortorum Cultus 2012, 11, 275–288. [Google Scholar]
- Gülçin, I.; Elmastaş, M.; Aboul-Enein, H.Y. Determination of antioxidant and radical scavenging activity of Basil (Ocimum basilicum L. Family Lamiaceae) assayed by different methodologies. Phytother. Res. 2007, 21, 354–361. [Google Scholar] [CrossRef]
- Labra, M.; Miele, M.; Ledda, B.; Grassi, F.; Mazzei, M.; Sala, F. Morphological characterization, essential oil composition and DNA genotyping of Ocimum basilicum L. cultivars. Plant Sci. 2004, 167, 725–731. [Google Scholar] [CrossRef]
- Hikmasari, I. Handbook of Herbs and Spices, 2nd ed.; Woodhead Publishing Limited: Cambridge, UK, 2012; Volume 1. [Google Scholar]
- Bufalo, J.; Cantrell, C.; Astatkie, T.; Jeliazkov, V.; Gawde, A.; Boaro, C.S.F. Organic versus conventional fertilization effects on sweet basil (Ocimum basilicum L.) growth in a greenhouse system. Ind. Crop. Prod. 2015, 74, 249–254. [Google Scholar] [CrossRef] [Green Version]
- Chiang, L.-C.; Ng, L.-T.; Cheng, P.-W.; Chiang, W.; Lin, C.-C. Antiviral activities of extracts and selected pure constituents of Ocimum basilicum. Clin. Exp. Pharm. Physiol. 2005, 32, 811–816. [Google Scholar] [CrossRef]
- Phippen, W.B.; Simon, J.E. Anthocyanins in Basil (Ocimum basilicum L.). J. Agric. Food Chem. 1998, 46, 1734–1738. [Google Scholar] [CrossRef]
- Hess, M.; Barralis, G.; Bleiholder, H.; Buhr, L.; Eggers, T.; Hack, H.; Stauss, R. Use of the extended BBCH scale—General for the descriptions of the growth stages of mono; and dicotyledonous weed species. Weed Res. 1997, 37, 433–441. [Google Scholar] [CrossRef]
- Moran, R.; Porath, D. Chlorophyll Determination in Intact Tissues Using N,N-Dimethylformamide. Plant Physiol. 1980, 65, 478–479. [Google Scholar] [CrossRef] [Green Version]
- Wellburn, A.R. The Spectral Determination of Chlorophylls a and b, as well as Total Carotenoids, Using Various Solvents with Spectrophotometers of Different Resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Vidican, T.I.; Cachita-Cosma, D. Determination of assimilator pigment content in cladodes of Opuntia fragilis var. Fragilis exposed to light of different colors emitted by LEDs. Studia Univ. Vasile Goldis Arad 2010, 20, 49–54. [Google Scholar]
- Kálmán, K.; Nyakas, A.; Mihalik, E.; Nagy, E. Plantanatomical practicality. In Növényanatómiai Praktikum; JATE Press: Budapest, Hungary, 2019. [Google Scholar]
- Tukey, J.W. Comparing Individual Means in the Analysis of Variance. Biometrics 1949, 5, 99–114. [Google Scholar] [CrossRef]
- Reddy, G.U.; Deshmukh, V.R.; Joshi, R.N.; Kayama, R. Utilization of Alfalfa (Mediacago sativa L.) Whey as a Fertilizer in Irrigation. Jap. J. Grassl. Sci. 1987, 33, 32–37. [Google Scholar] [CrossRef]
- Bergstrand, K.-J.; Löfkvist, K.; Asp, H. Dynamics of nitrogen availability in pot grown crops with organic fertilization. Biol. Agric. Hortic. 2018, 35, 143–150. [Google Scholar] [CrossRef]
- Larimi, S.B.; Shakiba, M.; Mohammadinasab, A.D.; Vahed, M.M. Changes in nitrogen and chlorophyll density and leaf area of sweet basil (Ocimum basilicum L.) affected by biofertilizer and nitrogen application. Int. J. Biosci. 2014, 5, 256–265. [Google Scholar] [CrossRef]
- Ertani, A.; Schiavon, M.; Muscolo, A.; Nardi, S. Alfalfa plant-derived biostimulant stimulate short-term growth of salt stressed Zea mays L. plants. Plant Soil 2013, 364, 145–158. [Google Scholar] [CrossRef]
- El-Ziat, R.A.; Swaefy, H.M.; Esmail, S.E.A. The Response of Red Rubin Basil Plant to Organic Fertilizer and Humic Acid versus Chemical Fertilizers. Middle East J. Agric. Res. 2018, 7, 740–751. [Google Scholar]
- Venkateshappa, S.M.; Sreenath, K.P. Some Species of Lamiaceae- Comparative Anatomical Studies. Indo Am. J. Pharm. Res. 2013, 3, 6. [Google Scholar]
- Werker, E.; Putievsky, E.; Ravid, U.; Dudai, N.; Katzir, I. Glandular hairs and essential oil in developing leaves of Ocimum basilicum L. (Lamiaceae). Ann. Bot. 1993, 71, 43–50. [Google Scholar] [CrossRef]
- Nassar, M.A.; El-Sagai, M.U.; Azoz, S.N. Anatomical and Phytochemical studies on Ocimum basilicum L. plant (Lamiaceae). Int. J. Adv. Res. 2014, 2, 204–226. [Google Scholar]
- Ream, H.W.; Smith, D.; Walgenbach, R.P. Effects of Deproteinized Alfalfa Juice Applied to Alfalfa—Bromegrass, Bromegrass, and Corn1. Agron. J. 1977, 69, 685–689. [Google Scholar] [CrossRef]
- Anugoolprasert, O.; Kinoshita, S.; Naito, H.; Shimizu, M.; Ehara, H. Effect of low pH on the growth, physiological characteristics and nutrient absorption of sago palm in a hydroponic system. Plant Prod. Sci. 2012, 15, 125–131. [Google Scholar] [CrossRef]
- Long, A.; Zhang, J.; Yang, L.T.; Ye, X.; Lai, N.W.; Tan, L.L.; Lin, D.; Chen, L.S. Effects of low pH on photosynthesis, related physiological parameters, and nutrient profiles of citrus. Front. Plant Sci. 2017, 8, 185. [Google Scholar] [CrossRef] [Green Version]
- Porra, R.J.; Thompson, W.A.; Kriedemann, P.E. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: Verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim. Biophys. Acta 1989, 975, 384–394. [Google Scholar] [CrossRef]
- Ferreira, A.S.; Santos, M.A.D.; Corrêa, G.F. Soil microbial response to glucose and phosphorus addition under agricultural systems in the Brazilian Cerrado. An. Acad. Bras. Ciênc. 2013, 85, 395–403. [Google Scholar] [CrossRef]




| Chlorophyll-a | Chlorophyll-b | Chlorophyll-a/b Ratio | Total Chlorophyll | SPAD Value | |
|---|---|---|---|---|---|
| 0.0% | 6.91 b | 1.96 b | 3.52 a | 8.86 a | 27.00 b |
| 0.5% | 8.05 a (16.52%) | 2.46 ab (25.78%) | 3.26 ab | 10.51 ab (18.57%) | 34.60 a (28.15%) |
| 1.0% | 6.94 ab (0.56%) | 2.00 ab (2.26%) | 3.47 ab | 8.95 ab (0.93%) | 32.40 ab (20.00%) |
| 2.5% | 7.93 ab (14.86%) | 2.67 a (36.12%) | 2.97 b | 10.60 b (19.56%) | 31.60 ab (17.04%) |
| BJ Rate | Epidermis | Cortex | Pith | Vascular Tissue |
|---|---|---|---|---|
| 0.0% | 23.75 ± 5.55‡ b | 273.55 ± 48.18 a | 2202.89 ± 239.13 c | 234.41 ± 53.75 c |
| 0.5% | 27.92 ± 7.81 a | 259.39 ± 73.72 a | 2529.03 ± 280.56 b | 548.68 ± 107.39 a |
| 1.0% | 27.37 ± 8.22 ab | 260.97 ± 51.36 a | 2684.10 ± 259.04 a | 539.41 ± 96.54 a |
| 2.5% | 28.88 ± 7.54 a | 242.16 ± 56.36 a | 2101.83 ± 162.30 c | 290.38 ± 59.46 b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kisvarga, S.; Barna, D.; Kovács, S.; Csatári, G.; O. Tóth, I.; Fári, M.G.; Makleit, P.; Veres, S.; Alshaal, T.; Bákonyi, N. Fermented Alfalfa Brown Juice Significantly Stimulates the Growth and Development of Sweet Basil (Ocimum basilicum L.) Plants. Agronomy 2020, 10, 657. https://doi.org/10.3390/agronomy10050657
Kisvarga S, Barna D, Kovács S, Csatári G, O. Tóth I, Fári MG, Makleit P, Veres S, Alshaal T, Bákonyi N. Fermented Alfalfa Brown Juice Significantly Stimulates the Growth and Development of Sweet Basil (Ocimum basilicum L.) Plants. Agronomy. 2020; 10(5):657. https://doi.org/10.3390/agronomy10050657
Chicago/Turabian StyleKisvarga, Szilvia, Döme Barna, Szilvia Kovács, Gábor Csatári, Ibolya O. Tóth, Miklós Gábor Fári, Péter Makleit, Szilvia Veres, Tarek Alshaal, and Nóra Bákonyi. 2020. "Fermented Alfalfa Brown Juice Significantly Stimulates the Growth and Development of Sweet Basil (Ocimum basilicum L.) Plants" Agronomy 10, no. 5: 657. https://doi.org/10.3390/agronomy10050657







